Abstract
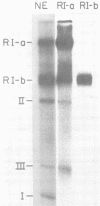
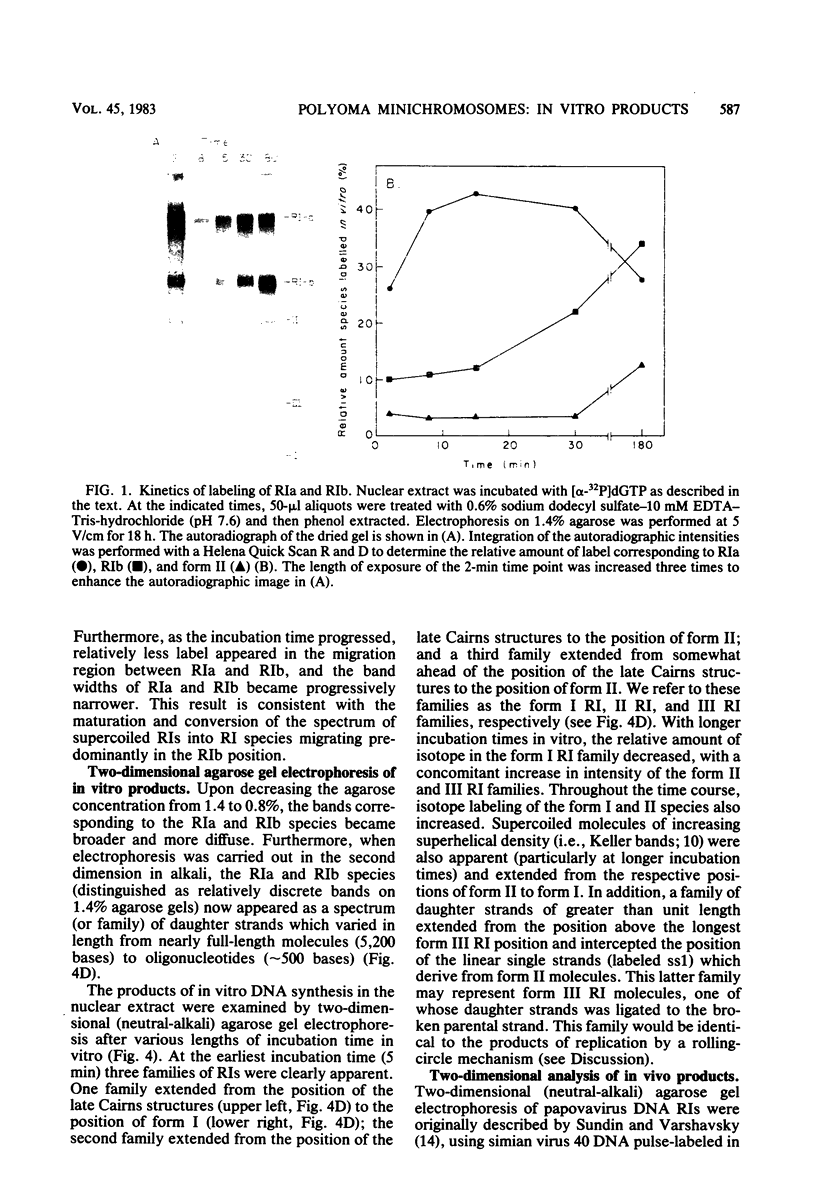
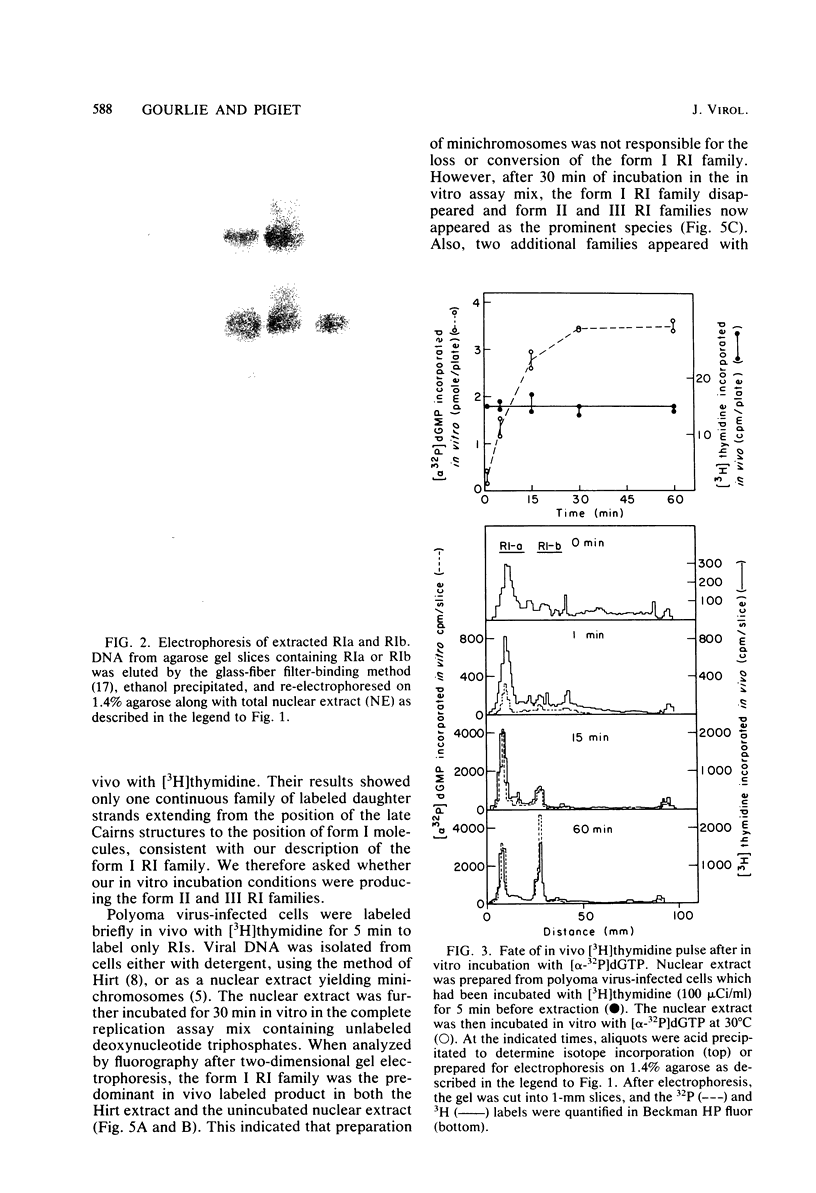
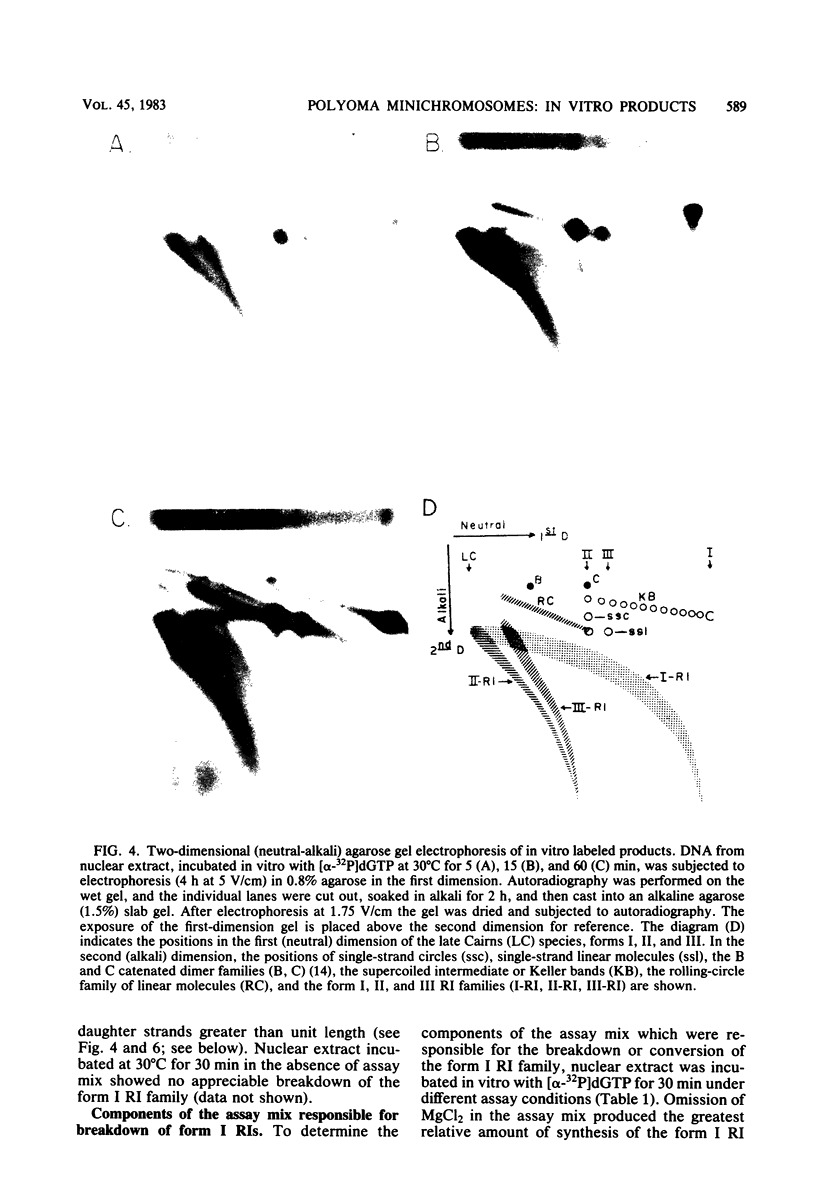
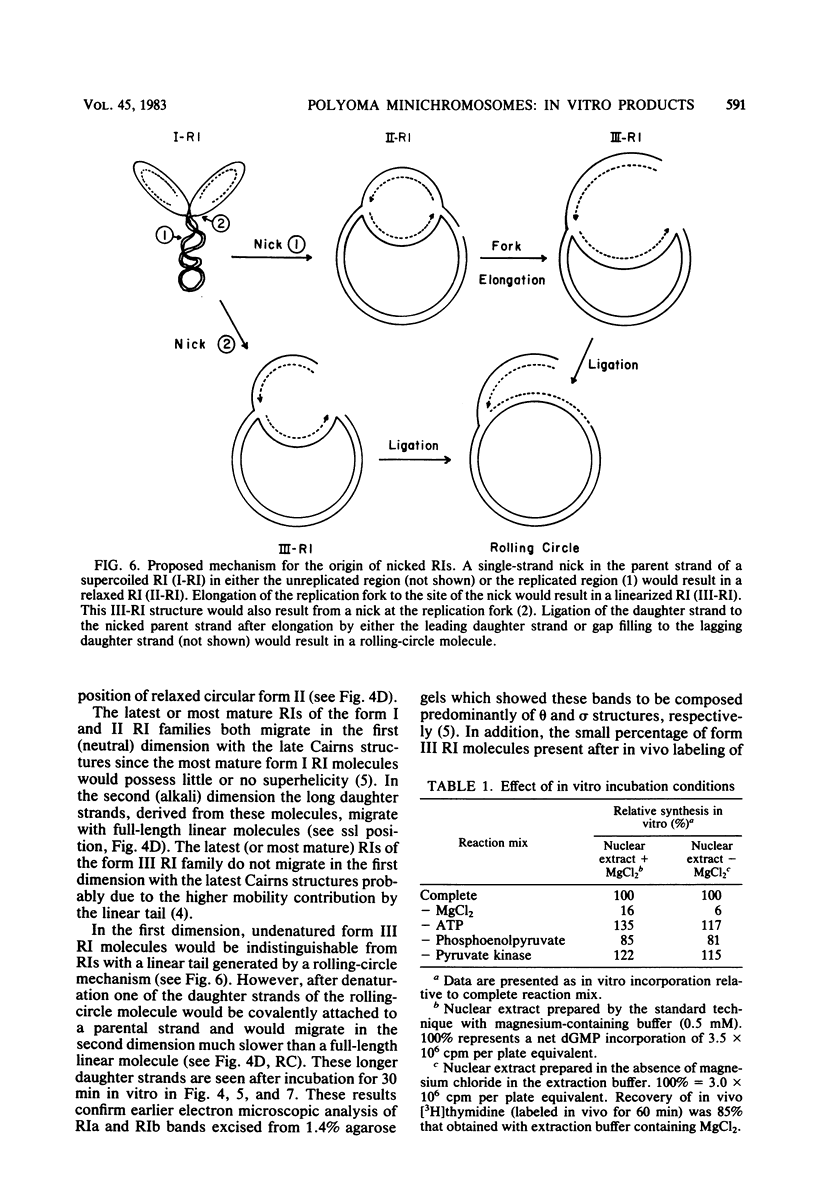
A two-dimensional (neutral-alkali) agarose gel electrophoretic system was used to separate three families of replicative intermediate (RI) polyoma virus DNA molecules (form I, form II, and form III RIs). Two of these families, form II and III RIs, are the result of artifactual nicking of one of the parental strands of supercoiled RIs (form I RIs) during in vitro replication of soluble minichromosomes. Kinetic studies in vitro showed that the nicked RIs serve as templates for limited DNA synthesis. The nicked species are not converted into normal products, however. The nicking reaction, which appears to be specific for the parental strands, is dependent on magnesium ions and occurs concurrently with the in vitro synthesis of DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjursell G. Effects of 2'-deoxy-2'-azidocytidine on polyoma virus DNA replication: evidence for rolling circle-type mechanism. J Virol. 1978 Apr;26(1):136–142. doi: 10.1128/jvi.26.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Anderson S., Bar-Shavit R., Collins E., Edenberg H., Herman T., Karas B., Kaufmann G., Krokan H., Shelton E. Replication and structure of simian virus 40 chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):679–692. doi: 10.1101/sqb.1979.043.01.076. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Fisher M. P., Dingman C. W. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide composite gels. Biochemistry. 1971 May 11;10(10):1895–1899. doi: 10.1021/bi00786a026. [DOI] [PubMed] [Google Scholar]
- Gourlie B. B., Krauss M. R., Buckler-White A. J., Benbow R. M., Pigiet V. Polyoma virus minichromosomes: a soluble in vitro replication system. J Virol. 1981 Jun;38(3):805–814. doi: 10.1128/jvi.38.3.805-814.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlie B. B., Pigiet V., Breaux C. B., Krauss M. R., King C. R., Benbow R. M. Polyoma virus minichromosomes: associated enzyme activities. J Virol. 1981 Jun;38(3):826–832. doi: 10.1128/jvi.38.3.826-832.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. E. Fine structure of polyoma virus DNA. J Mol Biol. 1977 Dec 5;117(2):447–471. doi: 10.1016/0022-2836(77)90137-1. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Keller W. Characterization of purified DNA-relaxing enzyme from human tissue culture cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2550–2554. doi: 10.1073/pnas.72.7.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. F. Analysis of polyoma virus DNA replicative intermediates by agarose gel electrophoresis. J Virol. 1977 Sep;23(3):827–832. doi: 10.1128/jvi.23.3.827-832.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Wigmore D. J. Sites in simian virus 40 chromatin which are preferentially cleaved by endonucleases. Cell. 1978 Dec;15(4):1511–1518. doi: 10.1016/0092-8674(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. In vitro replication of simian virus 40 DNA in a nucleoprotein complex. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3466–3470. doi: 10.1073/pnas.73.10.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Tsubota Y., Waqar M. A., Burke J. F., Milavetz B. I., Evans M. J., Kowalski D., Huberman J. A. Association of enzymes with replicating and nonreplicating simian virus 40 chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):693–704. doi: 10.1101/sqb.1979.043.01.077. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Föhring B., Chowdhury K., Gruss P., Sauer G. Origin of DNA replication in papovavirus chromatin is recognized by endogenous endonuclease. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5964–5968. doi: 10.1073/pnas.75.12.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W., Spaeren U., Mastromei G., Eliasson R., Reichard P. Replication of polyoma DNA in nuclear extracts and nucleoprotein complexes. J Mol Biol. 1979 Dec 15;135(3):675–689. doi: 10.1016/0022-2836(79)90171-2. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]