Abstract
Background
A gene for Larsen syndrome was recently described, and mutations were reported in five cases.
Objective
To test whether mutations in this gene, FLNB, could explain the disease in our independent collection of sporadic and dominant Larsen syndrome cases; and to test whether mutations occurred in a non‐random pattern.
Results
Missense mutations were found in each of five cases. Four of the five were new; one was reported in a sporadic case in the original Larsen syndrome study of five cases. All mutations from the two studies were compiled. Clustered mutations were observed within three filamin B protein domains: the calponin homology 2 domain, repeat 14, and repeat 15. This suggested that as few as five (of the total of 46) coding exons of FLNB could be screened to detect Larsen syndrome mutations. Four of these exons were screened in a sixth (sporadic) case and a previously reported G1691S substitution mutation detected.
Conclusions
Mutations in FLNB may be responsible for all cases of Larsen syndrome. They appear to occur in specific functional domains of the filamin B protein. This should simplify diagnostic screening of the FLNB gene. Analyses in larger patient series are warranted to quantify this. The study confirmed the extreme variability in clinical presentation and the presence of unaffected carriers. A molecular screen would be valuable for diagnosis and genetic counselling.
Keywords: Larsen syndrome, skeletal/craniofacial development, genetic heterogeneity, mutation detection
Larsen syndrome was first recognised and described in 1950 as a congenital disorder involving characteristic facial features, ligamentous laxity, multiple joint dislocations, and foot deformities.1 Craniofacial features are pathognomonic and include hypertelorism, a prominent forehead, and a depressed nasal bridge. Extensive orthopaedic management is often required to treat dislocations of hips, knees, and elbows, and the foot deformities, which include talipes equinovarus (club feet), “serpentine” or “z‐foot”, and pes equinovalgus.2 An accessory ossification centre in the calcaneus is also thought to be a consistent feature of the disorder.3,4,5 Scoliosis and cervical kyphosis may be secondary to failure of spinal segmentation; the latter deformity is of particular concern because of potentially fatal impingement on the spinal cord.6 The incidence of Larsen syndrome is estimated to be 1 in 100 000,7 but it is well recognised that diagnosis is problematic because of the wide variability in severity and clinical symptoms.3,8,9,10
Genetic heterogeneity in Larsen syndrome has been suggested. Many sporadic cases have been described in addition to autosomal dominant forms.1,3,4,11,12 A recessive form of Larsen syndrome has been observed in an inbred population from the island of La Réunion; however, the diagnosis in this population has been disputed.6,12 A dominant form of Larsen syndrome (LRS1; MIM 150250) was mapped to chromosome 3p14 through a genome‐wide linkage scan in an extended Swedish pedigree.10 “Larsen‐like” features, including facial dysmorphism and multiple joint dislocations, have also been reported in two patients with unbalanced translocations involving human chromosomes 6p and 1q42‐qter.13 Fluorescence in situ hybridisation (FISH) studies of the same patients revealed that each was monosomic for distal 6p25, and it was suggested that this region and chromosome 1q42‐qter may harbour genes for Larsen syndrome.14
Recently, mutations in the filamin B (FLNB) gene encoded in the 3p14 region linked to LRS1 were described in four sporadic and one dominant case of Larsen syndrome.15 Filamin B is a member of the filamin family of cytoskeletal proteins that induce actin polymerisation and participate in its interaction with signal transduction pathways.16,17,18 It is a high molecular weight protein containing 2602 amino acids that are organised into an N‐terminal actin‐binding domain (ABD) followed by 24 repeating, antiparallel β‐sheet units. Two calponin homology domains (CHD) comprise the ABD. The 24 repeating domains are interrupted by two “hinge” regions, one between repeats 15 and 16, and the second between repeats 23 and 24. The five mutations were found in the filamin B ABD (CHD2), repeat 14, or repeat 15. Four were heterozygous missense changes predicted to create single amino acid substitutions, and a fifth was an in‐frame deletion predicting loss of an amino acid. FLNB mRNA is expressed in many tissues, and the protein has been detected in human fetal chondrocytes throughout the epiphyseal growth plate and in condensing chondrocytes of embryonic mouse vertebral bodies.15 These observations support a critically important role for filamin B in early chondrogenesis, but the cellular consequences of disease causing mutations in this process, and in other cell types, are unknown.
We previously ascertained Larsen syndrome probands presenting with both sporadic and dominant forms. These earlier results, in which FLNB mutations were found in all (five) independent cases, suggested to us that the majority of, if not all, Larsen syndrome cases could be caused by mutations in this gene. We also hypothesised that the clinical manifestations of Larsen syndrome might arise as a result of alterations in specific functional domains of filamin B. If so, mutations should cluster in a non‐random pattern. To test these ideas we characterised the FLNB gene in our collection of patients.
Methods
Six probands were ascertained in the orthopaedic clinics of Texas Scottish Rite Hospital for Children. Two cases were sporadic from non‐consanguineous parents, and the remaining families showed a dominant inheritance. One proband (LR6‐1) was African American and all the others were white American. Diagnoses were made by JAH, CEJ, or KER; criteria were facial features typical of Larsen syndrome and one or more associated orthopaedic findings (spine, hips, knees, feet). Unrelated healthy controls without a family history of musculoskeletal disorders were ascertained locally. Informed consent was obtained from all participants in accordance with protocols approved by local institutions, the University of Texas Southwestern Medical Center institutional review board, and the Office of Human Research Protection. We collected blood samples from participating individuals by venepuncture. DNA was isolated from whole blood lymphocytes by standard procedures.
Forty six coding exons of FLNB were identified from public databases. We designed flanking primer sequences or used those kindly provided (Krakow D, personal communication). Primer sequences and conditions are available on request. Each exon was amplified from subject DNA and initially searched for heterozygous variants by one of two methods: either denaturing high performance liquid chromatography (dHPLC) or DNA capillary sequencing using the 3730 XL instrument (Applied Biosystems, Foster City, California, USA). Samples with dHPLC results different from normal control were subsequently sequenced. Common variants were identified by comparison with the reference human genomic sequence (hg17) using Sequencher software. Potential mutations were tested for segregation with disease in families by sequencing or restriction endonuclease cleavage. Segregating variants in the inherited cases were analysed by the same methods in at least 100 chromosomes from ethnically matched, healthy control individuals to further exclude them as common polymorphisms. For the sporadic case LR7‐1, parental genotypes were confirmed by analysis of five additional unlinked loci. Sequence alignments in vertebrate species were obtained from http://www.ensembl.org.
Results and comment
We originally ascertained five families through probands treated at this institution. Individuals with facial features typical of Larsen syndrome and clinical orthopaedic involvement were considered affected. One case (LR7‐1) was apparently sporadic, and the remaining cases were familial, with dominant inheritance. We tested linkage to chromosome 3p14 loci in the three multigeneration families LR1, LR9, and CF16 by genotyping polymorphic microsatellite loci in available individuals. Results were consistent with linkage to this region when a dominant inheritance model was applied (data not shown). Next, the 46 coding exons of FLNB were screened for variants in affected probands of each family. We detected FLNB missense mutations in all cases. Co‐segregation with disease was confirmed in the familial cases. Parental genotypes for sporadic case LR7‐1 were confirmed by analysis of additional unlinked polymorphic loci. Variants not previously described were also screened in at least 50 unrelated, ethnically matched healthy control individuals to exclude them as common polymorphisms. As expected, the 4640C→A mutation discovered in the sporadic case was not found in the unaffected parents. None of the changes we report here is polymorphic as determined from searches of public sequence databases.
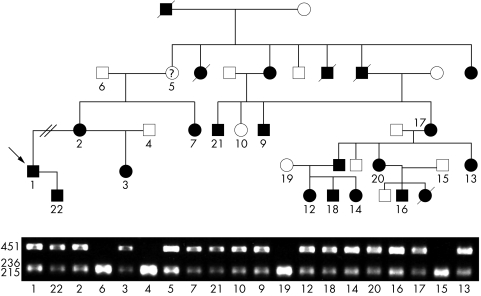
Family CF16 was of particular interest, as the proband, his mother, aunt, half sister, and son were originally diagnosed with isolated talipes equinovarus. The extended family showed significant clinical variability. Mutational analysis revealed a co‐segregating 4604G→A variant predicting a S1535N missense substitution. This mutation was also detected in one member scored as “possibly affected”, and one individual (CF16‐10) originally considered unaffected (fig 1).
Figure 1 Mutation detection in family CF16. AccI restriction endonuclease digestion produced a hemizygous diagnostic 451 base pair (bp) fragment in affected individuals. Order of samples is: CF16‐1, ‐22, ‐2, ‐6, ‐3, ‐4, ‐5, ‐7, ‐21, ‐10, ‐9, ‐19, ‐12, ‐18, ‐14, ‐20, ‐16, ‐17, ‐15, ‐13. Individual CF16‐5 was considered possibly affected because of her mild features. Note that the 451 bp fragment is present in individual CF16‐10 who was originally considered unaffected. All mutations co‐segregated with disease in the respective families.
In family LR1 we detected a co‐segregating 679G‐A mutation predicting E227K substitution. This mutation was previously reported as a de novo event in a sporadic case; however, the four mutations discovered in the remaining families were new. We noticed that, together with the previous report, all mutations occurred in one of only five exons comprising distinct domains in the filamin B protein. We screened only four exons (2, 4, 19, 20) in a newly ascertained sixth (sporadic) case and discovered a missense G1691S mutation in exon 29. This mutation was also observed by Krakow et al in a sporadic case.15 All reported mutations in Larsen syndrome are summarised in table 1. We found that each of the mutated amino acids, with the exception of N1571 in Fugu rubripes, displays orthologous conservation in vertebrate (human, mouse, rat, dog, chicken, blowfish) species. Paralogous conservation within the filamin protein family was also observed (fig 2). These findings further underscore the functional importance of amino acids altered in Larsen syndrome.
Table 1 Summary of FLNB missense mutations.
| Family ID | DNA mutation | Exon | Predicted amino | Domain | Reference |
|---|---|---|---|---|---|
| acid change | |||||
| 319 | 482T→G | 2 | F161C | CHD2 | 15 |
| LR9 | 479A→C | 2 | N160T | CHD2 | This study |
| LR1 | 679G→A | 4 | E227K** | CHD2 | This study; 15 |
| CF16 | 4604G→A | 27 | S1535N | 14 | This study |
| LR7 | 4640C→A | 27 | A1547D | 14 | This study |
| 318 | 4711_4713del AAT | 28 | 1571delN | 14 | 15 |
| 334 | 4756CG→A | 28* | G1586R | 14 | 15 |
| LR6 | 4930G →T | 29 | G1644W | 15 | This study |
| LR11 | 5071G →T | 29 | G1691S** | 15 | This study; 15 |
Mutations and their predicted amino acid substitutions are shown in exon order. *Exons are numbered as depicted in the Ensembl genome browser (www.ensembl.org) and differ slightly from numbering reported by Krakow et al.15
**Previously reported.17
Figure 2 LRS mutations alter conserved amino acids and cluster in the FLNB protein. Alignment of portions of the actin binding domain (ABD) and repeat domains. All reported amino acid mutations in Larsen syndrome are shown in bold. Top: Alignment with vertebrate orthologues. Bottom: Alignment with filamin paralogues.
The three filamins in humans—A, B, and C—are encoded on chromosomes Xq28, 3p14, and 7q32, respectively. Genomic organisation is highly conserved among the three genes, and proteins share 60–80% homology except in the hinge regions that are unique to each protein. Filamin A and filamin B in particular display similar patterns of expression in many tissues.16,17,21,22,23 Despite these similarities, the two clearly have distinct functional roles as evidenced by the pathologies now associated with each. Mutations in filamin A are known to cause five X linked disorders: periventricular nodular heterotopia (PVNH; MIM 300049), and the clinically overlapping diseases otopalatodigital syndromes 1 and 2 (OPD1, MIM 311300; OPD2, MIM 304120), Melnick‐Needles syndrome (MNS, MIM 309350), and frontometaphyseal dysplasia (FMD, MIM 305620).24,25,26,27 PVNH results from loss of function (or partial loss of function) mutations in FLNA. The predominant clinical symptom in these patients is late onset epilepsy, which is apparently secondary to failure of neurones to migrate to the correct cortical site during brain development.28 OPD1 patients show conduction deafness, congenital dislocations of hips and knees, long metacarpals, spatulate thumbs, scoliosis, and craniofacial anomalies. OPD2 is similar but with more severe features including microcephaly and mental retardation.19 MNS is similar to OPD but also includes deformities in ribs, clavicles, scapulae, and pelvis, and bowed long bones. Interestingly this is usually seen in females and is fatal in affected males.29 FMD patients show striking overgrowth of frontal facial bones and may also display features of OPD including deafness, bony dysplasia, and digital anomalies.20 OPD1, OPD2, MNS, and FMD all are caused by missense point mutations that cluster in specific regions of FLNA. OPD1 is caused by mutations in the ABD of FLNA and OPD2 may be caused by mutations in the ABD or in repeats 3, 14, or 15. MNS is caused by mutations in repeat 10, and FMD is caused by mutations in repeat 10 or in repeat 14.
In the present study we have shown that, like diseases associated with FLNA, Larsen syndrome is caused by missense mutations that show a striking non‐random distribution in the filamin B protein. It is interesting that mutations in the ABD and repeats 14 and 15 of the two paralogues give rise to clinically overlapping phenotypes. Clearly these domains in both proteins mediate participation in pathways that are critically important during skeletal development. Also, as with FLNA, mutations in FLNB are responsible for additional human skeletal disorders.15 Homozygous or compound heterozygous apparent loss of function mutations in FLNB cause recessive spondylocarpotarsal syndrome (SCT, MIM 272460), a disease that involves vertebral, carpal, and tarsal fusions and short stature.30 Three perinatal lethal conditions are caused by missense point mutations within FLNB. The atelosteogenesis I (AOI) (MIM 108720) and atelosteogenesis III (AOIII) (MIM 08721) are characterised by vertebral anomalies, joint dislocations, absent or hypoplastic bones, and deficiencies in ossification.31 One AOI/AOIII mutation has been described in repeat domain six, and the remaining mutations described occur in the CHD2 portion of the ABD which also harbours mutations causing Larsen syndrome. More recently, two missense mutations in CHD2 were identified in boomerang dysplasia, characterised by irregular and deficient ossification resulting in, among other features, boomerang shaped bones of the femur.32 The dramatic effects of seemingly subtle missense mutations in phylogenetically conserved residues suggest a very specific role for these domains in skeletogenesis.
Larsen syndrome has been described as genetically heterogeneous. Our results, combined with previous data, suggest that the disease may be entirely explained by mutations in FLNB, and that these mutations cluster in specific regions of the FLNB gene. Additional studies in a larger patient series are needed to quantify this. The potential to detect disease causing mutations in a few selected regions of a single gene should greatly simplify diagnostic testing for Larsen syndrome. A molecular diagnosis will be particularly useful in this disorder in the light of the significant frequency of de novo mutations, and the clinical heterogeneity and subtle findings that can confound the diagnosis. Both these issues were illustrated by the cases in this series. It is unlikely that phenotype will correlate with genotype, however, given the extreme variability even within families. A molecular diagnosis for Larsen syndrome also will have implications in genetic counselling for patients with the disorder.
Acknowledgements
We wish to thank the families for their participation. The contributions of C Thomas and L Edwards are gratefully acknowledged. We thank R Gross for diagnostic information. This work was supported by TSRH Research Fund No 4‐96‐353 to CAW.
Abbreviations
ABD - actin binding domain
CHD - calponin homology domain
MNS - Melnick‐Needles syndrome
OPD - otopalatodigital syndrome
PVNH - periventricular nodular heterotopia
Footnotes
Conflicts of interest: None declared
References
- 1.Larsen L J, Schottstaedt E R, Bost F C. Multiple congenital dislocations associated with characteristic facial abnormality. J Pediatr 195037574–581. [DOI] [PubMed] [Google Scholar]
- 2.Herring J A. Larsen syndrome. In: Tachdjian's pediatric orthopaedics, vol 3. Philadelphia: WB Sanders, 20021602–1615.
- 3.Latta R J, Graham C B, Aase J, Scham S M, Smith D W. Larsen's syndrome: a skeletal dysplasia with multiple joint dislocations and unusual facies. J Pediatr 197178291–298. [DOI] [PubMed] [Google Scholar]
- 4.Steel H H, Kohl E J. Multiple congenital dislocations associated with other skeletal anomalies (Larson's syndrome) in three siblings. J Bone Joint Surg 197254‐A75–82. [PubMed] [Google Scholar]
- 5.Robertson F W, Kozlowski K, Middleton R W. Larsen's syndrome: three cases with multiple congenital joint dislocations and distinctive facies. Clinic Pediatr 19751453–60. [DOI] [PubMed] [Google Scholar]
- 6.Johnston C E, Birch J G, Daniels J L. Cervical kyphosis in patients who have Larsen syndrome. J Bone Joint Surg 199678‐A538–545. [DOI] [PubMed] [Google Scholar]
- 7.Bonaventure J, Lasselin C, Mellier J, Cohen Solal L, Maroteaux P. Linkage studies of four fibrillar collagen genes in three pedigrees with Larsen‐like syndrome. J Med Genet 199229465–470. [PMC free article] [PubMed] [Google Scholar]
- 8.Houston C S, Reed M H, Desautels J E. Separating Larsen syndrome from the “arthropryposis basket. ” J Can Assoc Radiol 198132206–212. [PubMed] [Google Scholar]
- 9.Chen H, Chang C ‐ H, Perrin E, Perrin J. A lethal, Larsen‐like multiple joint dislocation syndrome. Am J Med Genet 198213149–161. [DOI] [PubMed] [Google Scholar]
- 10.Vujic M, Hallstensson K, Wahlström J, Lundberg A, Langmaack C, Martinsson T. Localization of a gene for autosomal dominant Larsen syndrome to chromosome region 3p21.1–14.1 in the proximity of, but distinct from, the COL7A1 locus. Am J Hum Genet 1995571104–1113. [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley D, Seymour N. The Larsen syndrome occurring in four generations of one family. Int Orthop 19858267–272. [DOI] [PubMed] [Google Scholar]
- 12.Laville J M, Lakermance P, Limouzy F. Larsen's syndrome: review of the literature and analysis of thirty‐eight cases.J Pediatr Orthop 19941463–73. [DOI] [PubMed] [Google Scholar]
- 13.Pierquin G, van Regemorter N, Hayez‐Delatte. Fourneau C, Bormans J, Foerster M, Damis E, Cremer‐Perlmutter N, Lapiere C M, Vamos E. Two unrelated children with partial trisomy 1q and monosomy 6p, presenting with the phenotype of the Larsen syndrome. Hum Genet 199187587–591. [DOI] [PubMed] [Google Scholar]
- 14.Davies A F, Mirza G, Sekhon G, Turnpenny P, Leroy F, Speleman F, Law C, van Regemorter N, Vamos E, Flinter F, Ragoussis J. Delineation of two distinct 6p deletion syndromes. Hum Genet 199910464–72. [DOI] [PubMed] [Google Scholar]
- 15.Krakow D, Robertson S P, King L M, Morgan T, Sebald E T, Bertolotto C, Wachsmann‐Hogiu S, Acuna D, Shapiro S S, Takafuta T.et al Mutations in the gene encoding filamin B disrupt vertebral segmentation, joint formation and skeletogenesis. Nat Genet 200436405–410. [DOI] [PubMed] [Google Scholar]
- 16.Takafuta T, Wu G, Murphy G F, Shapiro S S. Human β‐filamin is a new protein that interacts with the cytoplasmic tail of glycoprotein Ibα. J Biol Chem 199827317531–17538. [DOI] [PubMed] [Google Scholar]
- 17.Stossel T P, Condeelis J, Cooley L, Hartwig J H, Noegel A, Schleicher M, Shapiro S S. Filamins as integrators of cell mechanics and signaling. Nat Rev Mol Cell Biol 20012138–145. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Walsh C A. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol 200461034–1038. [DOI] [PubMed] [Google Scholar]
- 19.Herring J A. Otopalatodigital syndrome. In: Tachdjian's pediatric orthopaedics, vol 3. Philadelphia: WB Sanders, 20021666–1667.
- 20.Gorlin R J, Cohen M M. Frontometaphyseal dysplasia. A new syndrome. Am J Dis Child 1969118487–494. [DOI] [PubMed] [Google Scholar]
- 21.Gorlin J B, Henske E, Warren S T, Kunst C B, D'Urso M, Palmieri G, Hartwig J H, Bruns G, Kwiatkowski D J. Actin‐binding protein (ABP‐280) filamin gene (FLN) maps telomeric to the color vision locus (R/GCP) and centromeric to G6PD in Xq28. Genomics 199317496–498. [DOI] [PubMed] [Google Scholar]
- 22.Maestrini E, Patrosso C, Mancini M, Rivella S, Rocchi M, Repetto M, Villa A, Frattini A, Zoppé M, Vezzoni P, Toniolo D. Mapping of two genes encoding isoforms of the actin binding protein ABP‐280, a dystrophin like protein, to Xq28 and to chromosome 7. Hum Mol Genet 19932761–766. [DOI] [PubMed] [Google Scholar]
- 23.Chakarova C, Wehnert M S, Uhl K, Sakthivel S, Vosberg H ‐ P, van der Ven P F M, Fürst D O. Genomic structure and fine mapping of the two human filamin gene paralogues FLNB and FLNC and comparative analysis of the filamin gene family. Hum Genet 2000107597–611. [DOI] [PubMed] [Google Scholar]
- 24.Fox J W, Lamperti Ed, Eksioglu Y Z, Hong S E, Feng Y, Graham D A, Scheffer I E, Dobyns W B, Hirsch B A, Radtke R A, Berkovic S F, Huttenlocher P R, Walsh C A. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 1998211315–1325. [DOI] [PubMed] [Google Scholar]
- 25.Sheen V L.et al Mutations in the X‐linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum Mol Genet 2001101775–1783. [DOI] [PubMed] [Google Scholar]
- 26.Moro F, Carrozzo R, Veggiotti P, Tortorella G, Toniolo D, Volzone A, Guerrini R. Familial periventricular heterotopia: missense and distal truncating mutations of the FLN1 gene. Neurology 200258916–921. [DOI] [PubMed] [Google Scholar]
- 27.Robertson S P, Twigg S R, Sutherland‐Smith A J, Biancalana V, Gorlin R J, Horn D, Kenwrick S J, Kim C A, Morava E, Newbury‐Ecob R.et al Localized mutations in the gene encoding the cytoskeletal protein filamin a cause diverse malformations in humans.Nat Genet 200333487–491. [DOI] [PubMed] [Google Scholar]
- 28.Eksioglu Y Z, Scheffer I E, Cardenas P, Knoll J, DiMario F, Ramsby G, Berg M, Kamuro K, Verkovic S F, Duyk Gm, Parisi J, Huttenlocher P R, Walsh C A. Periventricular heterotopia: an X‐linked dominant epilepsy locus causing aberrant cerebral cortical development. Neuron 19961677–87. [DOI] [PubMed] [Google Scholar]
- 29.Melnick J C, Needles C F. An undiagnosed bone dysplasia. A 2 family study of 4 generations and 3 generations. Am J Roentgenol 19669739–48. [DOI] [PubMed] [Google Scholar]
- 30.Wiles C R, Taylor T F K, Sillence D O. Congenital synspondylism. Am J Med Genet 199242288–295. [DOI] [PubMed] [Google Scholar]
- 31.Sillence D, Worthington S, Dixon J, Osborn R, Kozlowski K. Atelosteogenesis syndromes: a review, with comments on their pathogenesis. Pediatr Radiol 199727388–396. [DOI] [PubMed] [Google Scholar]
- 32.Bicknell L S, Morgan T, Bonafe L, Wessels M W, Bialer M G, Willems P J, Cohn D H, Krakow D, Robertson S P. Mutations in FLNB cause boomerang dysplasia. J Med Genet 200542e43. [DOI] [PMC free article] [PubMed] [Google Scholar]