Abstract
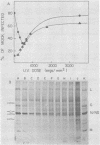
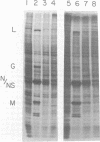
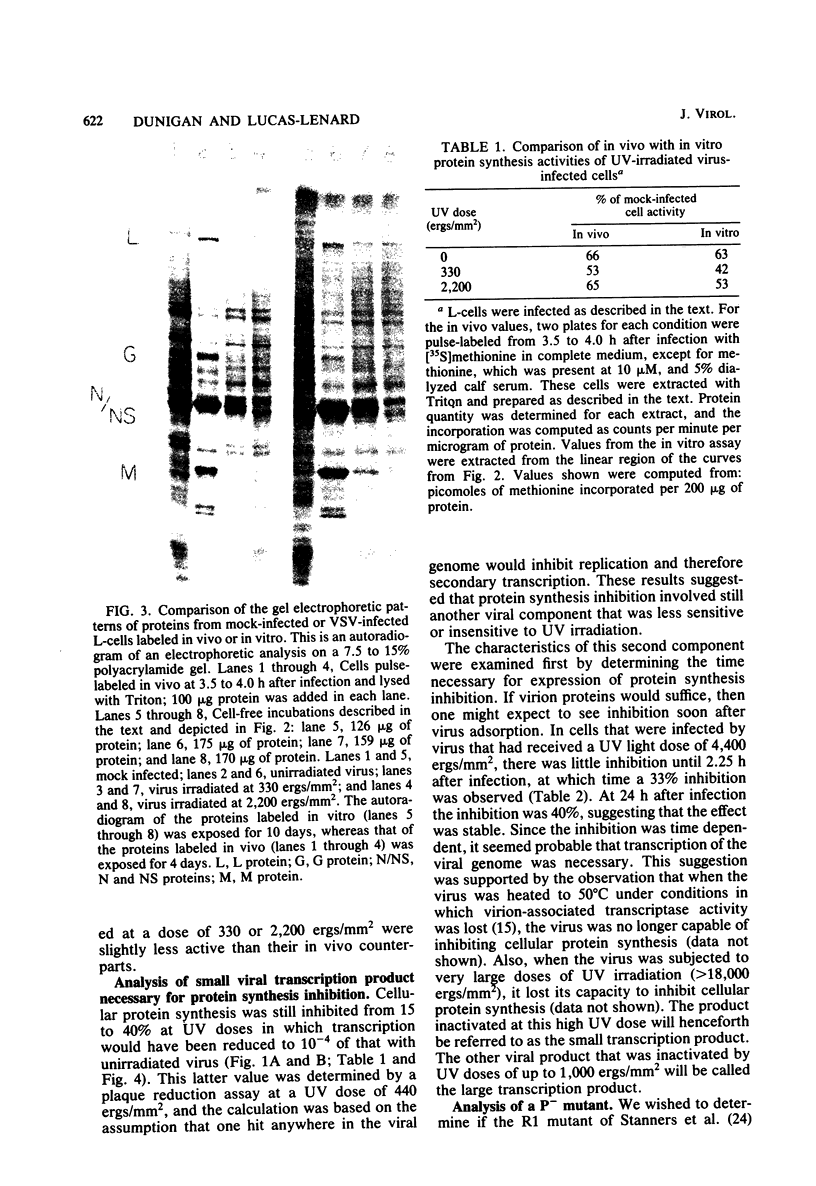
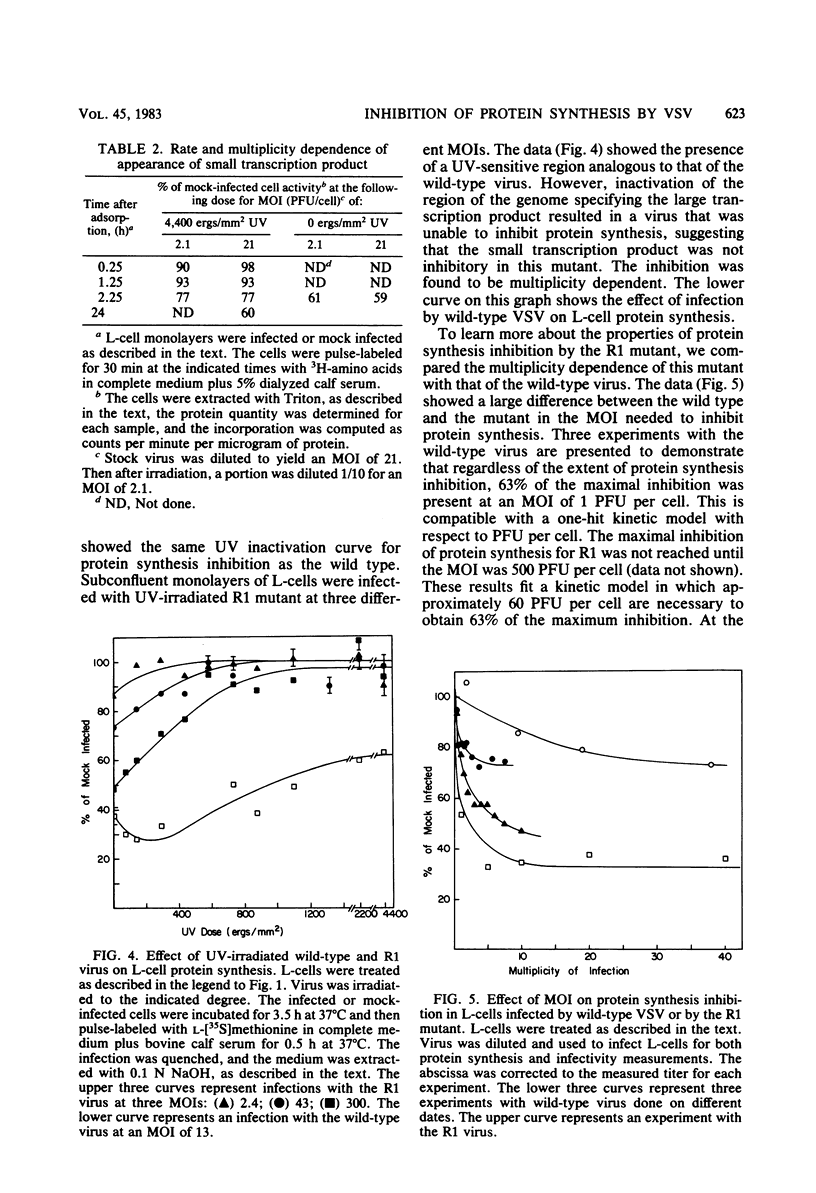
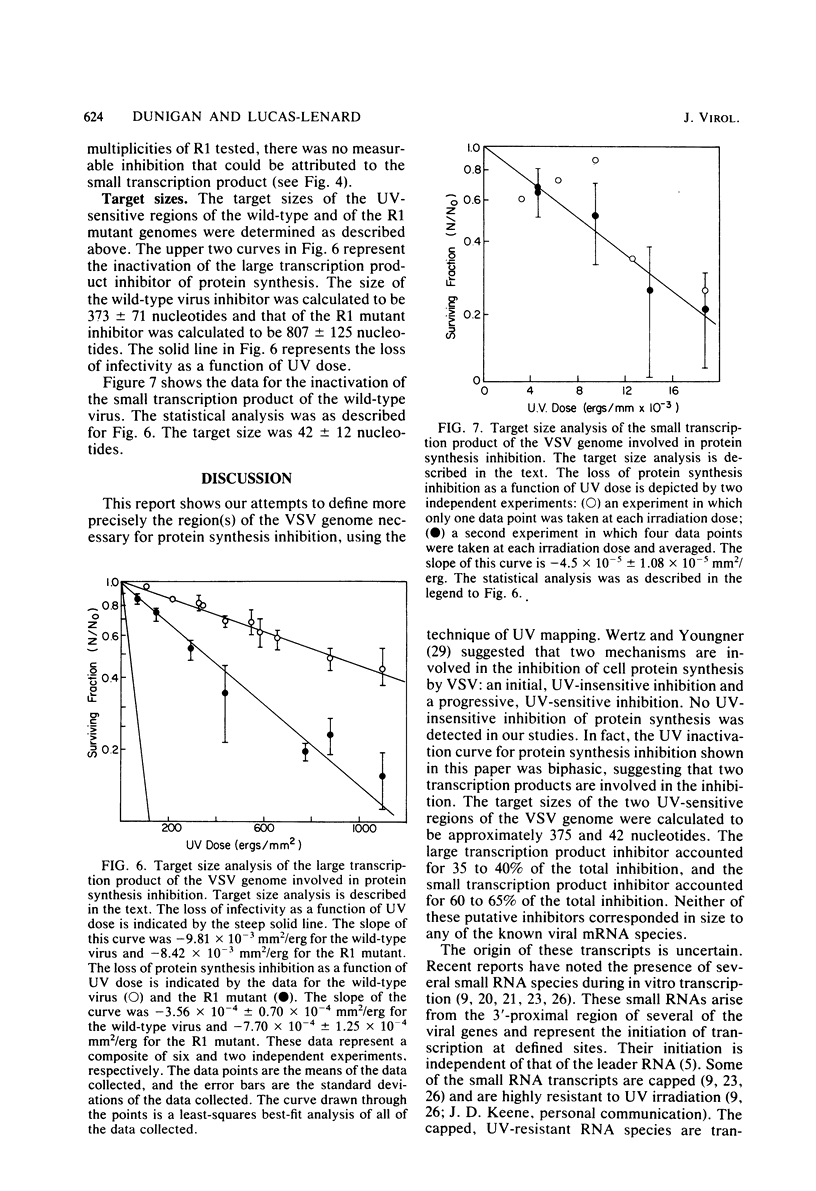
When mouse L-cells are infected with vesicular stomatitis virus, there is a decrease in the rate of protein synthesis ranging from 20 to 85% of that in mock-infected cells. Vesicular stomatitis virus, irradiated with increasing doses of UV light, eventually loses this capacity to inhibit protein synthesis. The UV inactivation curve was biphasic, suggesting that transcription of two regions of the viral genome is necessary for the virus to become inactivated in this capacity. The first transcription product corresponded to about 373 nucleotides, and the second corresponded to about 42 nucleotides. Inhibition of transcription of the larger product by irradiating the virus with low doses of UV light left a residual inhibition of protein synthesis consisting of approximately 60 to 65% of the total inhibition. This residual inhibition could be obviated by irradiating the virus with a UV dose of greater than 20,000 ergs/mm2 and was thus considered to represent the effect of the smaller transcription product. In the R1 mutant of C. P. Stanners et al. (Cell 11:273-281, 1977), inhibition of transcription of the larger product sufficed to restore protein synthesis to the mock-infected level, suggesting that the smaller transcription product is nonfunctional with respect to protein synthesis inhibition. It thus appears that the inhibition of protein synthesis by wild-type vesicular stomatitis virus involved at least two separate viral transcription products, and the inhibition by the R1 mutant involved only one. Extracts from cells infected with virus irradiated with low doses of UV light showed a protein synthesis capacity quite similar to that of their in vivo counterparts, indicating that these extracts closely reflect the in vivo effects of virus infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Centrella M., Lucas-Lenard J. Regulation of protein synthesis in vesicular stomatitis virus-infected mouse L-929 cells by decreased protein synthesis initiation factor 2 activity. J Virol. 1982 Mar;41(3):781–791. doi: 10.1128/jvi.41.3.781-791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Flamand A., Bishop D. H. In vivo synthesis of RNA by vesicular stomatitis virus and its mutants. J Mol Biol. 1974 Jul 25;87(1):31–53. doi: 10.1016/0022-2836(74)90558-0. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway A. F., Wong P. K., Cormack D. V. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus. Virology. 1970 Dec;42(4):917–926. doi: 10.1016/0042-6822(70)90340-5. [DOI] [PubMed] [Google Scholar]
- Iverson L. E., Rose J. K. Sequential synthesis of 5'-proximal vesicular stomatitis virus mRNA sequences. J Virol. 1982 Oct;44(1):356–365. doi: 10.1128/jvi.44.1.356-365.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M. G., Piwnica-Worms H., Keene J. D. Rapid and transient localization of the leader RNA of vesicular stomatitis virus in the nuclei of infected cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5240–5244. doi: 10.1073/pnas.79.17.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Translational control of protein synthesis after infection by vesicular stomatitis virus. J Virol. 1980 Dec;36(3):719–733. doi: 10.1128/jvi.36.3.719-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Vesicular stomatitis virus mRNA and inhibition of translation of cellular mRNA--is there a P function in vesicular stomatitis virus? J Virol. 1981 May;38(2):504–517. doi: 10.1128/jvi.38.2.504-517.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. II. Cell killing by vesicular stomatitis virus: a requirement for virion-derived transcription. Virology. 1975 Jan;63(1):176–190. doi: 10.1016/0042-6822(75)90383-9. [DOI] [PubMed] [Google Scholar]
- Marvaldi J. L., Lucas-Lenard J., Sekellick M. J., Marcus P. I. Cell killing by viruses. IV. Cell killing and protein synthesis inhibition by vesicular stomatitis virus require the same gene functions. Virology. 1977 Jun 15;79(2):267–280. doi: 10.1016/0042-6822(77)90354-3. [DOI] [PubMed] [Google Scholar]
- Marvaldi J., Sekellick M. J., Marcus P. I., Lucas-Lenard J. Inhibition of mouse L cell protein synthesis by ultraviolet-irradiated vesicular stomatitis virus requires viral transcription. Virology. 1978 Jan;84(1):127–133. doi: 10.1016/0042-6822(78)90224-6. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Differential inhibition of host protein synthesis in L cells infected with RNA - temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1976 May;18(2):550–558. doi: 10.1128/jvi.18.2.550-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. Identification and characterization of a group of discrete initiated oligonucleotides transcribed in vitro from the 3' terminus of the N-gene of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):889–896. doi: 10.1128/jvi.42.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. doi: 10.1128/jvi.42.3.897-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Nucleotide sequences of ribosome recgonition sites in messenger RNAs of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3672–3676. doi: 10.1073/pnas.74.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Sprague J., Condra C. S., Lazzarini R. A. In vitro transcription of vesicular stomatitis virus: initiation with GTP at a specific site within the N cistron. J Virol. 1982 Jul;43(1):166–173. doi: 10.1128/jvi.43.1.166-173.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P., Francoeur A. M., Lam T. Analysis of VSV mutant with attenuated cytopathogenicity: mutation in viral function, P, for inhibition of protein synthesis. Cell. 1977 Jun;11(2):273–281. doi: 10.1016/0092-8674(77)90044-7. [DOI] [PubMed] [Google Scholar]
- Talib S., Banerjee A. K. Covalent attachment of psoralen to a single site on vesicular stomatitis virus genome RNA blocks expression of viral genes. Virology. 1982 Apr 30;118(2):430–438. doi: 10.1016/0042-6822(82)90362-2. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. S., Lucas-Lenard J. M. Inhibition of ribonucleic acid accumulation in mouse L cells infected with vesicular stomatitis virus requires viral ribonucleic acid transcription. Biochemistry. 1980 Feb 19;19(4):804–810. doi: 10.1021/bi00545a029. [DOI] [PubMed] [Google Scholar]