Abstract
Background
Cornelia de Lange syndrome (CdLS) is a multiple congenital anomaly syndrome characterised by a distinctive facial appearance, prenatal and postnatal growth deficiency, psychomotor delay, behavioural problems, and malformations of the upper extremities. Recently mutations in NIPBL, the human homologue of the Drosophila Nipped‐B gene, were found to cause CdLS. Mutations have been found in 39% of reported cases.
Methods
Patients were enrolled in the study and classified into one of four groups based on clinical examination: classic, mild, possible, or definitively not CdLS. Three dimensional photography was taken of 20 subjects, and compared between groups. Behaviour was assessed with specific attention to autism. We searched for mutations in NIPBL and correlated genotype with phenotype.
Results
: We found mutations in 56% of cases.
Conclusions
Truncating mutations were generally found to cause a more severe phenotype but this correlation was not absolute. Three dimensional facial imaging demonstrated the potential for classifying facial features. Behavioural problems were highly correlated with the level of adaptive functioning, and also included autism. No correlation of behaviour with the type of mutation was found
Keywords: Cornelia de Lange syndrome, behaviour, 3D scanning, NIPBL, genotype‐phenotype correlations
Cornelia de Lange syndrome (CdLS MIM #122470), also called typus amstelodamensis or Brachmann‐de Lange syndrome, is a multiple congenital anomaly syndrome characterised by a distinctive facial appearance, prenatal and postnatal growth deficiency, psychomotor delay, behavioural problems, and malformations of the upper extremities.1 Cardiac defects and gastrointestinal anomalies are common, and many additional physical features occur, including hearing loss, myopia, palatal abnormalities, genitourinary abnormalities, and congenital diaphragmatic hernias.
CdLS has a variable phenotype, including classic and mild types, which also evolve with age.2,3,4,5,6,7 In an extensive review of 310 cases, Jackson et al8 reported mildly affected cases to be more common than anticipated. Various behavioural phenotypes have been associated with CdLS, especially involving self injurious,9 aggressive, and self restraining behaviour, and autism (‐like) features.10,11,12,13
In 2004, two groups independently found NIPBL gene mutations to be responsible for CdLS.14,15 All types of mutation were identified. NIPBL is the human homologue of the Drosophila Nipped‐B gene, which belongs to the family of chromosomal adherins involved in chromatid cohesion processes and enhancer promoter communication in Drosophila.16,17 The exact function of the human NIPBL gene product, delangin, is unknown. To date, NIPBL mutations have been identified in 20–50% of CdLS cases.14,15,18,19,20 In total, 161 patients were studied molecularly, of whom 63 (39%) were found to have a mutation. Genotype‐phenotype correlations in the study of Gillis et al19 showed significant differences between patients with and without mutations in terms of the degree of growth retardation and developmental delay. Expressed limb abnormalities were reported by Miyake et al in three of four mutation positive patients,20 and Gillis et al19 found a similar but not significant correlation. Gillis et al also observed a more strongly expressed clinical phenotype among the truncating mutation carriers compared with those with a missense mutation.
Here we report on the clinical, molecular, and behavioural phenotype of 39 Dutch CdLS patients, and correlations between genotype and phenotype.
MATERIALS AND METHODS
Clinical evaluation of subjects
The study was approved by the medical ethics committee of the Academic Medical Centre in Amsterdam, and by the board of the Dutch CdLS Support Group.
Patients enrolled in the study through the cooperation of the Dutch CdLS Support Group. All patients were evaluated by one of two clinical geneticists (AvH, RCMH) specifically for this study. This evaluation included clinical history, family history (three generations), physical examination, and anthropometry. In older patients, photographs taken at a younger age were evaluated, and pertinent data regarding medical history and psychological testing were retrieved. Patients were classified at first contact (before molecular studies were performed) as having a phenotype that was either classic, mild, atypical but did not definitively exclude the diagnosis CdLS ("possible CdLS"), or definitively not CdLS. The various features used in establishing this severity score are summarised in table 1, with examples shown in fig 1. Criteria for postnatal growth and limb anomalies were as reported previously.19 Normal values of prenatal growth and skull circumference were those for the general population.21,22 As it has been reported that individuals with CdLS have a more expressed developmental delay if birth weight is below 1500 g,23,24 prenatal growth retardation was categorised as lesser delay (birth weight 1500–2500 g) and expressed delay (<1500 g). If necessary, data were normalised for gestational age. In prematurely born children, the cut off levels were adapted according to gestational age. To allow distinction between the usually mild microcephaly in mild CdLS type (−3SD) and more severe microcephaly in classic CdLS (−6SD),2 microcephaly was divided into subgroups above and below −4SD. Adaptive functioning was scored according to the results of formal testing using the Vineland Adaptive Behaviour Scale (VABS) (see below). Criteria for the various facial phenotypes have been reported elsewhere.2
Table 1 Severity classification of the phenotype.
| Prenatal growth | Postnatal growth* | Skull growth | Limbs | Face | Adaptive functioning | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 = >2500 g | 1 = >P75 | 1 = OFC>−2SD | 1 = no reduction defect | 1 = possible CdLS | 1 = normal/borderline | |||||
| 2 = 1500–2500 g | 2 = P25−P75 | 2 = −2SD>OFC>−4SD | 2 = partial reduction defects (absence of 1 or 2 fingers) | 2 = mild type | 2 = mild/moderately impaired | |||||
| 3 = <1500 g | 3 = <P25 | 3 = OFC<−4SD | 3 = severe reduction defects (absence of 3 of more fingers or complicated oligo/polydactyly) | 3 = classic type | 3 = severely/profoundly impaired |
*CdLS standard growth curves were used.41
Figure 1 Prototypic CdLS patient from the classic (A), mild (B), and possible CdLS (C) groups. Written permission was obtained from the parents for publication of these pictures.
In total, 42 patients participated in the study. Three patients were found not to have CdLS and were excluded from the study, leaving 39 participants. All were sporadic.
Three dimensional photography
Thee dimensional (3D) images of 20 individuals (7 female, 13 male; all white) with a putative diagnosis of CdLS were captured using a commercial photogrammetric face scanner (Surfim/MedEIM Ltd). The age range was 1.3 to 23.8 years (mean 10.8 years). In addition, 195 images of healthy white individuals with an age range of 2 weeks to 24.2 years (mean 9.6 years) were collected using the same scanner or a similar device (3dMD Inc). These controls were recruited either from staff at our institutions or from healthy family members at meetings of support groups for a variety of genetic conditions. Each image was annotated manually with 21 reproducible landmarks by one individual, as described elsewhere.25 Ten individuals had a confirmed mutation, and five had no mutation but were clinically diagnosed, and each was classified as “classic” or “mild” before mutation analysis was undertaken. The remaining five were labelled “possible” as they had no mutation and their clinical diagnosis was inconclusive. Using software developed in house, a dense surface model of facial shape26 was constructed from 210 landmarked images comprising the 195 controls and the 15 individuals classified with a classic or mild CdLS phenotype. Approximately 16 000 points on each face were used in the dense correspondence. The corresponding dense surface model (DSM) contained 65 principal components covering 99% of all shape variation.
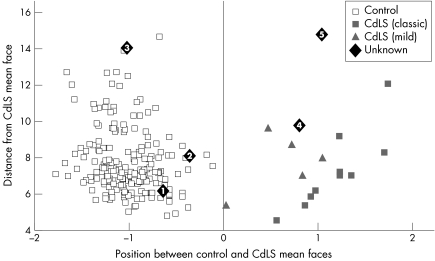
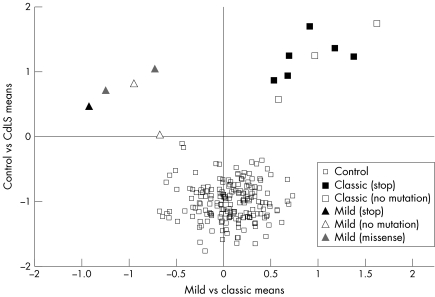
The mean face shape was derived for the control and CdLS subgroups (fig 2). The images of the five individuals classified as “possible” were compared with the DSM unseen. Using the 65 principal components to synthesise each face, the position of each face relative to the control and CdLS mean faces was normalised to the interval −1 to +1, with the control mean at −1 and the CdLS mean at +1. A scatter plot of relative position between the means against the distance from the CdLS mean is shown in fig 3. Each face is shown labelled as either control, CdLS (mild), CdLS (classic), or possible CdLS. Similarly, a scatter plot (fig 4) of mild mean versus classic mean (horizontally) against control mean versus CdLS mean (vertically) was generated for all images except for possible CdLS patients. In fig 4, the labelling included was in terms of mutation.

Figure 2 Profile and portrait views of the average face of the CdLS group with the classic type compared with the control group. Written permission was obtained from the parents for publication of these pictures.
Figure 3 Scatter plot of position of the faces of the patients with classic and mild CdLS. Overlaid are the positions of the faces of five possible CdLS patients (see text for description). Written permission was obtained from the parents for publication of these pictures.
Figure 4 Scatter plot of position of each face relative to average faces of the control group and the CdLS group (y axis), and relative to the average CdLS face of the mild and the classic type (x axis). The various results of molecular analysis are also shown.
Psychological assessments
The present study aimed to assess general behaviour, adaptive behaviour, and autistic features. Of the 39 patients, one had died in the meantime, and two were unavailable for psychological studies for practical reasons only. The remaining 36 individuals were assessed for adaptive behaviour using the VABS (expanded version),27 for behavioural problems with the Dutch version of the Developmental Behaviour Checklist (DBC),28,29 and for autism using the Diagnostic Interview for Social and Communication Disorders (DISCO),30 and the autism algorithm score of the DBC.29 More extensive psychological data, including data on the burden of parenthood of a child with CdLS, will be published elsewhere (Klein et al, in preparation).
Data regarding formal testing of cognitive functioning of patients were available from earlier assessments. However, we did not include these, as a variety of intelligence tests had been used and the results did not compare well. Moreover, the levels of adaptive functioning are of more importance in relation to challenging behaviour and autistic features.
Molecular investigations
Genomic DNA was isolated from peripheral blood lymphocytes (Gentra Systems) from both patients and parents of patients who had a detectable NIPBL mutation or polymorphism. The entire NIPBL coding region (exons 2–47) was screened for mutations (primer sequences and PCR conditions are available at http://www.jmedgenet.com/supplemental). Mutational analysis of the amplimers was performed by denaturing high performance liquid chromatography (DHPLC) (Transgenomic Wave). PCR products with altered DHPLC peak were purified using a QiaQuick PCR purification kit (Qiagen) and sequenced bidirectionally on an ABI 377 sequencer. The NIPBL sequence from the NCBI (NM_015384) was used as reference sequence to name mutations and polymorphisms. As no single case with a larger deletion detected by fluorescent in situ hybridisation has been reported, we did not perform this analysis. All patients were investigated using standard cytogenetic techniques (450 bands or more).
Genotype‐phenotype correlations
Genotype‐phenotype correlations were analysed using contingency table analysis. This was performed for the criteria used to construct the severity score (table 1), and also for all minor features, with a focus on the presence versus the absence of a mutation in NIPBL, on the nature of the mutation (missense mutations versus frameshift/splice site/nonsense (truncating) mutations), and on the three clinical phenotypes (classic, mild, possible). For analysis of the non‐continuous fixed variables, the χ2 test was used. For analysing continuous variables (age, gestation, weight, prenatal growth, postnatal growth, skull growth, and hand and foot length) a non‐parametric test (Mann‐Whitney U test) was employed. The significance threshold was set at p⩽0.05.
RESULTS
Clinical phenotype
In total, 39 patients entered the study. The clinical data found in the present group are summarised in table 2, grouped as findings in all patients, in various groups of patients depending on the results of molecular studies (truncating mutations, missense mutations, no detectable mutations) and clinical phenotype (classic type, mild type, possible CdLS). The main clinical features were similar to those previously reported in the literature. Gestation was shorter, and length and weight at birth were lower in patients with the classic phenotype. Hands were shorter and reflux was more prominent in the classic type (p<0.05), as was a depressed nasal bridge (p<0.01), but small nipples were more common in the mild type (p<0.05). A hitherto infrequently reported finding was the presence of a short fourth metatarsal (either unilaterally or bilaterally present) in 50% of the total group under study, both in the classic and the mild type.
Table 2 Major features in 39 Dutch patients with CdLS, depending on the result of the molecular studies (middle three columns) and the clinical phenotype (last three columns).
| Feature | Total | Molecular findings | Clinical phenotype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Truncating mutations | Missense mutations | No mutations | Classic type | Mild type | Possible CdLS | |||||||||
| Number of patients | 39 | 19 | 5 | 15 | 27 | 8 | 4 | |||||||
| Sex (M/F) | 19/20 | 13/6 | 1/4 | 6/9 | 14/11 | 6/1 | 1/3 | |||||||
| Mean weight (g) at birth (M/F) | 2454/2234 | 2377/ 1842 | 3100/2433 | 24602461 | 2277*/1967 | 2716/ 2600 | 2650/ 3000 | |||||||
| Mean adult weight (kg) | 50.91 | 38.97** | 66.6 | 56.98 | 42.05 | 51.4 | 68.5 | |||||||
| Mean adult height (m) | 1.44 | 1.42 | 1.47 | 1.48 | 1.26* | 1.53 | 1.35 | |||||||
| Postnatal growth | ||||||||||||||
| >P75 | 7 | 2 | 2 | 3 | 1 | 4 | 2 | |||||||
| P25−P75 | 19 | 9 | 3 | 8 | 15** | 2 | 2 | |||||||
| <P25 | 8 | 6 | 1 | 1 | 7** | 0 | 0 | |||||||
| Skull growth | ||||||||||||||
| >−2SD | 6 | 2 | 1 | 3 | 2 | 2 | 2 | |||||||
| <−2SD and >−4SD | 17 | 7 | 4 | 6 | 10 | 4 | 1 | |||||||
| <−4SD | 12 | 7 | 0 | 5 | 10** | 1 | 1 | |||||||
| Limb | ||||||||||||||
| No reduction defect | 31 | 9 | 5 | 17 | 20 | 6 | 4 | |||||||
| Partial reduction defect | 1 | 1 | 0 | 0 | 1 | 0 | 0 | |||||||
| Severe reduction defect | 5 | 4 | 0 | 1 | 4 | 1 | 0 | |||||||
| Face | ||||||||||||||
| Classic type | 27 | 17 | 3 | 7 | ||||||||||
| Mild type | 8 | 2 | 2 | 4 | ||||||||||
| Possible CdLS | 4 | 0 | 0 | 4 | ||||||||||
| VABS | ||||||||||||||
| Normal/borderline | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Mildly/moderately impaired | 11 | 6 | 0 | 5 | 5 | 3 | 2 | |||||||
| Severely/profoundly impaired | 25 | 12 | 4 | 9 | 18** | 5 | 2 | |||||||
| DISCO | ||||||||||||||
| Autism | 24 | 13 | 2 | 9 | 20** | 1 | 2 | |||||||
| No autism | 12 | 5 | 2 | 5 | 3 | 6 | 2 | |||||||
| DBC | ||||||||||||||
| Autism | 22 | 10 | 2 | 10 | 16 | 3 | 2 | |||||||
| No autism | 14 | 7 | 3 | 4 | 7 | 4 | 2 | |||||||
*p<0.05; **p<0.01.
As expected, the mean total severity score in the patients with a classic phenotype (11.96) was higher than the score in patients with a mild type (10.14; p = 0.008) or those who possibly have CdLS (8.25; p = 0.004). The correlations between the various components of the severity score varied from −0.374 (prenatal growth to postnatal growth) to −0.702 (adaptive functioning to face), indicating that even for the components with the highest value, one correlation could be explained by the other for less than 50%. The independence of the various components was therefore sufficiently high to allow inclusion of each component.
Three dimensional scanning
Using a classification criterion of closest mean, as determined by horizontal position relative to the y axis in fig3, the faces of patients 1, 2, and 3 with possible CdLS were classified as controls and 4 and 5 as CdLS. Patients 1 and 2 are well within the control group and 3 is an outlier, suggesting that the face, although not CdLS‐like, is definitely dysmorphic. Patient 4 is on the periphery of the CdLS group in terms of similarity to the CdLS mean. This image was captured while the individual was sleeping, however, and so may not be reliable. Patient 5 is considerably further from the CdLS mean, suggesting the face has CdLS‐like features but is not very similar to the rest of the CdLS group. Fig 4 shows clear separation between the mild and classic subgroups, thus confirming the pregenotyping manual phenotype classification and indicating the potential for subclassifying on facial features. The plot shows no obvious subgrouping in relation to mutation analysis apart from the previously stated and independently confirmed conclusion that a truncating mutation is associated with a classic facial phenotype. The number of 3D images is too small to validate this statistically, however.
Psychological assessments
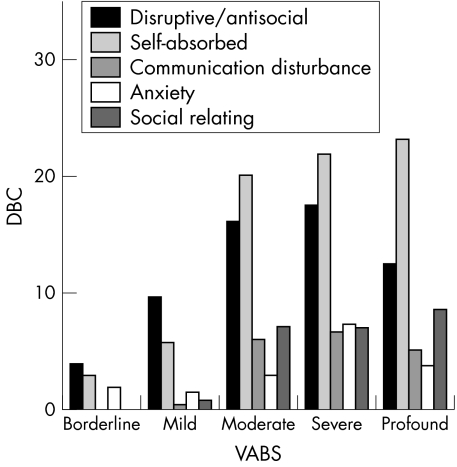
The results of psychological testing are summarised in table 2 and in part shown in fig 5. The raw scores of the adaptive functioning in the VABS were categorised at six levels.27 Of the 36 CdLS patients, 1 was functioning at borderline level, 4 at mild, 6 at moderate, 6 at severe, and 19 at profound level .
Figure 5 Relationship between adaptive functioning (as measured through the VABS) and various behavioural problems (as measured through the DBC). It shows that the more disturbed the adaptive functioning is, the more expressed are the behavioural problems.
There was a significant positive correlation (p<0.05) between age and severity of adaptive malfunctioning; with increasing age the adaptive level decreased. The most expressed behavioural problems concerned self absorbed behaviour (such as preoccupation with trivial items, pica, biting, screaming, head banging, stereotypical movements), and disruptive behaviour (kicking and hitting, noisiness, mood change, impatience, impulsiveness). There was a high correlation between the level of adaptive behaviour and the severity of behaviour problems; patients with the lowest level of adaptive functioning had more behavioural problems (p<0.01) (fig 5).
Autistic traits are found at the four lowest levels of adaptive functioning, and concern in particular individuals with a profoundly affected adaptive level. Of the 19 individuals in this group, 17 achieved a score compatible with autism spectrum disorder using the DISCO and 15 of them on the DBC autism algorithm scale also. According to the DISCO scale, two individuals with severely affected adaptive functioning and five individuals with moderately affected adaptive functioning meet the criteria of autism. On the DBC scale, four patients with severely and three with moderately affected adaptive functioning were classified as autistic. However, these results are established using cut off scores and do not take qualitative data into account. A comparison of present qualitative results with those obtained in individuals with classic autism falls beyond the scope of this report and will be presented elsewhere (Klein et al in preparation). Patients with a classic phenotype were more often found to be autistic on both the DBC (p<0.05) and DISCO (p<0.01) scales.
NIPBL mutations
NIPBL mutations were identified in 22 patients (56%) with CdLS (table 3), and comprised splice site mutations, 5 missense mutations, 3 nonsense mutations, and 11 frameshift mutations. All identified mutations were private, except for a 2 bp deletion in exon 10 (c.2479_2480delAG) in two unrelated individuals, which has previously been described in two patients,19 and a splice site mutation in the upstream of exon 7 (c.610 −6T→C) in two unrelated patients. Missense mutations p.Pro29Gln, p.Lys775Arg, p.Glu2052Asp, p.Arg2298Ser, and p.Lys2550Ile altered residues highly conserved across species (rat, mouse, and Drosophila), and the changes were not detected in 150 control subjects (300 alleles). All mutations were found to have occurred de novo. Biological relationships were confirmed within each family of patients both with and without a detectable mutation. We identified six novel polymorphisms, both exonic and intronic (table 3), all inherited from a normal parent.
Table 3 Results of molecular studies in 39 Dutch patients with CdLS .
| Type of mutation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Missense | Splice site | Nonsense | Frameshift | Polymorphisms | ||||
| Exon 3 | Intron 6 | Exon10 | Exon 10 | Intron 5 | ||||
| c.86 C→A | c.611 −6T→C | c.2341A→T | c.1513‐1514insA | c.458 −49G→C | ||||
| p.Pro29Gln | c.610 +5G→C | p.Lys781X | c.1784delA | Exon 6 | ||||
| Exon 10 c.2324A→Gp.Lys775Arg | Exon 28 c.5427G→TIntron 31 | p.Arg834XExon 45c.7849C→T | c.2358 duplication 22 nucleotidesc.2479_2480delAG* | c.614G→A Exon 10c.2324A→G | ||||
| Exon 35 | c.5808 +5G→C | p.Gln2617X | c.2587_2588insA | Intron 11 | ||||
| c.6156 G→C | c.2771delA | c.3304 −92insT | ||||||
| p.Glu2052Asp | c.3047_3048delTT | c.4422 −23C→T | ||||||
| Exon 40 | c.3060‐3063delAGAG | Intron 20 | ||||||
| c.6893G→A | c.3367delA | Intron 27 | ||||||
| p.Arg2298Ser | Exon 47 | c.5329 −15A→G | ||||||
| Exon 44 | c.8137‐8138insA | |||||||
| c.7649A→T | ||||||||
| p.Lys2550Ile | ||||||||
*Previously reported by Gillis et al19
Genotype‐phenotype correlations
The results of genotype‐phenotype correlation analysis, performed for each phenotypic parameter, with a focus on the presence versus absence of a mutation in NIPBL and on the nature of the mutation are summarised in table 2. Only symptoms constituting the severity score are shown. The analysis shows that the patients with classic facial features of CdLS are more likely to have a mutation in the NIPBL gene, especially a truncating mutation (p<0.023). One patient with a clinically uncertain diagnosis (possibe CdLS) was found to have a mutation. Other phenotypic parameters significantly different between mutation carriers and those without detectable mutation were the presence of a depressed nasal bridge, widely spaced teeth, a short neck, gastro‐oesophageal reflux (p<0.05), and squint (p<0.01). Significant differences were observed between the carriers of missense versus truncating mutations; CdLS patients with a truncating mutation in NIPBL are prone to lower height and weight (table 2) compared with individuals with missense mutations. Squint and depressed nasal bridges were observed more in carriers of truncating NIPBL mutations (truncating 56%; missense 40%), while cutaneous toe syndactyly (missense 40%; truncating 5%) and wide teeth spacing (missense 100%; truncating 70%) were observed more in carriers of missense NIPBL mutations. No significant phenotypic effect was observed to depend on the position of the mutation, either in the missense or the truncating mutation group. Except for the correlation between adaptive functioning (see above), no correlation of age with any other parameter was found, nor were significant differences found between the sexes. The total severity score in patients with a truncating mutation (12.0) was significantly higher (p = 0.013) than the total score in patients with a missense mutation (10.4) or those without a detectable mutation (10.5).
DISCUSSION
We have evaluated 39 patients with CdLS originating from the Netherlands. We divided the participating patients into three groups: classic, mild,2,31,32,33 and possible CdLS, the latter when a clinical diagnosis remained uncertain. Clinical features among the patients were similar to those reported previously for the classic and mild groups.8,24 The absence of significant differences between the three groups for individual symptoms indicates that CdLS is a diagnosis that relies more on the total Gestalt. However, numbers reported here are small, and confirmation is needed from additional studies. An as yet infrequently reported feature was that shortening of the metatarsal bones, usually the fourth, but sometimes the second or third, became more expressed with age.
Mutations in the NIPBL gene were found in 56% of the patients, which is somewhat higher than the frequency reported in other studies. The careful clinical examination of each patient before inclusion in the study may partly explain this, although a mutation was also found in the possible CdLS group. The nature of the mutations was similar to those from other reports: 22% missense mutations and 78% truncating mutations. Although there were a number of recurrent or previously reported mutations, no true hot spot in NIPBL was evident. We found an excessive number of mutations clustered in exon 10, as did Tonkin et al15 and Gillis et al,19 but this may be partly explained by the relatively large size. Strachan34 speculated about a nuclear localisation signal (NLS) in NIPBL at position 1108–1124, just downstream of exon 10. Any mutation in the immediate region upstream of the NLS would perturb this function. Further NIPBL functional studies are needed to explain this.
The percentage of patients with mutations is now just above half, but there is still a considerable number of patients in whom no mutation is detected. We expect that further studies, which may include techniques such as multiplex ligation dependent probe amplification, will allow detection of mutations in a somewhat higher percentage of patients. It remains possible, however, that another gene or other genes play a role in the cause of CdLS.
The genotype was compared with the phenotype in various ways. For the total groups the severity of the phenotype was found to be more strongly expressed in patients with a truncating mutation than in those with a missense mutation. For an individual patient this may not be true, however; the splice site mutation c.611 −6T→C was present twice, once in a patient with obvious classic features (total severity score 13) and once in a patient with a mild phenotype (total severity score 10), suggesting that other (endogenous or exogenous) factors are important in the phenotype. Patients with truncating mutations in NIPBL frequently have the characteristic, classic face, but not always. As in earlier studies,19,20 we observed the majority of patients with truncating mutations to have expressed limb reduction defects, but this was not statistically significant.
The 3D facial imaging clearly demonstrates the potential for classifying facial features in terms of comparisons such as control versus CdLS and classic versus mild. The former classification will enable unknown or doubtful cases to be compared against a validated dense surface model containing controls and individuals who have been mutationally or clinically confirmed as having CdLS. The latter will be useful in further studies of genotype‐phenotype correlations when more images become available. Once sufficient data have been collected, the dense surface models constructed will need to be evaluated using randomised, unseen test sets, for example in the form of multifold cross validation studies as have been carried out for other conditions.23,35
The present study confirms that CdLS individuals have a considerable risk of developing severe behavioural problems. The data regarding the nature of the behavioural problems generally correspond well with the findings in other studies.9,36 Severity of the behavioural problems is highly correlated with the level of adaptive functioning. Earlier studies have shown that these behavioural problems are often the result of poor communication skills.37,38 Such communication problems do not appear in table 2, as the communication subscale in the DBC is mainly applicable to individuals with speech, which excludes most CdLS individuals with profoundly lowered adaptive functioning.
The question arises whether a subgroup of CdLS patients falls within the autism spectrum. Differentiation between autism and profoundly impaired adaptive retardation is well known to be difficult and sometimes impossible.38,39 In the same way, it remains uncertain in the CdLS group whether the autistic features are caused by the low level of functioning or whether they can be considered part of autism. However, this does not alter the result that the number of autistic symptoms in CdLS individuals increases if the level of adaptive behaviour decreases. Gillberg40 has suggested that there are behavioural phenotypes within the autism diagnosis that are typical for the underlying medical condition, such as fragile X syndrome, tuberous sclerosis, and Rett syndrome. CdLS was not included in Gilberg's research, but the present study indicates that this may also apply to CdLS. The recognition of autistic features has consequences for treatment. The combination of autistic traits and mental retardation form a high risk for challenging behaviour.38,39 The motivation for identifying autism in CdLS patients is not one of labelling as such but rather an attempt to determine appropriate treatment. An autism specific approach can have beneficial effects, for instance in preventing behavioural problems.
The present study is hampered by its limited size, which makes several of the conclusions above only tentative. For most similar studies in individual countries, this will also hold true. Only through international collaborations, including registries that contain data on phenotype, genotype, and behaviour, will firmer conclusions be possible. The recent establishment of an International CdLS Scientific Advisory Council (chair: A Levin, Hospital for Sick Children, Toronto) may be the first step in that direction.
In conclusion, in this study of a major proportion of the CdLS population in a single country, a mutation could be found in 56% of patients who received careful clinical investigation. Although truncating mutations were generally found cause a more severe phenotype, this correlation was not absolute and it seems likely that other genetic or environmental factors influence the phenotype. 3D face scanning will be a powerful tool in genotype‐phenotype analyses. Behavioural problems showed the same variation; usually truncating mutations cause more pronounced problems, including autism, but similar problems also occur in missense mutations. It may be that patients with CdLS show a specific, unique autistic behaviour as has been found in several other entities. International collaborations will be needed to obtain patient samples of sufficient size to allow reliable (behavioural) phenotype‐ genotype correlations.
The primer sequences and PCR conditions are available as addendum at http://www.jmedgenet.com/supplemental.
ACKNOWLEDGMENTS
The authors thank the patients and their parents for their generous co‐operation, Mrs M Van Leeuwen for her help in organising the study, M Otter, J Bliek, and P Boorsma for laboratory assistance, Dr J Ruiter (AMC, Amsterdam) for statistical advice, and the many colleagues who initially established the diagnoses in patients.
Abbreviations
3D - three dimensional
CdLS - Cornelia de Lange syndrome
DBC - Developmental Behaviour Checklist
DHPLC - denaturing high performance liquid chromatography
DISCO - Diagnostic Interview for Social and Communication Disorders
DSM - dense surface model
NLS - nuclear localisation signal
VABS - Vineland Adaptive Behaviour Scale
Footnotes
Competing interests: there are no competing interests
The primer sequences and PCR conditions are available as addendum at http://www.jmedgenet.com/supplemental.
References
- 1.Gorlin R J, Cohen M M, Hennekam R C M.Syndromes of the head and neck. 4th ed. Oxford Medical Press, New York 2001
- 2.Allanson J E, Hennekam R C M, Ireland M. De Lange syndrome: subjective and objective comparison of the classical and mild phenotypes. J Med Genet 199734645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraitser M, Papavasiliou A S. Mild de Lange syndrome–does it exist? Clin Dysmorphol 19932147–150. [PubMed] [Google Scholar]
- 4.Greenberg F, Robinson L K. Mild Brachmann‐de Lange syndrome: Changes of phenotype with age. Am J Med Genet 19893290–92. [DOI] [PubMed] [Google Scholar]
- 5.Halal F, Silver K. Syndrome of microcephaly, Brachmann‐de Lange‐like facial changes, severe metatarsus adductus, and developmental delay: mild Brachmann‐de Lange syndrome? Am J Med Genet 199224381–386. [DOI] [PubMed] [Google Scholar]
- 6.Leroy J G, Van de Weghe V, Van Hecke R, Oostra A, De Bie S, Craen M. On the variability of the Brachmann‐de Lange syndrome in seven patients. Am J Med Genet 199347983–991. [DOI] [PubMed] [Google Scholar]
- 7.Selicorni A, Lalatta F, Livini E, Briscioli V, Pigurry T, Clerici Bagozzi D, Mastroiacovo P, Zampino G, Gaeta G, Pugliese A, Cerutti‐Mainaroli P, Guala A, Zelante L, Stabile M, Belli S, Franceschini P, Gianotti A, Scarano G. Variability of the Brachmann‐de Lange syndrome. Am J Med Genet 199347977–982. [DOI] [PubMed] [Google Scholar]
- 8.Jackson L, Kline A D, Barr M A, Koch S. De Lange syndrome: a clinical review of 310 individuals. Am J Med Genet 199347940–946. [DOI] [PubMed] [Google Scholar]
- 9.Hyman P, Oliver C, Hall S. Self‐injurious behaviour, self‐restraint, and compulsive behaviours in Cornelia de Lange syndrome. Am J Ment Retard 2002107146–154. [DOI] [PubMed] [Google Scholar]
- 10.Bay C, Mauk J, Radcliffe J, Kaplan P. Mild Brachmann‐de Lange syndrome. Delineation of the clinical phenotype, and characteristic behaviors in a six‐year‐old boy. Am J Med Genet 199347965–968. [DOI] [PubMed] [Google Scholar]
- 11.Opitz J M. The Brachmann‐de Lange syndrome. Am J Med Genet 19852289–102. [DOI] [PubMed] [Google Scholar]
- 12.Sarimski K. Communication, social‐emotional development and parenting stress in Cornelia‐de‐Lange syndrome. J Intellect Disabil Res 19974170–75. [DOI] [PubMed] [Google Scholar]
- 13.Udwin O, Dennis J. Psychological and behavioural phenotypes in genetically determined disorders: a review of research findings. In: O'Brien G, Yule W, eds. Behavioural phenotypes. London: Mac Keith Press, 1995
- 14.Krantz I D, McCallum J, DeScipio C, Kaur M, Gillis L A, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris C A, Nowaczyk M J, Toriello H, Bamshad M J, Carey J C, Rappaport E, Kawauchi S, Lander A D, Calof A L, Li H H, Devoto M, Jackson L G. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped‐B. Nature Genet 200436631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonkin E T, Wang T J, Lisgo S, Bamshad M J, Strachan T. NIPBL, encoding a homolog of fungal Scc2‐type sister chromatid cohesion proteins and fly Nipped‐B, is mutated in Cornelia de Lange syndrome. Nature Genet 200436636–641. [DOI] [PubMed] [Google Scholar]
- 16.Rollins R A, Morcillo P, Dorsett D. Nipped‐B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics 1999152577–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollins R A, Korom M, Aulner N, Martens A, Dorsett D. Drosophila nipped‐B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long‐range activation of the cut gene. Mol Cell Biol 2004243100–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borck G, Redon R, Sanlaville D, Rio M, Prieur M, Lyonnet S, Vekemans M, Carter N P, Munnich A, Colleaux L, Cormier‐Daire V. NIPBL mutations and genetic heterogeneity in Cornelia de Lange syndrome. J Med Genet 200441e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillis L A, McCallum J, Kaur M, DeScipio C, Yaeger D, Mariani A, Kline A D, Li H H, Devoto M, Jackson L G, Krantz I D. NIPBL mutational analysis in 120 individuals with Cornelia de Lange syndrome and evaluation of genotype‐phenotype correlations. Am J Hum Genet 200475610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake N, Visser R, Kinoshita A, Yoshiura K I, Niikawa N, Kondoh T, Matsumoto N, Harada N, Okamoto N, Sonoda T, Naritomi K, Kaname T, Chinen Y, Tonoki H, Kurosawa K. Four novel NIPBL mutations in Japanese patients with Cornelia de Lange syndrome. Am J Med Genet A 2005135103–105. [DOI] [PubMed] [Google Scholar]
- 21.Nellhaus G G. Head circumference from birth to eighteen years. Pediatrics 196841106–111. [PubMed] [Google Scholar]
- 22.Van Wieringen J C, Roede M J, Wit J M. Growth diagrams for patient care. Tijdschr Kindergeneeskd 198553147–152. [PubMed] [Google Scholar]
- 23.Saal H M, Samango‐Sprouse C A, Rodnan L A, Rosenbaum K N, Custer D A. Brachmann‐de Lange syndrome with normal IQ. Am J Med Genet 199347995–998. [DOI] [PubMed] [Google Scholar]
- 24.Hawley P P, Jackson L G, Kurnit D M. Sixty‐four patients with Brachmann‐de Lange syndrome: a survey. Am J Med Genet 198520453–459. [DOI] [PubMed] [Google Scholar]
- 25.Hammond P, Hutton T, Allanson J, Buxton B, Campbell L, Clayton‐Smith J, Donnai D, Karmiloff‐Smith A, Metcalfe K, Murphy K, Patton M A, Pober B, Prescott K, Shaw A, Scambler P, Smith A, Temple K, Hennekam R C M, Tassabehji M. Discriminating power of localized 3D facial morphology. Am J Hum Genet 200577999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutton T J, Buxton B F, Hammond P, Potts H W W. Estimating average growth trajectories in shape‐space using kernel smoothing. IEEE Transact Med Imaging 200322747–753. [DOI] [PubMed] [Google Scholar]
- 27.Sparrow S S, Cicchetti D V. The behavior inventory for rating development (BIRD): assessments of reliability and factorial validity. Appl Res Ment Retard 19845219–231. [DOI] [PubMed] [Google Scholar]
- 28.Dekker M C, Nunn R, Koot H M. Psychometric properties of the revised Developmental Behaviour Checklist scales in Dutch children with intellectual disability. J Intellect Disabil Res 20024661–75. [DOI] [PubMed] [Google Scholar]
- 29.Einfeld S L, Tonge B J. The Developmental Behavior Checklist: the development and validation of an instrument to assess behavioral and emotional disturbance in children and adolescents with mental retardation. J Autism Dev Disord 19952581–104. [DOI] [PubMed] [Google Scholar]
- 30.Wing L.Diagnostic interview for social and communication disorders. 10th ed. Bromsley, UK: Centre for Social and Communication Disorders, 1999
- 31.Clericuzio C L. Mild mental retardation with classic somatic phenotype in the Brachmann‐de Lange syndrome. Am J Med Genet 199347992–994. [DOI] [PubMed] [Google Scholar]
- 32.Moeschler J B, Graham J M., Jr Mild Brachmann‐de Lange syndrome. Phenotypic and developmental characteristics of mildly affected individuals. Am J Med Genet 199347969–976. [DOI] [PubMed] [Google Scholar]
- 33.Saul R A, Rogers R C, Phelan M C, Stevenson R E. Brachmann‐de Lange syndrome: diagnostic difficulties posed by the mild phenotype. Am J Med Genet 199347999–1002. [DOI] [PubMed] [Google Scholar]
- 34.Strachan T. Cornelia de Lange syndrome and the link between chromosomal function, DNA repair and developmental gene regulation. Curr Opin Genet Dev 200515258–264. [DOI] [PubMed] [Google Scholar]
- 35.Hammond P, Hutton T J, Allanson J E, Campbell L E, Hennekam R C M, Holden S. Murphy KC, Patton MA, Shaw A, Temple IK, Trotter M, Winter RM. 3D analysis of facial morphology. Am J Med Genet 2004126A339–348. [DOI] [PubMed] [Google Scholar]
- 36.Berney T P, Ireland M, Burn J. Behavioural phenotype of Cornelia de Lange syndrome. Arch Dis Child 199981333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigafoos J. Communication development and aberrant behavior in children with developmental disabilities. Educat Train Mental Retardat Develop Disab 200235168–176. [Google Scholar]
- 38.Van Berckelaer‐Onnes I A, Van Loon J, Peelen A. Challenging behaviour: A challenge to change. Autism 20026259–270. [DOI] [PubMed] [Google Scholar]
- 39.Cox R D, Mesibov G B. Relationship between autism and learning disabilities. In: Schopler E, Mesibov GB, eds. Learning and cognition in autism. New York: Plenum Press, 1995
- 40.Gillberg C L. Subgroups in autism: are there behavioural phenotypes typical of underlying medical conditions? J Intellect Disabil Res 199236201–214. [DOI] [PubMed] [Google Scholar]
- 41.Kline A D, Barr M, Jackson L G. Growth manifestations in the Brachmann‐de Lange syndrome. Am J Med Genet 1993471042–1049. [DOI] [PubMed] [Google Scholar]