Abstract
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is a tumour predisposition syndrome caused by heterozygous germline mutations in the fumarate hydratase (FH) gene. The condition is characterised by predisposition to benign leiomyomas of the skin and the uterus, renal cell carcinoma (RCC), and uterine leiomyosarcoma (ULMS). To comprehensively examine the cancer risk and tumour spectrum in Finnish FH mutation positive families, genealogical and cancer data were obtained from 868 individuals. The cohort analysis of the standardised incidence ratios (SIR) was analysed from 256 individuals. FH mutation status was analysed from all available individuals (n = 98). To study tumour spectrum in FH mutation carriers, loss of the wild type allele was analysed from all available tumours (n = 22). The SIR was 6.5 for RCC and 71 for ULMS. The overall cancer risk was statistically significantly increased in the age group of 15–29 years, consistent with features of cancer predisposition families in general. FH germline mutation was found in 55% of studied individuals. Most RCC and ULMS tumours displayed biallelic inactivation of FH, as did breast and bladder cancers. In addition, several benign tumours including atypical uterine leiomyomas, kidney cysts, and adrenal gland adenomas were observed. The present study confirms with calculated risk ratios the association of early onset RCC and ULMS with FH germline mutations in Finns. Some evidence for association of breast and bladder carcinoma with HLRCC was obtained. The data enlighten the organ specific malignant potential of HLRCC.
Keywords: HLRCC, fumarate hydratase, cancer risk, renal cell cancer, uterine leiomyosarcoma
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is a recently recognised tumour predisposition syndrome caused by heterozygous germline mutations in the fumarate hydratase (FH; fumarase) gene (HLRCC, MIM 605839; MCUL, MIM 150800).1,2 To date, 114 mutation positive families have been reported, mainly in Europe and North America.1,3,4,5,6,7,8,9,10 Leiomyomas of the skin and uterus are the most common feature of HLRCC. RCC has been reported in families from North America, the UK, Finland, and Poland.1,3,5,8,9,10,11 Predisposition to uterine leiomyosarcoma has been observed only in Finnish HLRCC families. In HLRCC patients from other populations, one case of leiomyosarcoma of the skin, a single case of bladder and brain cancer, and one malignant and two benign breast tumours have been reported.8,10,12 Of note, no clear correlation between the type of FH mutation and phenotype has been found.10,11 Previously, homozygous FH mutations have been shown to cause a recessive condition with progressive encephalomyopathy, referred to as fumarase deficiency (MIM 606812). Phenotypic similarities between HLRCC and FH deficiency have been observed in one family with FH deficiency, in which a patient's heterozygous parent had cutaneous leiomyomas.2 To date, no cancer has been reported in these patients or their first degree relatives.
MATERIALS AND METHODS
Studies on high risk populations provide a unique opportunity to examine FH related cancer predisposition. To gain comprehensive insight into potential cancer phenotype in FH mutation positive families, the risk of cancer and the tumour spectrum was examined in seven Finnish families with HLRCC (FAM1‐7) and one kindred with FH deficiency (FAM8) (table 1). The characteristic leiomyomatosis phenotype was not further scrutinised in this study. The study was approved by the Helsinki University Central Hospital authorised ethics review committee and all samples were derived after obtaining the individual's informed consent or authorisation from National Authority for Medicolegal Affairs.
Table 1 Family data.
| Family | No. of family members | Phenotype | Germline mutation | |||
|---|---|---|---|---|---|---|
| FAM1* | 137 | RCC | 541delAG | |||
| FAM2* | 97 | RCC | 541delAG | |||
| FAM3† | 59 | Leiomyomas | R300X | |||
| FAM4 | 208 | ULMS | H153R | |||
| FAM5 | 37 | RCC | H153R | |||
| FAM6‡ | 42 | Leiomyomas | H153R | |||
| FAM7‡ | 6 | ULMS | 541delAG | |||
| FAM8§ | 282 | FHD | Q333P |
RESULTS AND DISCUSSION
Genealogical and cancer data from church parish registries, death certificates, the Population Register Centre, and the Finnish Cancer Registry were obtained from 868 family members. To avoid selection bias, only individuals of the completely traced generations (n = 256) were included in the cohort analysis of the standardised incidence ratios (SIR). No selection according to mutation status was performed. Furthermore, all cancers in the index patients (five cancers in eight patients) and other patients with RCC or ULMS used for identification of the families (seven cancers in six patients) were excluded. The risk analysis provided statistically significant results, with a 6.5 fold risk (95% confidence interval (CI) 2.1 to 15.0) for RCC and a 71 fold risk (95% CI 8.6 to 260) for ULMS compared with the general population (table 2). The excess was concentrated in the age groups 15–29 and 30–44 years. Moreover, the overall risk of cancer was statistically significantly increased in the age group of 15–29 years (SIR 6.60, 95% CI 1.40 to 19.0) (table 2), although of note, this group included only three cancers (RCC, uterine leiomyosarcoma, and Hodgkin's lymphoma). The risk for other tumour types did not reach statistical significance (data not shown).
Table 2 Risk of cancer in families positive for FH mutation.
| Observed | Expected | SIR | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|
| RCC | ||||||||
| All | 5 | 0.78 | 6.50 | 2.10 to 15.0 | ||||
| 0–14 | – | 0.01 | 0.00 | 0.00 to 260 | ||||
| 15–29 | 1 | 0.00 | 230 | 5.90 to 1300 | ||||
| 30–44 | 2 | 0.04 | 45.0 | 5.50 to 160 | ||||
| 45–59 | – | 0.22 | 0.00 | 0.00 to 17.0 | ||||
| 60–74 | 1 | 0.35 | 2.80 | 0.07 to 16.0 | ||||
| 75+ | 1 | 0.14 | 7.00 | 0.18 to 39.0 | ||||
| ULMS | ||||||||
| All | 2 | 0.02 | 71.0 | 8.60 to 260 | ||||
| 0–14 | – | 0.00 | – | – | ||||
| 15–29 | 1 | 0.00 | 2100 | 52.0 to 11 000 | ||||
| 30–44 | 1 | 0.00 | 180 | 4.60 to 1000 | ||||
| 45–59 | – | 0.01 | 0.00 | 0.00 to 540 | ||||
| 60–74 | – | 0.00 | 0.00 | 0.00 to 840 | ||||
| 75+ | – | 0.00 | 0.00 | 0.00 to 2100 | ||||
| Total cancer | ||||||||
| All | 34 | 24.73 | 1.40 | 0.95 to 1.90 | ||||
| 0–14 | – | 0.23 | 0.00 | 0.00 to 16.0 | ||||
| 15–29 | 3 | 0.45 | 6.60 | 1.40 to 19.0 | ||||
| 30–44 | 5 | 1.97 | 2.50 | 0.82 to 5.90 | ||||
| 45–59 | 6 | 6.16 | 0.97 | 0.36 to 2.10 | ||||
| 60–74 | 15 | 10.40 | 1.40 | 0.81 to 2.40 | ||||
| 75+ | 5 | 5.51 | 0.91 | 0.29 to 2.10 |
Members were divided into six age groups. SIR, standardised incidence ratio.
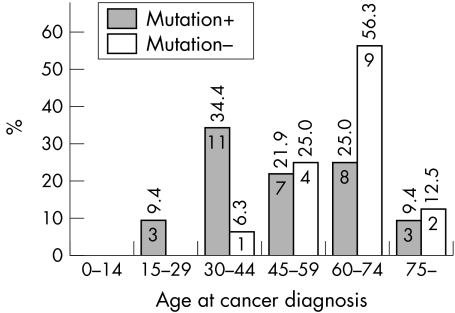
We next investigated the tumour spectrum in FH mutation positive individuals. Of 98 patients tested, 54 harboured a germline FH mutation, and approximately half of these patients had been diagnosed with cancer (table 3). Comparison of the age at cancer diagnosis of mutation positive and negative patients showed that mutation positive individuals are prone to cancer at a younger age (p = 0.009) (fig 1), although this difference did not remain significant after exclusion of the probands. In FH mutation positive patients, the most prominent cancer types were RCC (n = 12) and uterine ULMS (n = 5), affecting 22% of all mutation positive individuals and 15% of mutation positive women, respectively. In addition, four patients had atypical leiomyomas, a variant of leiomyoma sometimes difficult to discern from leiomyosarcoma. As both atypical leiomyomas and leiomyosarcomas were frequent in FH mutation positive individuals, it is possible that the underlying FH mutation promotes malignant transformation of leiomyomas. Several cases of breast carcinoma and hematopoietic or lymphoid malignancies were observed. Cancer cases detected in FH mutation positive individuals are summarised in table 3.
Table 3 Cancer cases in FH mutation‐positive individuals.
| Tumour | n | Age(s) at diagnosis, years (median) | Somatic second hit detected/ analysed (%) | |||
|---|---|---|---|---|---|---|
| RCC | 12* | 26, 32, 33, 35, 36, 39, 42, 48, 49, 68, 71, 90 (40.5) | 10/12 (83) | |||
| ULMS | 5* | 27, 30, 32, 35, 39 (32) | 3/3 (100) | |||
| Breast cancer | 4 | 50, 53, 55, 61 (52.5) | 3/3 (100) | |||
| Bladder | 1 | 71 | 1/1 (100) | |||
| Non‐Hodgkin's lymphoma | 1 | 63 | 0/1 | |||
| Hodgkin's lymphoma | 1 | 24 | 0/1 | |||
| Chronic lymphatic leukaemia | 1 | 48 | 0/1 | |||
| Oesophageal cancer | 1 | 53 | 0/1 | |||
| Basal cell cancer | 2 | 70, 83 | 0/1 | |||
| Multiple myeloma | 1 | 61 | Not analysed | |||
| Prostate cancer | 1 | 63 | Not analysed | |||
| Liver/bile duct cancer (obligatory carrier) | 1 | 82 | Not analysed | |||
| Unknown origin | 1 | 42 | Not analysed |
*Seven RCCs and two ULMSs have previously been published and have been reviewed by Kiuru and Launonen 2004.11RCC, renal cell cancer; ULMS, uterine leiomyosarcoma.
Figure 1 Age at cancer diagnosis in mutation positive and negative patients. The age distribution of cancer cases by percentage value is calculated within groups with different mutation status. The value inside the bar indicates number of cancer cases in the age group.
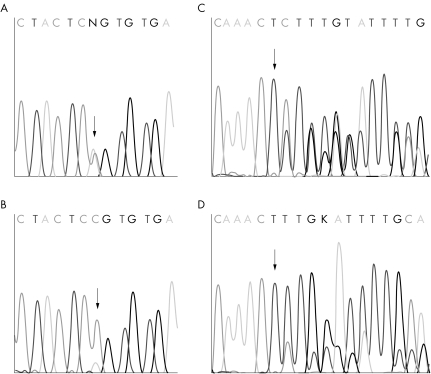
To get insight into which tumours might have arisen due to the FH germline mutation, we examined somatic (biallelic) inactivation of the gene in 22 tumours (table 3, fig 2). Almost all analysed RCC and ULMS tumours as well as the one bladder and all three studied breast carcinomas had lost the wild type FH allele. One of the breast carcinomas and the bladder carcinoma were detected in the family with FH deficiency. These results suggest possible association of these two tumour types with FH germline mutations. Two of the patients with breast cancer had also been diagnosed with ULMS, supporting this concept. Strikingly, one patient with ULMS and breast cancer was also affected with multiple myeloma and non‐Hodgkin's lymphoma.
Figure 2 Examples of LOH detected in breast cancer. Chromatograms A and C illustrates normal breast tissues displaying germline mutations Q333P and 541delAG, respectively. LOH in breast tumours (B, D) is observable by the lower wild type allele signal in the presence of normal tissue contamination.
In addition to the malignant tumours, data on benign tumours other than skin and uterine leiomyomas were obtained. Radiology reports available from 33 mutation positive individuals revealed benign kidney cysts in 14 individuals (42%). Scanning was mainly performed by ultrasound, but in 10 cases alternatively or in addition by computed tomography (CT). Reported prevalence of kidney cysts in general population varies, probably due to detection method used. Ultrasound and CT screenings have indicated prevalence to vary between 11.9–17.6% and 24–41%, respectively.15,16,17,18,19 Thus, cystic lesions in the present study were observed somewhat more frequently than usual, especially at a young age. For comparison, in individuals younger than 40 years, prevalence was 36% compared with 4.6–8.2% (ultrasound and CT, respectively) in the general population.18,19 Five of the 14 individuals with cysts were diagnosed with RCC. These included four multiple cyst cases, of which one was bilateral. Seven RCC patients had no cysts or the presence of them was not scrutinised. Thus, correlation between RCC and cysts is not probable. The remaining nine individuals with cysts displayed both single (n = 6) and multiple lesions (n = 3), of which two were bilateral. Diameter of these lesions was 20–60 mm in the cases where the information was available in the radiology report.
In addition, one liver haemangioma and adrenal gland adenomas including one bilateral tumour in four individuals (12%) were reported. Of note, the frequency of adenomas was higher than in the general population (0.5–2%).20 Interestingly, RCC, kidney cysts, adrenal gland pheochromocytomas, and liver haemangiomas are associated with von Hippel‐Lindau syndrome, and RCC and adrenal gland pheochromocytomas in hereditary paraganglioma syndrome (PGL). The molecular background in these syndromes also overlaps. The predisposing genes in the three syndromes have been implicated in the hypoxia pathway, and the genes predisposing to HLRCC and PGL operate in the mitochondrial Krebs cycle.21,22,23 These similarities might provide clues to further understand the pathways of tumorigenesis in these diseases.
The present study has for the first time, using the exceptional databases and resources available in Finland, thoroughly evaluated the spectrum and relative risk of different cancers in patients with FH mutation. The results of the study confirm with calculated risk values the association of early onset RCC and ULMS with FH germline mutations. In addition to Finland, several cases of RCC have also been reported in North American HLRCC families; In a recent study, the frequency of HLRCC families with RCC was as high as 62%.10 The average frequency of North American HLRCC kindreds with RCC is 35%, indicating high cancer risk in populations also other than Finns,8,10 although criteria for family recruitment obviously affects the results of the different studies. However, no cases of ULMS have been identified in HLRCC families in North America. Data suggesting correlation of certain mutations to RCC have been reported,10,24 but no such evidence concerning ULMS has been obtained. Difference in incidence of ULMS, however, may be due to the high frequency of myomectomy and hysterectomy at a young age carried out in affected women in North America; for example, in one cohort, 57% of women with cutaneous or uterine leiomyomas had undergone hysterectomy before 30 years of age.8 Interestingly, as in Finnish families, those women included cases with uterine atypia (n = 2). The present study also provides evidence that other cancer types, including breast and bladder carcinoma, may be promoted by loss of FH. Of note, FH mutation carriers with bladder and breast tumours have been reported recently in HLRCC families in other populations.10,12 Although the observations of the present study have been derived from a high risk population, it is likely to reflect that of other populations. This study provides insight into organ specific malignant potential associated HLRCC, and should promote further studies in families with FH germline mutations and awareness in patient management.
ACKNOWLEDGEMENTS
We thank S Marttinen for gathering the genealogy data. The study was supported by grants from the Academy of Finland (44870/Finnish Center of Excellence Program 2000–2005, 76227, and 77547), the Sigrid Juselius Foundation, the Cancer Society of Finland, and Helsinki University Central Hospital.
Abbreviations
CT - computed tomography
FH - fumarate hydratase
HLRCC - hereditary leiomyomatosis and renal cell cancer
PGL - paraganglioma syndrome
RCC - renal cell cancer
SIR - standardised incidence ratio
ULMS - uterine leiomyosarcoma
Footnotes
Competing interests: there are no competing interests
References
- 1.Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E, Sistonen P, Herva R, Aaltonen L A. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci USA 2001983387–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomlinson I P, Alam N A, Rowan A J, Barclay E, Jaeger E E, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance R R, Olpin S, Bevan S, Barker K, Hearle N, Houlston R S, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomaki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen L A, Multiple Leiomyoma Consortium Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 200230406–410. [DOI] [PubMed] [Google Scholar]
- 3.Kiuru M, Launonen V, Hietala M, Aittomaki K, Vierimaa O, Salovaara R, Arola J, Pukkala E, Sistonen P, Herva R, Aaltonen L A. Familial cutaneous leiomyomatosis is a two‐hit condition associated with renal cell cancer of characteristic histopathology. Am J Pathol 2001159825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiuru M, Lehtonen R, Arola J, Salovaara R, Jarvinen H, Aittomaki K, Sjoberg J, Visakorpi T, Knuutila S, Isola J, Delahunt B, Herva R, Launonen V, Karhu A, Aaltonen L A. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res 2002624554–4557. [PubMed] [Google Scholar]
- 5.Alam N A, Rowan A J, Wortham N C, Pollard P J, Mitchell M, Tyrer J P, Barclay E, Calonje E, Manek S, Adams S J, Bowers P W, Burrows N P, Charles‐Holmes R, Cook L J, Daly B M, Ford G P, Fuller L C, Hadfield‐Jones S E, Hardwick N, Highet A S, Keefe M, MacDonald‐Hull S P, Potts E D, Crone M, Wilkinson S, Camacho‐Martinez F, Jablonska S, Ratnavel R, MacDonald A, Mann R J, Grice K, Guillet G, Lewis‐Jones M S, McGrath H, Seukeran D C, Morrison P J, Fleming S, Rahman S, Kelsell D, Leigh I, Olpin S, Tomlinson I P. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet 2003121241–1252. [DOI] [PubMed] [Google Scholar]
- 6.Chuang G S, Martinez‐Mir A, Horev L, Glaser B, Geyer A, Landau M, Waldman A, Gordon D, Spelman L J, Hatzibougias I, Engler D E, Cserhalmi‐Friedman P B, Green J, Garcia Muret M P, Prieto Cid M, Brenner S, Sprecher E, Christiano A M, Zlotogorski A. Germline fumarate hydratase mutations and evidence for a founder mutation underlying multiple cutaneous and uterine leiomyomata. Am J Hum Genet 200373(suppl)P577 [Google Scholar]
- 7.Martinez‐Mir A, Glaser B, Chuang G S, Horev L, Waldman A, Engler D E, Gordon D, Spelman L J, Hatzibougias I, Green J, Christiano A M, Zlotogorski A. Germline fumarate hydratase mutations in families with multiple cutaneous and uterine leiomyomata. J Invest Dermatol 2003121741–744. [DOI] [PubMed] [Google Scholar]
- 8.Toro J R, Nickerson M L, Wei M H, Warren M B, Glenn G M, Turner M L, Stewart L, Duray P, Tourre O, Sharma N, Choyke P, Stratton P, Merino M, Walther M M, Linehan W M, Schmidt L S, Zbar B. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet 20037395–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan I, Wong T, Martinez‐Mir A, Christiano A M, McGrath J A. Familial multiple cutaneous and uterine leiomyomas associated with papillary renal cell cancer. Clin Exp Dermatol 20053075–78. [DOI] [PubMed] [Google Scholar]
- 10.Wei M H, Toure O, Glenn G, Pithukpakorn M, Neckers L, Stolle C, Choyke P, Grubb R, Middleton L, Turner M L, Walther M, Merino M, Zbar B, Linehan W M, Toro J R. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet 20064318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiuru M, Launonen V. Hereditary leiomyomatosis and renal cell cancer (HLRCC). Curr Mol Med 20044869–875. [DOI] [PubMed] [Google Scholar]
- 12.Alam N A, Barclay E, Rowan A J, Tyrer J P, Calonje E, Manek S, Kelsell D, Leigh I, Olpin S, Tomlinson I P. Clinical features of multiple cutaneous and uterine leiomyomatosis: an underdiagnosed tumor syndrome. Arch Dermatol 2005141199–206. [DOI] [PubMed] [Google Scholar]
- 13.Remes A M, Rantala H, Hiltunen J K, Leisti J, Ruokonen A. Fumarase deficiency: two siblings with enlarged cerebral ventricles and polyhydramnios in utero. Pediatrics 199289730–734. [PubMed] [Google Scholar]
- 14.Remes A M, Filppula S A, Rantala H, Leisti J, Ruokonen A, Sharma S, Juffer A H, Hiltunen J K. A novel mutation of the fumarase gene in a family with autosomal recessive fumarase deficiency. J Mol Med 200482550–554. [DOI] [PubMed] [Google Scholar]
- 15.Laucks S P, Jr, McLachlan M S. Aging and simple cysts of the kidney. Br J Radiol 19815412–14. [DOI] [PubMed] [Google Scholar]
- 16.Ravine D, Gibson R N, Donlan J, Sheffield L J. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am J Kidney Dis 199322803–807. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda M, Masai M, Shimazaki J. A simple renal cyst. Nippon Hinyokika Gakkai Zasshi 199384251–257. [DOI] [PubMed] [Google Scholar]
- 18.Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol 200216721–23. [PubMed] [Google Scholar]
- 19.Carrim Z I, Murchison J T. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol 200358626–629. [DOI] [PubMed] [Google Scholar]
- 20.Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 2003149273–285. [DOI] [PubMed] [Google Scholar]
- 21.Pavlovich C P, Schmidt L S. Searching for the hereditary causes of renal‐cell carcinoma. Nat Rev Cancer 20044381–393. [DOI] [PubMed] [Google Scholar]
- 22.Pollard P, Wortham N, Barclay E, Alam A, Elia G, Manek S, Poulsom R, Tomlinson I. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol 200520541–49. [DOI] [PubMed] [Google Scholar]
- 23.Selak M A, Armour S M, MacKenzie E D, Boulahbel H, Watson D G, Mansfield K D, Pan Y, Simon M C, Thompson C B, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF‐alpha prolyl hydroxylase. Cancer Cell 2005777–85. [DOI] [PubMed] [Google Scholar]
- 24.Alam N A, Olpin S, Leigh I M. Fumarate hydratase mutations and predisposition to cutaneous leiomyomas, uterine leiomyomas and renal cancer. Br J Dermatol 200515311–17. [DOI] [PubMed] [Google Scholar]