Abstract
Background
Mutations in the BRCA1 (MIM 113705) gene are found in many families with multiple cases of breast and ovarian cancer, and women with a BRCA1 mutation are at significantly higher risk of developing breast and ovarian cancer than are the general public.
Methods
We obtained blood samples and pedigree information from 3568 unselected cases of early‐onset breast cancer and 609 unselected patients with ovarian cancer from hospitals throughout Poland. Genetic testing was performed for three founder BRCA1 mutations. We also calculated the risk of breast and ovarian cancer to age 75 in the first degree relatives of carriers using Kaplan‐Meier methods.
Results
The three founder BRCA1 mutations were identified in 273 samples (187 with 5382insC, 22 with 4153delA, and 64 with C61G). A mutation was present in 4.3% of patients with breast cancer and 12.3% of patients with ovarian cancer. The overall risk of breast cancer to age 75 in relatives was 33% and the risk of ovarian cancer was 15%. The risk for breast cancer was 42% higher among first degree relatives of carriers of the C61G missense mutation compared to other mutations (HR = 1.42; p = 0.10) and the risk for ovarian cancer was lower than average (OR = 0.26; p = 0.03). Relatives of women diagnosed with breast cancer had a higher risk of breast cancer than relatives of women diagnosed with ovarian cancer (OR = 1.7; p = 0.03).
Conclusions
The risk of breast cancer in female relatives of women with a BRCA1 mutation depends on whether the proband was diagnosed with breast or ovarian cancer.
Keywords: BRCA1 , breast cancer, ovarian cancer, penetrance
Mutations in the BRCA1 (MIM 113705) gene are found in a substantial proportion of families with multiple cases of breast and ovarian cancer, and women with a BRCA1 mutation are at significantly higher risk of developing breast and ovarian cancer than are the general public. However, uncertainty remains as to the best estimate of risk for gene mutation carriers. It is also uncertain which factors influence the penetrance of the BRCA1 gene. Different methods have been used to measure penetrance, but it is generally accepted that studies based on unselected patient series are superior to those based on cases selected for family history.1,2 This is because familial cases may be enriched for predisposing cofactors and because families with multiple cases are more likely to be ascertained than are families with a smaller number of affected women. If there are predisposing risk factors that cluster within families, then we expect the risk of cancer in close relatives to depend on whether the proband was affected with breast or ovarian cancer, as well as with the mutation itself.
Three founder BRCA1 mutations (5382insC, C61G, and 4153delA) comprise over 90% of all detectable BRCA1 mutations in Poland.3,4 We have conducted population surveys of unselected cases of breast (diagnosed under the age of 50) and ovarian (diagnosed at any age) cancer in Poland. The patients provided details about cancers in first degree relatives and underwent genetic testing for the three founder BRCA1 mutations. Using these data, we were able to estimate the cumulative incidence of all cancers in the first degree relatives of carriers, and to compare the risks by BRCA1 mutation and by the disease status of the index case (breast or ovarian cancer).
Methods
Study subjects
In the course of a national breast cancer survey we identified 4596 women diagnosed with breast cancer at the age of 50 or below at one of 18 centres in Poland from 1996 to 2003.5 We were able to obtain a DNA sample for BRCA1 analysis from 3568 of these women. A total of 609 patients with ovarian cancer were interviewed from 1999 to 2004 at eight centres in Poland. The ovarian cancer patients have been described in detail previously.6 The study was approved by the ethics board of the Pomeranian Medical University.
Pedigree data
The patient was interviewed in person by a member of the research team. The woman was asked to provide information about cancers in all her first degree relatives, including type of cancer, age at onset, age at death, and current age for relatives without cancer. None of the diagnoses of cancer in the relatives was confirmed by reference to pathology reports. Patients were unaware of their mutation status at the time of interview.
Laboratory analyses
Three founder mutations in the BRCA1 gene (5382insC, C61G, and 4153delA), which cover about 90% of BRCA1 mutations in Poland, were studied. Methods have been described elsewhere.3,4,5,6
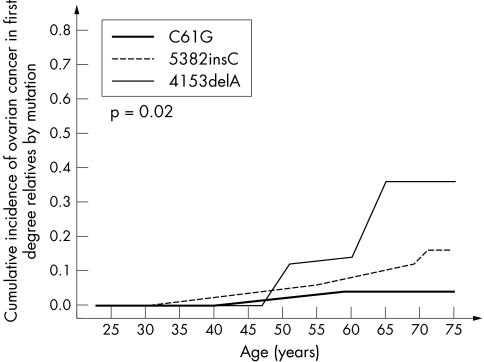
Figure 3 Cumulative incidence of ovarian cancer in female first degree relatives of 263 BRCA1 mutation carriers, by mutation.
Statistical analysis
We estimated the age specific breast, ovarian, and total cancer risks for first degree relatives of mutation carriers for each mutation separately, using Kaplan‐Meier survival analyses. Patients were considered to be at risk of cancer from birth until either the development of cancer, death from another cause, the date of patient interview, or until age 75. Cumulative incidence curves were computed separately for breast cancer, ovarian cancer, and all cancers combined (any cancer). Curves were computed separately for each of the three founder mutations. We also calculated the cumulative incidence of breast (and ovarian) cancer for women who were the relatives of probands with breast (or ovarian) cancer. Penetrance curves were compared for mothers and sisters. Statistical significance between curves was compared using the log‐rank test. To establish which of these factors were the most relevant for predicting lifetime cancer risk, a Cox proportional hazards model was used which incorporated mutation, proband disease status, and relationship (mother versus sisters). In this model the baseline categories were 5382insC, breast cancer diagnosed in the proband, and breast cancer diagnosed in sisters of the proband.
Results
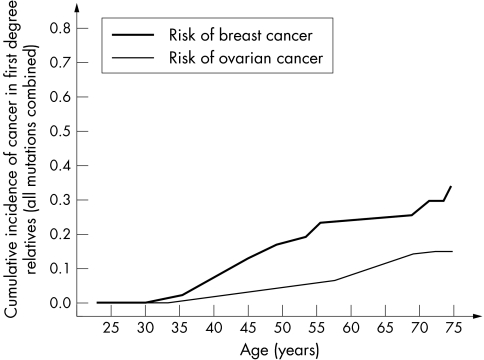
Among the unselected Polish women with cancer in this study, 273 mutation carriers were identified, including 198 probands with breast cancer and 75 probands with ovarian cancer. A mutation was present in 4.3% of the patients with breast cancer and 12.3% of the patients with ovarian cancer. There were sufficient data for 263 of the pedigrees for statistical analysis (96%). The cumulative risks of cancer among the first degree relatives of the BRCA1 mutation carriers are shown in table 1 and fig 1. The risk of breast cancer for all female first degree relatives of all mutation carriers was estimated to be 33% to age 75, and 67% of female relatives had been diagnosed with some type of cancer by the age of 75, compared with 35% of male relatives.
Table 1 Estimated cumulative risks for breast, ovarian, and other cancers for first degree relatives of patients with founder BRCA1 mutations.
| Cancer site in relatives | BRCA1 mutation type | First degree relatives with cancer/total | Cancer risk to age of 50 | Cancer risk to age of 75 | |
|---|---|---|---|---|---|
| Women | Breast | 5382insC | 60/557 | 0.15 | 0.29 |
| C61G | 32/226 | 0.23 | 0.46 | ||
| 4153delA | 8/68 | 0.25 | 0.25 | ||
| Any BRCA1 | 100/851 | 0.18 | 0.33 | ||
| Ovary | 5382insC | 29/557 | 0.04 | 0.17 | |
| C61G | 3/226 | 0.01 | 0.05 | ||
| 4153delA | 6/68 | 0.08 | 0.38 | ||
| Any BRCA1 | 38/851 | 0.03 | 0.15 | ||
| Other | 5382insC | 22/557 | 0.03 | 0.15 | |
| C61G | 17/226 | 0.05 | 0.36 | ||
| 4153delA | 1/68 | 0.02 | 0.02 | ||
| Any BRCA1 | 40/851 | 0.04 | 0.19 | ||
| Any | 5382insC | 111/557 | 0.22 | 0.61 | |
| C61G | 52/226 | 0.29 | 0.87 | ||
| 4153delA | 15/68 | 0.36 | 0.66 | ||
| Any BRCA1 | 178/851 | 0.25 | 0.67 | ||
| Men | Any | 5382insC | 47/553 | 0.03 | 0.36 |
| C61G | 16/193 | 0.05 | 0.33 | ||
| 4153delA | 7/70 | 0.00 | 0.39 | ||
| Any BRCA1 | 70/816 | 0.03 | 0.35 |
Figure 1 Cumulative incidence of breast and ovarian cancer in female first degree relatives of 263 BRCA1 mutation carriers.
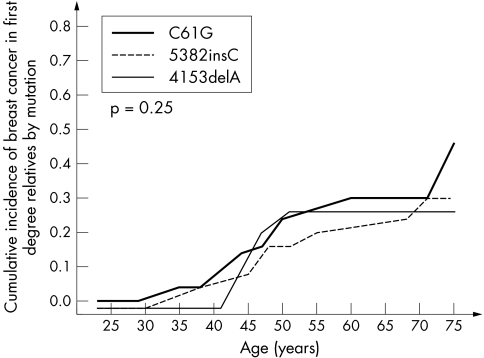
We observed moderate differences in cancer risk for the subgroups of relatives with each of the three different mutations (table 2, figs 2 and 3). In the Cox proportional hazard model, the breast cancer risk for relatives of women with the missense mutation C61G was about 40% higher than that conferred by the more common mutation 5382insC (OR = 1.44; p = 10−4).
Table 2 Comparison of risks of cancer in relatives, by proband mutation.
| Mutation in proband | HR (95% CI) p value | ||
|---|---|---|---|
| Breast cancer | Ovarian cancer | Any cancer | |
| 5382insC, n = 557 | 1 | 1 | 1 |
| 4153 del A, n = 68 | 1.12 (0.53 to 2.34) 0.77 | 1.85 (0.77 to 4.47) 0.17 | 1.17 (0.68 to 2.01) 0.58 |
| C61G, n = 226 | 1.44 (0.93 to 2.21) 0.10 | 0.28 (0.09 to 0.93) 0.04 | 1.28 (0.92 to 1.78) 0.15 |
Figure 2 Cumulative incidence of breast cancer in female first degree relatives of 263 BRCA1 mutation carriers, by mutation.
Differences in risk with different mutations were also seen for ovarian cancer. Only 5% of the female relatives of the C61G mutation carriers were affected with ovarian cancer by age 75, and this represented only 28% of the risk relative to the 5382insC mutation (OR = 0.28; p = 0.04). For all mutations combined, the risk of ovarian cancer to age 75 was 15%.
We compared the risk of cancer in sisters and mothers of the probands to establish if the risk appears to be changing with time. For both breast and ovarian cancer the lifetime risk for sisters exceeded that of mothers (table 3). The hazard ratio for breast cancer (sisters versus mothers) was 2.5 (p = 104) and the hazard ratio for ovarian cancer was 3.0 (p = 0.003).
Table 3 Estimated cumulative risks for breast and ovarian cancer for mothers and for sisters of patients with founder BRCA1 mutations.
| Cancer site in relatives | BRCA1 mutation type | First degree relatives with cancer/total | Cancer risk to age of 50 | Cancer risk to age of 75 |
|---|---|---|---|---|
| Breast | Sisters | 57/356 | 0.27 | 0.42 |
| Mothers | 41/254 | 0.10 | 0.25 | |
| Any BRCA1 | 100/851 | 0.18 | 0.33 | |
| Ovary | Sisters | 18/356 | 0.06 | 0.21 |
| Mothers | 20/254 | 0.01 | 0.12 | |
| Any BRCA1 | 38/1158 | 0.02 | 0.15 |
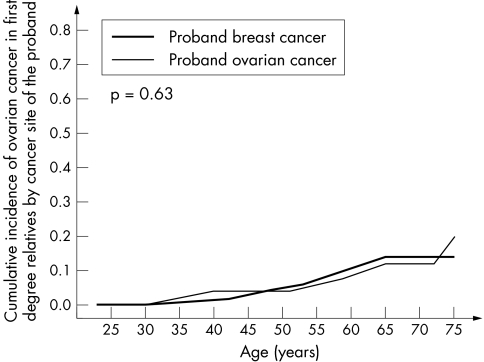
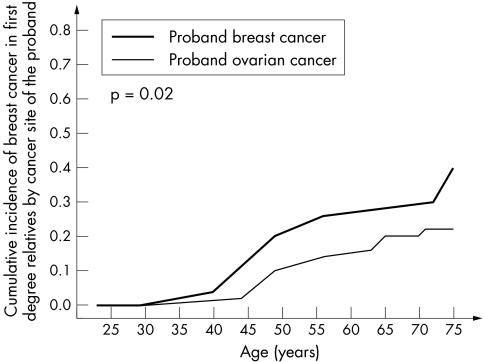
The data were then analysed with respect to the type of cancer diagnosed in the proband (breast or ovarian). The underlying null hypothesis is that the cancer risk is entirely dependent on the mutation itself, and that other familial factors (genetic and/or environmental) do not play a significant role. The risk of cancer was therefore compared for relatives of probands with breast and ovarian cancer. For ovarian cancer risk in relatives, there was no appreciable difference seen whether the proband had breast or ovarian cancer (table 2, fig 4). In contrast, the relatives of breast cancer patients experienced a much greater risk of breast cancer than did the relatives of ovarian cancer patients. A survival analysis using Cox regression estimated that the relatives of ovarian cancer patients experienced approximately one half of the risk of breast cancer experienced by relatives of breast cancer patients (HR = 0.58; p = 0.03) (table 4, fig 5).
Figure 4 Cumulative incidence of breast cancer in female first degree relatives of 263 BRCA1 mutation carriers, by cancer type in proband.
Table 4 Comparison of risks in relatives by proband cancer type.
| Proband cancer type | RR (95% CI) P | ||
|---|---|---|---|
| Breast cancer | Ovarian cancer | Any cancer | |
| Breast cancer, n = 624 | 1 | 1 | 1 |
| Ovarian cancer, n = 227 | 0.58 (0.36 to 0.94) 0.03 | 1.18 (0.60 to 2.31) 0.63 | 0.82 (0.58 to 1.14) 0.23 |
Figure 5 Cumulative incidence of ovarian cancer in female first degree relatives of 263 BRCA1 mutation carriers, by cancer type in proband.
Finally, we wished to establish whether the observed effect of the proband cancer type on breast cancer risk was due to a different distribution of mutations in the two groups. A multivariate Cox regression was carried out. In this analysis the hazard ratios were adjusted for specific mutation and relationship to the proband (sister versus mother). After adjustment the effect of proband cancer type was still significant; the hazard ratio for breast cancer in relatives of women with ovarian cancer was 0.51 (95% CI 0.31 to 0.84; p = 0.008). Similarly, in the multivariate analysis the effects of the mutation types on cancer risk were essentially unchanged. Because of the possible confounding influence of the C61G mutation on these results, we repeated this regression analysis but restricted the probands to women who carried the 5382insC mutation. The results were essentially unchanged.
Discussion
Although it is clear that BRCA1 mutations confer a substantial risk for both breast and ovarian cancer, the cancer risk appears to vary between populations, and within populations between individual carriers. Undoubtedly, some of the variance in the risk estimates can be attributed to biases in the methods of the different studies, but there are several reasons to believe that the differences are real. There are three potential sources of variation: first, variation in risk between mutations; second, modifying genetic background; and third, environmental/lifestyle factors.
In our study we have shown that the risks of breast and ovarian cancer appear to differ significantly with the mutation itself. We found a higher risk of breast cancer and a lower risk of ovarian cancer associated with the missense mutation C61G. The C61G mutation is the most frequent missense change reported to the Breast Cancer Information Core Database (http://research.nhgri.nih.gov/bic/). It was first reported in two families, one of which was Polish, and is one of the few missense changes in BRCA1 that has been determined to be linked to disease using segregation analysis.7 This mutation targets a critical residue that coordinates binding to a zinc atom in the RING finger domain of BRCA1.8 Mutation at this residue abrogates the ubiquitin ligase activity of BRCA1.9,10 Importantly, this mutant seems to be unable to reverse gamma radiation hypersensitivity of BRCA1 null cells.10 We observed that the highest risk of breast cancer and the lowest risk of ovarian cancer were associated with this mutation. It is not clear of this is a general characteristic of other pathogenic missense mutations or of other mutations which disrupt the RING finger. To our knowledge this is the only common founder mutation in the RING finger.
Several studies have proposed the notion of a genotype‐phenotype correlation for BRCA1 and BRCA2 in which risk for ovarian or breast cancer varies with mutation position.11,12,13,14 However, these studies have looked at mutations as a group, with most mutations being frameshift or nonsense mutations which generate truncated proteins. It will be of interest to functionally evaluate the C61G mutation by comparing its activity in breast and ovarian cell lines.
There is also some evidence that families with mutations in the centre of BRCA1 (nucleotides 2401–4190) have a lower than average risk of breast cancer.12 The risks of breast and ovarian cancers associated with particular mutations are difficult to estimate, because of the high costs of genetic testing, the rarity of mutations in the general population, and the large number of distinct mutations in the gene. At this stage it is only practical to perform mutation specific analyses for founder populations. Antoniou et al estimated the risks of breast and ovarian cancer in a (mostly) Jewish study population with two BRCA1 founder mutations.15 They reported that the risk of ovarian cancer to age 70 was substantially higher among carriers of the BRCA1 5382insC mutation (33%) than the 185 del AG mutation (14%), but the difference was not statistically significant. No apparent differences were noted for breast cancer risks with the two BRCA1 mutations.
We recently reported that the breast cancer risk associated with the Polish 4153delAG mutation was much lower than that associated with the other two mutations, based on odds ratios generated from unselected Polish breast cancer patients and controls.16 In the present study, which was based on reported family histories, we did not observe that the breast cancer risk for relatives of probands with the 4153delAG mutation was significantly lower than for the other two mutations; however, the number of patients in this group of relatives was small (n = 8). The reason for the difference between the two studies is not yet clear, but in both the actual number of subjects was small.
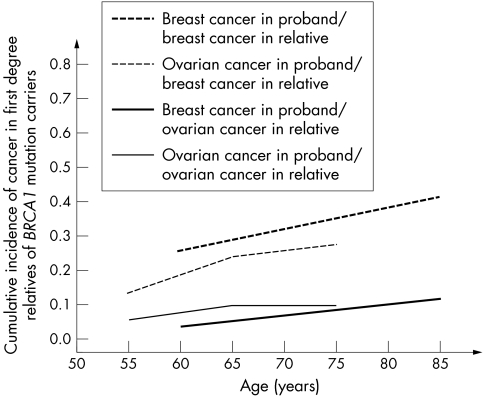
We found that the risk of breast cancer in first degree relatives differed significantly, depending on whether the proband was diagnosed with breast or ovarian cancer. This was not the case for ovarian cancer risk. These observations are consistent with the earlier reports of Moslehi et al17 and Warner et al,18 who used identical methods in Jewish populations of unselected ovarian cancer and breast cancer patients, respectively. They also found that the risk of breast cancer varied, depending on the type of cancer in the proband, but the risk of ovarian cancer was similar. The data from these two studies are summarised in fig 6. Together the studies suggest that it is likely that there are modifying genetic risk factors for breast cancer, but not necessarily for ovarian cancer. There are modifying non‐genetic risk factors for ovarian cancer in BRCA1 mutation carriers, such as oral contraceptives19 and breastfeeding (unpublished data), but these risk factors do not necessarily cluster within families.
Figure 6 Summary of cancer risk estimates in Ashkenazi Jewish women. Cancer risks in first degree relatives of mutation carriers, by cancer type in proband. (From Moslehi et al17 and Warner et al18)
We also observed that the risks of breast and ovarian cancer were higher in the sisters of probands than in their mothers, confirming previous reports.2,20 This observation suggests that non‐genetic factors are also important in influencing the risk of both breast and ovarian cancer and that the prevalence of these risk factors has increased in Poland over the last generation.
The baseline cancer risks to age 75 in Poland are approximately 1.5% for ovarian cancer and 5% for breast cancer.21 Therefore, based on the observed risks in first degree relatives, the average penetrances of the BRCA1 founder mutations are estimated to be 46% for breast cancer and 25% for ovarian cancer. However, the means for individual mutations vary considerably.
Our study has several strengths. It is a large single centre study of unselected breast and ovarian cancer patients from a well defined, ethnically homogeneous group residing in one country. We believe this to be close to the ideal study design. Very few patients refused to participate when approached. Subjects were unaware of their genetic status at the time the family history was obtained, and both patients populations were studied using the same methods and by the same investigators and interviewers. Our total number of mutation carriers in this study was 273, compared to a total of 196 in the Antoniou study (which was based on a meta‐analysis of 22 population based studies from many different countries).15 Nevertheless, some of our individual risk estimates were imprecise and it will be important to continue to accrue patients to these two studies and to re‐examine this question in the future.
Acknowledgements
We would like to thank M Chosia, E Grzybowska, D Lange, B Mika, A Mackiewicz, A Karczewska, K Lamperska, M Stawicka, J Breborowicz, S Gozdecka‐Grodecka, M Bebenek, D Sorokin, A Wojnar, O Haus, J Sir, T Mierzwa, S Niepsuj, K Gugala, S Gozdz, J Sygut, B Kozak‐Klonowska, B Musiatowicz, M Posmyk, R Kordek, M Morawiec, O Zambrano, B Wasko, L Fudali, D Surdyka, K Urbański, J Mituś, J Rys, M Swiec, A Rozmarek, I Dziuba, C Szczylik, A Kozak, A Kozlowski for contributing patients to this study.
Electronic‐database information
The Breast Cancer Information Core Database web site is at http://research.nhgri.nih.gov/bic/
Footnotes
Work in the Monteiro Laboratory is supported by NIH grant CA92309
Competing interests: none declared
The Breast Cancer Information Core Database web site is at http://research.nhgri.nih.gov/bic/
References
- 1.Risch H A, Narod S A. On the use of familial aggregation in population‐based case probands for calculating penetrance. J Natl Cancer Inst 20039573–74. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou A, Pharoah P D, Narod S A, Risch H A, Eyfjord J E, Hopper J L, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles D M, Tang N, Olah E, Anton‐Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi O P, Thompson D, Evans C, Peto J, Lalloo F, Evans D G, Easton D F. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003721117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gyorski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J, Gronwald J, Pluzanska A, Bebenek M, Fischer‐Maliszewska L, Grzybowska E, Narod S A, Lubinski J. Founder mutations in the BRCA1 gene in Polish families with breast‐ovarian cancer. Am J Hum Genet 2000661963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorski B, Jakubowska A, Huzarski T, Byrski T, Gronwald J, Grzybowska E, Mackiewicz A, Stawicka M, Bebenek M, Sorokin D, Fiszer‐Maliszewska L, Haus O, Janiszewska H, Niepsuj S, Gozdz S, Zaremba L, Posmyk M, Pluzanska M, Kilar E, Czudowska D, Wasko B, Miturski R, Kowalczyk J R, Urbanski K, Szwiec M, Koc J, Debniak B, Rozmiarek A, Debniak T, Cybulski C, Kowalska E, Toloczko‐Grabarek A, Zajaczek S, Menkiszak J, Medrek K, Masojc B, Mierzejewski M, Narod S A, Lubinski J. A high proportion of founder BRCA1 mutations in Polish breast cancer families. Int J Cancer 2004110683–686. [DOI] [PubMed] [Google Scholar]
- 5.Lubiński J, Górski B, Huzarski T, Byrski T, Gronwald J, Serrano‐Fernández P, Domagala W, Chosia M, Uciński M, Grzybowska E, Lange D, Mika B, Mackiewicz A, Karczewska A, Breborowicz J, Lamperska K, Stawicka M, Gozdecka‐Grodecka S, Bębenek M, Sorokin D, Wojnar A, Haus O, Sir J, Mierzwa T, Niepsuj S, Gugala K, Goźdź S, Sygut J, Kozak‐Klonowska B, Musiatowicz B, Posmyk M, Kordek R, Morawiec M, Zambrano O, Waśko B, Fudali L, Skręt J, Surdyka D, Urbański K, Mituś J, Ryś J, Szwiec M, Rozmiarek A, Dziuba I, Wandzel P, Wiśniowski R, Szczylik C, Kozak A, Kozlowski A, Narod SA BRCA1‐positive Breast Cancers in Young Women in Poland. Breast Cancer Res Treat., accepted
- 6.Menkiszak J, Gronwald J, Gorski B, Jakubowska A, Huzarski T, Byrski T, Foszczynska‐Kloda M, Haus O, Janiszewska H, Perkowska M, Brozek I, Grzybowska E, Zientek H, Gozdz S, Kozak‐Klonowska B, Urbanski K, Miturski R, Kowalczyk J, Pluzanska A, Niepsuj S, Koc J, Szwiec M, Drosik K, Mackiewicz A, Lamperska K, Strozyk E, Godlewski D, Stawicka M, Wasko B, Bebenek M, Rozmiarek A, Rzepka‐Gorska I, Narod S A, Lubinski J. Hereditary ovarian cancer in Poland. Int J Cancer 2003106942–945. [DOI] [PubMed] [Google Scholar]
- 7.Friedman L S, Ostermeyer E A, Szabo C I, Dowd P, Lynch E D, Rowell S E, King M C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet 19948399–404. [DOI] [PubMed] [Google Scholar]
- 8.Brzovic P S, Rajagopal P, Hoyt D W, King M C, Klevit R E. Structure of a BARD1 heterodimeric RING‐RING complex. Nat Struct Biol 20018833–837. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T. The RING heterodimer BRCA1‐BARD1 is a ubiquitin ligase inactivated by a breast cancer‐derived mutation. J Biol Chem 200127614537–14540. [DOI] [PubMed] [Google Scholar]
- 10.Ruffner H, Joazeiro C A P, Hemmati D, Hunter T, Verma I M. Cancer‐predisposing mutations within the RING domain of BRCA1: loss of ubiquitin protein ligase activity and protection from radiation sensitivity. Proc Natl Acad Sci U S A 2001985134–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gayther S A, Warren W, Mazoyer S, Russell P A, Harrington P A, Chiano M, Seal S, Hamoudi R, van Rensburg E J, Dunning A M. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype‐phenotype correlation. Nat Genet 199511428–433. [DOI] [PubMed] [Google Scholar]
- 12.Thompson D, Easton D. Breast Cancer Linkage Consortium. Variation in BRCA1 cancer risks by mutation position. Cancer Epidemiol Biomarkers Prev 200211329–336. [PubMed] [Google Scholar]
- 13.Lubinski J, Phelan C M, Ghadirian P, Lynch H T, Garber J, Weber B, Tung N, Horsman D, Isaacs C, Monteiro A N, Sun P, Narod S A. Cancer variation associated with the position of the mutation in the BRCA2 gene. Fam Cancer 200431–10. [DOI] [PubMed] [Google Scholar]
- 14.Thompson D, Easton D, Breast Cancer Linkage Consortium Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet 200168410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniou A C, Pharoah P D, Narod S, Risch H A, Eyfjord J E, Hopper J L, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles D M, Tang N, Olah E, Anton‐Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi O P, Thompson D, Evans C, Peto J, Lalloo F, Evans D G, Easton D F. Breast and ovarian cancer risks to carriers of the BRCA1 5382insC and 185delAG and BRCA2 6174delT mutations: a combined analysis of 22 population based studies. J Med Genet 200542602–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorski B, Menkiszak J, Gronwald J, Lubinski J, Narod S A. A protein truncating BRCA1 allele with a low penetrance of breast cancer. J Med Genet 200441e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moslehi R, Chu W, Karlan B, Fishman D, Risch H, Fields A, Smotkin D, Ben‐David Y, Rosenblatt J, Russo D, Schwartz P, Tung N, Warner E, Rosen B, Friedman J, Brunet J S, Narod S A. BRCA1 and BRCA2 mutation analysis of 208 Ashkenazi Jewish women with ovarian cancer. Am J Hum Genet 2000661259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, Ozcelik H, Goss P, Allingham‐Hawkins D, Hamel N, Di Prospero L, Contiga V, Serruya C, Klein M, Moslehi R, Honeyford J, Liede A, Glendon G, Brunet J S, Narod S. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 1999911241–1247. [DOI] [PubMed] [Google Scholar]
- 19.Narod S A, Risch H, Moslehi R, Dorum A, Neuhausen S, Olsson H, Provencher D, Radice P, Evans G, Bishop S, Brunet J S, Ponder B A. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N Engl J Med 1998339424–428. [DOI] [PubMed] [Google Scholar]
- 20.King M C, Marks J H, Mandell J B, New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003302643–646. [DOI] [PubMed] [Google Scholar]
- 21.Parkin D M, Whelan S L, Ferlay J, Raymond L, Young J. eds. Cancer incidence in five continents. Vol VII. Lyon, France: IARC Scientific Press, 1997