Abstract
Background
Delayed puberty is common among individuals with cystic fibrosis (CF) and is usually attributed to chronic disease and/or poor nutrition. However, it has recently been recognised that pubertal delay can occur even in the setting of good nutritional and clinical status. This finding, along with evidence that Cftr is expressed in rat brain, human hypothalamus, and a gonadotropin releasing hormone secreting cell line, raises the possibility that some of the pubertal delay in CF could stem directly from alterations in Cftr function that affect the hypothalamic‐pituitary‐gonadal axis.
Methods
To examine this hypothesis, we investigated pubertal timing (as assessed by vaginal opening (VO)) in a mouse model of CF (Cftrtm1Unc) engineered to produce a truncated Cftr mRNA and referred to as S489X. Homozygous knockout, heterozygote, and wild type (WT) female mice were examined.
Results
As expected, the S489X−/S489X− knockout mice, which have chronic inflammation and gastrointestinal disease, grew more slowly and had later onset of puberty than WT animals. We anticipated that the S489X−/S489X+ heterozygotes, which have no clinical CF phenotype, might display an intermediate timing of puberty. Surprisingly, however, these mice had earlier VO than WT. These findings were confirmed in a second, independent model of CF engineered to generate the ΔF508 mutation in mice. Again, the homozygotes displayed later pubertal timing, while the heterozygotes displayed earlier VO than the WT animals.
Conclusions
These data provide further evidence that Cftr can directly modulate the reproductive endocrine axis and raise the possibility that heterozygate mutation carriers may have a reproductive advantage.
Keywords: cystic fibrosis, delayed puberty, GnRH, gonadotropin releasing hormone
Cystic fibrosis (CF) is the most common life shortening autosomal recessive disease among Caucasians.1 It has a calculated carrier frequency of 5% and a disease frequency of 1 in 2000–3000 live births.2 CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR), located on chromosome 7.3,4,5 CFTR functions as a cyclic adenosine monophosphate (cAMP) regulated chloride channel,6 but the protein may have other functions as well.7,8,9 Mutations in CFTR affect organ systems with epithelial surfaces: the lungs, pancreas, intestinal mucous glands, liver, reproductive tracts, and sweat glands. The clinical features of CF vary but include progressive lung disease, exocrine pancreatic insufficiency, nasal polyposis, sinusitis, rectal prolapse, diabetes, biliary cirrhosis, infertility, delayed puberty, and slowed growth.2,10
Delayed puberty is common in both males and females with CF, with young women often being more dramatically affected than young men.11,12 The onset of puberty in females with CF is typically delayed at least 1 year and often more. For example, one study has reported a mean age at onset of secondary sexual characteristics of 14.2 years13 and another has reported mean age at menarche of 14.9 years.14 Reproductive endocrine function is intact in these women, but the maturation of the hypothalamic‐pituitary‐gonadal (HPG) axis and age related increments of follicle stimulating hormone (FSH) and luteinising hormone (LH) are delayed. This pubertal delay has classically been attributed to effects of chronic disease and/or suboptimal nutritional status.2,15
However, recent studies question whether all delayed growth and puberty in CF patients can be attributed to the effects of chronic disease. Delayed puberty has been reported as a feature of CF even in the setting of good nutritional and clinical status.14 Expression of CFTR has been documented in the rat brain and the human hypothalamus.16,17,18 In addition, in a gonadotropoin releasing hormone (GnRH) secreting hypothalamic neuronal cell line (GT1‐7), Cftr is functional and involved in GnRH secretion.19 Taken together, these data suggest that some of the delayed growth and puberty seen in CF could stem from alterations in Cftr function that might directly affect the HPG axis. To examine further the relationship between Cftr function and growth and development, we assessed pubertal timing in two different mouse models of CF.
Methods
Mouse strains
Different lines of mice with C57BL/6J (B6) genetic backgrounds were assessed. First, B6 mice heterozygous for the S489X mutation that generates a stop codon in the coding sequence of exon 10 of Cftr (B6.129P2‐Cftrtm1Unc/J),20 were purchased from Jackson Laboratory (Bar Harbor, ME; stock no. 002196) and crossed onto C57BL/6J mice for a minimum of 12 generations. This mutation leads to a truncated protein product similar to many human CF mutations.20 Heterozygous S489X mice (referred to as S489X−/S489X+) were bred and the progeny homozygote, heterozygote, or negative (wild type, WT) for the S489X mutation were studied.
Second, mice heterozygous for the ΔF508 mutation that generates a 3 bp (CTT) deletion between bases 1656 and 1660 of Cftr and results in the loss of a phenylalanine residue in exon 10 (B6.129S6‐Cftrtm1Kth/J), were a gift from Dr Kirk Thomas (University of Utah).21 The ΔF508 mutation disrupts the processing of Cftr resulting in the retention and degradation of the protein in the endoplasmic reticulum, which causes the absence of Cftr in the apical membrane.22,23 These ΔF508 mice were bred with B6 mice purchased from Jackson Laboratory (stock no. 000664). The heterozygous (ΔF508−/ΔF508+) progeny were selected and repeatedly backcrossed with WT B6 animals for more than 12 generations to ensure a uniform B6 background. The resultant heterozygous ΔF508 mice were bred for this study, and the progeny homozygous, heterozygous, or negative (WT) for the ΔF508 mutation were evaluated.
In the colony described here, the ΔF508 and S489X lines are backcrossed to B6 mice every five generations to ensure genetic fidelity and prevent genetic drift. Live animals were genotyped at 7 days after date of birth by PCR analysis of DNA extracted from a toe removed for mouse identification.24 WT mice from the S489X or ΔF508 matings are both predicted to be indistinguishable from B6 mice due to the backcrossing of these alleles. Because there were no observable phenotypic differences between the WT mice generated from the two mating strategies (mean timing of vaginal opening (VO) 31.3±1.4 days for S489X WT (n = 14) and 31.3±1.5 days for ΔF508 WT (n = 12)), WT animals were assessed as a single group. We did, however, verify that the differences we observed between homozygote, heterozygote, and WT mice were statistically significant when comparisons were limited to the progeny from each individual strain. For the S489X mice, the p values for comparison of homozygote and heterozygote mice to WT mice were <0.00001 and 0.00004, respectively; for the ΔF508 mice, the p values for comparison of homozygote and heterozygote mice to WT mice were 0.00007 and 0.0002, respectively.
Animals housing and protocol
All animals used in this study were cared for according to a Case Western Reserve University approved protocol and Institutional Animal Care and Use Committee (IACUC) guidelines. All animals were maintained on a 12 h light:12 h dark schedule (lights on at 0600 h) at a mean ambient temperature of 22°C (with deviations from 20°C to 23°C) and housed in standard polysulfone microisolator cages in ventilated units with corncob bedding. Mice were given access to irradiated chow (Harlan Teklad 7960; Harlan Teklad Global Diets, Madison, WI) and either sterile water or Colyte (Schwarz Pharma, Milwaukee, WI) ad libitum. To prevent intestinal obstruction,6,20,21 homozygous S489X−/S489X− and ΔF508−/ΔF508− mice were provided with Colyte25 solution instead of sterile water. Despite the use of Colyte, many of the homozygous CF mice died prior to or immediately after weaning.
Phenotyping was performed using a variation of the protocol employed in previous studies.26 Individual males were placed in a cage with two females for continuous harem breeding. Breeders were monitored daily, 7 days a week, between 0900 and 1300 h for birth, and the date of birth was designated as the day pups were first observed. To reduce the exposure of female pups to male pheromonal and hormonal signals, male littermates were weaned at 21 days. Females were then housed with their dams until 28 days when they were weaned. (Weaning for mice is typically performed at 20–21 days after birth, but later weaning is required to maximise survival of CF knockout mice. For uniformity of experimental conditions, all genotypes in our study were weaned at day 28). After weaning, no more than six female pups were housed per cage to ensure that access to food and water was unfettered. Beginning 21 days after birth, female mice were examined daily, 7 days a week, between 0900 and 1300 h, and the date of VO and concurrent body weight were recorded.
Data analysis
Two tailed, non‐parametric tests for independent variables (Mann‐Whitney U tests) were used for all comparisons. The p values for the comparison of the time of VO and weight among the S489X−/S489X−, S489X−/S489X+, ΔF508−/ΔF508−, and ΔF508−/ΔF508+ mice versus WT controls were corrected for multiple hypothesis testing using a factor of 4 because four different strains were compared with WT B6 mice during this study. Thus, significance was attributed to p<0.0125 (0.05/4 tests). Analyses were performed using the Complete Statistical System: Statistica from StatSoft (Tulsa, OK).
Results
VO occurred significantly later in S489X homozygous knockout (S489X−/S489X−) mice than in WT B6 mice (table 1, fig 1). The S489X CF mice also grew more slowly than WT mice and had not even reached a mean weight of 15 g by 50 days of age; thus, despite the delay in VO, the S489X−/S489X− mice experienced VO at significantly lower mean body weights than the WT animals.
Table 1 Timing of vaginal opening (VO).
| Strain | n (litters) | Age at VO (days) (mean±SD) | p v WT for VO | Body weight (g) at VO (mean±SD) | p v WT for body weight |
|---|---|---|---|---|---|
| WT | 26 (18) | 31.3±1.4 | NA | 15.7±1.4 | NA |
| Corrected* | Corrected* | ||||
| p v B6 (VO) | p v B6 (body weight) | ||||
| S489X−/S489X− | 14 (11) | 48.9±9.3 | <0.00001 | 13.9±1.5 | 0.0003 |
| S489X−/S489X+ | 21 (12) | 27.6±2.2 | <0.00001 | 14.5±1.8 | NS |
| ΔF508−/ΔF508− | 10 (7) | 47.9±14.7 | <0.00001 | 12.9±2.1 | 0.0002 |
| ΔF508−/ΔF508+ | 14 (8) | 27.1±2.5 | <0.00001 | 14.7±2.0 | NS |
*For the analysis of the timing of VO and body weight at VO in each strain versus homozygous non‐mutant Cftr (WT) mice, statistical significance was attributed to p<0.0125 to correct for multiple hypothesis testing; see Methods for details.
n, number of animals; NA, not applicable; NS, not significant; SD, standard deviation; VO, vaginal opening.
Figure 1 Comparison of the timing of vaginal opening (VO) for S489X mice. The cumulative percentage of animals with VO is shown. S489X−/S489X−, filled squares; S489X−/S489X+, open squares; and WT, filled circles.
To investigate the direct effects of Cftr on pubertal timing without the confounding effects of chronic disease, mice heterozygous for the S489X mutation were examined. We anticipated that the S489X−/S489X+ mice, which have no clinical CF phenotype,27 would display timing of VO similar to WT mice or be intermediate to the knockout and WT mice. Surprisingly, the heterozygous S489X−/S489X+ mice experienced VO significantly earlier than the WT mice (table 1, fig 1).
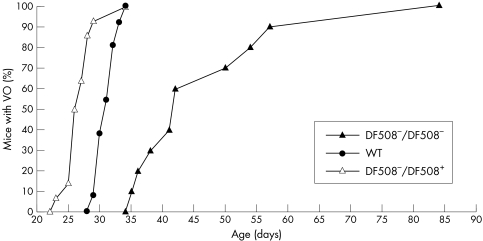
Similar results were seen with a second, independent CF mouse model engineered to generate the common ΔF508 mutation. Again, the CF ΔF508−/ΔF508− knockout mice had later timing of puberty and grew more slowly than the WT mice, while the heterozygous ΔF508−/ΔF508+ mice experienced VO significantly earlier than the WT mice (table 1, fig 2).
Figure 2 Comparison of the timing of vaginal opening (VO) for ΔF508 mice. The cumulative percentage of animals with VO is shown. ΔF508−/ΔF508−, filled triangles; ΔF508−/ΔF508+, open triangles; and WT, filled circles.
Discussion
VO is an oestrogen dependent process and is a reliable and rapid method of measuring the onset of puberty in mice. Although VO does not characterise fully the maturation of the reproductive endocrine axis,28 it is clear that the factors that control VO are among the important determinants of reproductive maturation in mice.26 Thus, our findings strongly suggest that Cftr mutations modulate the HPG axis in mice.
The analysis of growth and puberty in CF knockout mice is complicated by severe chronic disease, most notably a tendency for intestinal obstruction29 and malabsorption.30 In fact, despite the use of Colyte and postponing weaning until day 28, data from one ΔF508−/ΔF508− and six S489X−/S489X− mice were not included in the analyses because the animals died prior to VO. The health of the CF knockout mice also impacted on our experimental design. Because litter size had to be reduced, and the CF knockout mice required an extra week with their dams to enhance their chances of survival, it was impractical to compare timing of VO among all animals of different genotypes derived from the same litter. Instead, we needed to compare groups of animals of each genotype housed under uniform conditions. Therefore, heterozygote and WT animals were often from different litters than the CF mice, but had been left with their mothers until weaning at day 28. This uniform strategy should have guaranteed that differences observed in pubertal timing were due to differences in Cftr function among the mouse strains.
Mice from both CF knockout models displayed delayed puberty and slowed growth. As in the human disease, it is difficult to separate the direct effects of mutations in Cftr from those of chronic disease. Indeed, it has previously been shown that malnutrition and inflammation can each lead to pubertal delay in a rodent model of colitis.31 However, the data from the S489X−/S489X+ and ΔF508−/ΔF508+ heterozygous animals strongly suggest that Cftr may exert a direct effect on the HPG axis. While it is formally possible that the effect on the HPG axis is indirect and involves, for example, alterations in intestinal function or inflammation, this seems less likely as the sole mechanism since the heterozygote mice have no known clinical phenotype. The mechanism by which carrier status would directly affect the HPG axis is unclear. It could involve a role for Cftr in the development of a component of the HPG axis, as has been suggested for the lung,32 or in the regulation of GnRH secretion. It is also possible that the effects we observed could stem from the particular mutations generated in our mouse models of CF. This seems unlikely, however, since the two strains studied here were generated independently and involve distinct mechanisms. It will be important, though, to study other mouse models to determine if they display the same findings. Regardless of the mechanism, our data provide an important in vivo complement to the previous in vitro data16,17,18,19 and suggest that further investigation into the role that Cftr plays in the regulation of reproductive maturation in mice is warranted.
Previous epidemiologic data have suggested that carriers for CF mutations may have a reproductive advantage,33,34 a hypothesis that could explain the relatively high frequency of CF and CFTR mutations in the human population. One postulated mechanism is that CF carriers have more children than non‐carriers.35,36,37,38 In this context, our data regarding alterations in pubertal timing among the heterozygote mice are interesting. We do not yet know whether there is a human correlate of our data or whether it would manifest as a difference in pubertal timing, ovulatory function, fertility, or another aspect of reproductive endocrine function. However, our findings do highlight the importance of studying further the reproductive endocrine axis in human female CF carriers.
Acknowledgements
We thank Ms Pamela J Supelak and Dr Brandon M Nathan for assistance with phenotyping of mice for vaginal opening. We also thank Dr Matthew Warman for support and advice throughout these studies.
Abbreviations
CF - cystic fibrosis
GnRH - gonadotropoin releasing hormone
HPG axis - hypothalamic‐pituitary‐gonadal axis
VO - vaginal opening
WT - wild type
Footnotes
This work was supported by NIH grants K23 RR15544 (MRP) and HL68883 (MLD)
Competing interests: none declared
References
- 1.Moran A. Endocrine complications of cystic fibrosis. Adolesc Med 200213145–157. [PubMed] [Google Scholar]
- 2.Welsh M J, Tsui L, Boat T F, Beaudet A L. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic basis of inherited disease. New York: McGraw‐Hill, 19953799–3876.
- 3.Rommens J M, Iannuzzi M C, Kerem B, Drumm M L, Melmer G, Dean M, Rozmahel R, Cole J L, Kennedy D, Hidaka N.et al Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 19892451059–1065. [DOI] [PubMed] [Google Scholar]
- 4.Riordan J R, Rommens J M, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J L.et al Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 19892451066–1073. [DOI] [PubMed] [Google Scholar]
- 5.Kerem B ‐ S, Rommens J M, Buchanan J A, Markiewicz D, Cox T K, Chakravarti A, Buchwald M, Tsui L C. Identification of the cystic fibrosis gene: genetic analysis. Science 19892451073–1080. [DOI] [PubMed] [Google Scholar]
- 6.Clarke L L, Grubb B R, Gabriel S E, Smithies O, Koller B H, Boucher R C. Defective epithelial chloride transport in a gene‐targeted mouse model of cystic fibrosis. Science 19922571125–1128. [DOI] [PubMed] [Google Scholar]
- 7.Anderson M P, Gregory R J, Thompson S, Souza D W, Paul S, Mulligan R C, Smith A E, Welsh M J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 1991253202–205. [DOI] [PubMed] [Google Scholar]
- 8.Stutts M J, Canessa C M, Olsen J C, Hamrick M, Cohn J A, Rossier B C, Boucher R C. CFTR as a cAMP‐dependent regulator of sodium channels. Science 1995269847–850. [DOI] [PubMed] [Google Scholar]
- 9.Al‐Awqati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science 1995269805–806. [DOI] [PubMed] [Google Scholar]
- 10.Lai H C, Kosorok M R, Sondel S A, Chen S T, FitzSimmons S C, Green C G, Shen G, Walker S, Farrell P M. Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry data: evaluation of various criteria used to identify malnutrition. J Pediatr 1998132478–485. [DOI] [PubMed] [Google Scholar]
- 11.Stern R C, Boat T F, Doershuk C F, Tucker A S, Primiano F P, Jr, Matthews L W. Course of cystic fibrosis in 95 patients. J Pediatr 197689406–411. [DOI] [PubMed] [Google Scholar]
- 12.Shwachman H, Kulczzycki L L, Khaw K ‐ T. Studies in cystic fibrosis: a report on sixty‐five patients over 17 years of age. Pediatrics 196536689–699. [PubMed] [Google Scholar]
- 13.Mitchell‐Heggs P, Mearns M, Batten J C. Cystic fibrosis in adolescents and adults. Q J Med 1976179479–504. [PubMed] [Google Scholar]
- 14.Johannesson M, Gottlieb C, Hjelte L. Delayed puberty in girls with cystic fibrosis despite good clinical status. Pediatrics 19979929–34. [DOI] [PubMed] [Google Scholar]
- 15.Aswani N, Taylor C J, McGaw J, Pickering M, Rigby A S. Pubertal growth and development in cystic fibrosis: a retrospective review. Acta Paediatr 2003921029–1032. [PubMed] [Google Scholar]
- 16.Mulberg A E, Resta L P, Wiedner E B, Altschuler S M, Jefferson D M, Broussard D L. Expression and localization of the cystic fibrosis transmembrane conductance regulator mRNA and its protein in rat brain. J Clin Invest 199596210–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannesson A, Bogdanovic N, Nordqvist A S, Hjelte L, Schalling M. Cystic fibrosis mRNA expression in rat brain: cerebral cortex and medial preoptic area. Neuroreport 19978535–539. [DOI] [PubMed] [Google Scholar]
- 18.Mulberg A E, Weyler R T, Altschuler S M, Hyde T M. Cystic fibrosis transmembrane conductance regulator expression in human hypothalamus. Neuroreport 19989141–144. [DOI] [PubMed] [Google Scholar]
- 19.Welyer R T, Yurko‐Mauro K A, Rubenstein R, Kollen W J, Reenstra W, Altschuler S M, Egan M, Mulberg A E. CFTR is functionally active in GnRH‐expressing GT1‐7 hypothalamic neurons. Am J Physiol 1999277C563–C571. [DOI] [PubMed] [Google Scholar]
- 20.Snouwaert J N, Brigman K K, Latour A M, Malouf N N, Boucher R C, Smithies O, Koller B H. An animal model for cystic fibrosis made by gene targeting. Science 19922571083–1088. [DOI] [PubMed] [Google Scholar]
- 21.Zeiher B G, Elchwald E, Zabner J, Smith J J, Puga A P, McCray P B, Jr, Capecchi M R, Welsh M J, Thomas K R. A mouse model for the ΔF508 allele of cystic fibrosis. J Clin Invest 1995962051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng S H, Gregory R J, Marshall J, Paul S, Souza D W, White G A, O'Riordan C R, Smith A E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 199063(4)827–834. [DOI] [PubMed] [Google Scholar]
- 23.French P J, Doorninck J H, Peters R H, Verbeek E, Ameen N A, Marino C R, de Jonge H R, Bijman J, Scholte B J. A delta F508 mutation in mouse cystic fibrosis transmembrane conductance regulator results in a temperature‐sensitive processing defect in vivo. J Clin Invest 199698(6)1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truett G E, Heeger P, Mynatt R L, Truett A A, Walker J A, Warman M L. Preparation of PCR‐quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 200029(1)52–54. [DOI] [PubMed] [Google Scholar]
- 25.Clarke L L, Gawenis L R, Franklin C L, Harline M C. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci 199646(6)612–618. [PubMed] [Google Scholar]
- 26.Krewson T D, Supelak P J, Hill A E, Singer J B, Lander E S, Nadeau J H, Palmert M R. Chromosomes 6 and 13 harbor genes that regulate pubertal timing in mouse chromosome substitution strains. Endocrinology 2004145(10)4447–4451. [DOI] [PubMed] [Google Scholar]
- 27.Dorin J R, Farley R, Webb S, Smith S N, Farini E, Delaney S J, Wainwright B J, Alton E W, Porteous D J. A demonstration using mouse models that successful gene therapy for cystic fibrosis requires only partial gene correction. Gene Ther 19963(9)797–801. [PubMed] [Google Scholar]
- 28.Nelson J F, Karelus K, Felicio L S, Johnson T E. Genetic influences on the timing of puberty in mice. Biol Reprod 199042649–655. [DOI] [PubMed] [Google Scholar]
- 29.Davidson D J, Rolfe M. Mouse models of cystic fibrosis. Trends Genet 200117S29–S37. [DOI] [PubMed] [Google Scholar]
- 30.Bijvelds M J, Bronsveld I, Havinga R, Sinaasappel M, de Jonge H R, Verkade H J. Fat absorption in cystic fibrosis mice is impeded by defective lipolysis and post‐lipolytic events. Am J Physiol Gastrointest Liver Physiol 2005288(4)G646–G653. [DOI] [PubMed] [Google Scholar]
- 31.Azooz O G, Farthing M J, Savage M O, Ballinger A B. Delayed puberty and response to testosterone in a rat model of colitis. Am J Physiol Regul Integr Comp Physiol 2001281(5)R1483–R1491. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J C, Lundblad L K, Bates J H, Levitzky M, Larson J E. The “Goldilocks effect” in cystic fibrosis: identification of a lung phenotype in the cftr knockout and heterozygous mouse. BMC Genet 20045(1)21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serre J L, Simon‐Bouy B, Mornet E, Jaume‐Roig B, Balassopoulou A, Schwartz M, Taillandier A, Boue J, Boue A. Studies of RFLP closely linked to the cystic fibrosis locus throughout Europe lead to new considerations in populations genetics. Hum Genet 199084(5)449–454. [DOI] [PubMed] [Google Scholar]
- 34.Morral N, Bertranpetit J, Estivill X, Nunes V, Casals T, Gimenez J, Reis A, Varon‐Mateeva R, Macek M, Jr, Kalaydjieva L.et al The origin of the major cystic fibrosis mutation (delta F508) in European populations. Nat Genet 19947(2)169–175. [DOI] [PubMed] [Google Scholar]
- 35.Knudson A G, Jr, Wayne L, Hallett W Y. On the selective advantage of cystic fibrosis heterozygotes. Am J Hum Genet 196719(3)388–392. [PMC free article] [PubMed] [Google Scholar]
- 36.Conneally P M, Merritt A D, Yu P L. Cystic fibrosis: population genetics. Tex Rep Biol Med 197331(4)639–650. [PubMed] [Google Scholar]
- 37.Jorde L B, Lathrop G M. A test of the heterozygote‐advantage hypothesis in cystic fibrosis carriers. Am J Hum Genet 198842(6)808–815. [PMC free article] [PubMed] [Google Scholar]
- 38.Danks D M, Allan J, Anderson C M. A genetic study of fibrocystic disease of the pancreas. Ann Hum Genet 196528323–356. [Google Scholar]