Abstract
Background
The VACTERL with hydrocephalus (VACTERL‐H) phenotype is recognised to be a severe manifestation of autosomal recessive Fanconi anaemia. Several families have been described in which the VACTERL‐H phenotype segregates as an X linked syndrome. The mutations which cause X linked VACTERL‐H syndrome are not known.
Objective
To determine if mutations in FANCB, which are known to cause Fanconi anaemia complementation group B, are a cause of X linked VACTERL‐H syndrome.
Methods
A three generation pedigree with X linked VACTERL‐H syndrome was investigated. X inactivation was tested in carrier females, and fibroblasts from an affected male fetus were analysed for increased sensitivity to diepoxybutane. FANCB coding exons and flanking splice sites were screened for mutations by direct sequencing of polymerase chain reaction (PCR) fragments amplified from genomic DNA. cDNA from affected fetal fibroblasts was analysed by PCR and direct sequencing using specific exonic primers.
Results
A FANCB mutation which results in a premature stop codon by causing skipping of exon 7 was identified. Chromosomes from the affected fetus showed increased sensitivity to diepoxybutane, and carrier women were found to have 100% skewed X inactivation in blood.
Conclusions
Mutations in FANCB are a cause of X linked VACTERL‐H syndrome. The data presented are of relevance to the genetic counselling of families with isolated male cases of VACTERL‐H and Fanconi anaemia.
Keywords: Fanconi anaemia, FANCB, VACTERL, X linkage, X inactivation
Fanconi anaemia is a heterogeneous disorder associated with defective haemopoiesis and is caused by mutations in at least 12 different genes, 11 of which have been identified.1,2,3,4 The 11 known genes encode proteins which function in the Fanconi anaemia DNA damage response pathway which is important for genome maintenance. In addition to causing bone marrow failure, Fanconi anaemia causes a diverse spectrum of congenital anomalies and an increased risk of malignancy.1,2 Fanconi anaemia cells show increased sensitivity to DNA cross linking agents such as mitomycin C and diepoxybutane, which manifests as increased chromosome breakage. It is this sensitivity to DNA cross linking agents that provides the basis for a diagnostic test for this condition.2 Congenital anomalies affect over two thirds of individuals with Fanconi anaemia.1,2 These anomalies include limb (radial ray) defects, vertebral defects, urogenital abnormalities, and microphthalmia. The congenital anomalies are often asymmetrical and display both interfamilial and intrafamilial variability.
Some individuals with Fanconi anaemia have a clinical phenotype which overlaps with that seen in the VACTERL association.1,5 This association occurs sporadically and is characterised as a non‐random pattern of at least three of the following defects: vertebral anomalies, anal atresia, cardiovascular malformations, tracheo‐oesophageal fistula, renal and limb abnormalities (including radial ray defects). In contrast to Fanconi anaemia, growth retardation and pigmentary skin changes are not features of the VACTERL association, and true VACTERL association is not associated with brain abnormalities. These clinical features are, however, recognised as part of the VACTERL with hydrocephalus phenotype (VACTERL‐H), which behaves as a monogenic disease and not as a sporadic association.6
Like Fanconi anaemia, VACTERL‐H is also genetically heterogeneous, and familial recurrences consistent with both autosomal recessive and sex linked inheritance have been reported.7,8,9,10 Some cases of VACTERL‐H are associated with increased chromosome breakage and rearrangement, and are now known to represent severe manifestations of Fanconi anaemia.11,12 Until recently, Fanconi anaemia was thought to be inherited exclusively as an autosomal recessive condition. The finding that the defective gene causing Fanconi anaemia complementation group B (Fanconi anaemia‐B) maps to the X chromosome was therefore unexpected.13 To date the FANCB gene has been shown to be mutated in four unrelated males all of whom had growth retardation, radial ray defects, kidney abnormalities, and hypogonadism.13 Three of these individuals were also reported to have unspecified head abnormalities. The affected males from these pedigrees had no other affected male relatives and therefore presented as isolated cases. We have studied a family in which a male fetus and maternal uncle expressed the VACTERL‐H phenotype. This phenotype is associated with a mutation which causes abnormal splicing of the FANCB transcript and leads to a premature stop codon. This is the first mutation to be described in X linked VACTERL‐H syndrome.
Case report
We were referred a 23 year old female patient (II‐1) following the termination of a male fetus (III‐1) with multiple congenital abnormalities at 20 weeks' gestation (fig 1A). There was no history of consanguinity or exposure to drugs, teratogens, or alcohol in the pregnancy. The fetus weighed 240 g, and had a crown–rump length of 23.8 cm and a head circumference of 15.8 cm. The congenital anomalies included cervical vertebral defects, absent thumbs and radii, unilateral renal agenesis, and bilateral cerebral ventriculomegaly (fig 1B). The cerebral aqueduct was found to be patent at necropsy. Incomplete lung lobation was also found at necropsy. There were no other craniofacial abnormalities or dysmorphisms.
Figure 1 Identification of the FANCB mutation associated with the VACTERL‐H phenotype. (A) X linked VACTERL‐H pedigree. The proband II‐1 is indicated by an arrow. (B) Radiograph of the affected fetus III‐1 showing bilateral radial ray defects (absent thumbs and radii) and cervical vertebrae anomalies. (C) Complete skewing of X inactivation in peripheral blood leucocytes from carrier females at the HUMAR locus. I‐1 and II‐1 have inactivated different HUMAR alleles. This can be accounted for by a single recombination event between the HUMAR locus at Xq12 and the FANCB locus at Xp22.3 in II‐1. (D) The intronic G to A mutation in the splice donor site of intron 7 in genomic DNA sequence from the affected hemizygous fetus III‐1 and his heterozygous mother II‐1 (genomic sequence from I‐1 not shown).
In the family history, the patient's mother (I‐1) was found to have had a stillborn male fetus (II‐2) at 30 weeks' gestation, who had similar congenital abnormalities (fig 1A). The necropsy report of this affected fetus was reviewed. The fetus weighed 1038 g, and had a crown–rump length of 26 cm and a head circumference of 29 cm. He had hydrocephalus associated with an Arnold‐Chiari malformation, missing thumbs, and unilateral renal agenesis. In addition, he had multiple cardiac defects, tracheo‐oesophageal fistula, oesophageal atresia, and abnormal ears. Radiographic studies showed bilateral absent radii and a lumbar spina bifida occulta. These developmental abnormalities in two related male fetuses led to a diagnosis of X linked VACTERL‐H syndrome (table 1).
Table 1 Summary of congenital abnormalities associated with the X linked VACTERL‐H phenotype in the affected fetuses III‐1 and II‐2.
| Abnormality | Fetus III‐1 | Fetus II‐2 |
|---|---|---|
| Brain | Bilateral ventriculomegaly, patent aqueduct | Hydrocephalus, Arnold‐Chiari malformation |
| Vertebral | Abnormal cervical (C2–C6) vertebrae | Lumbar (L5/S1) spina bifida occulta |
| Alimentary system | Normal | Oesophageal atresia |
| Cardiac | Normal | ASD, VSD, aortic coarctation |
| Trachea and oeseophagus | Normal | Fistula |
| Renal | Agenesis left kidney and ureter | Agenesis right kidney, dysplastic left kidney |
| Limb | Absent radii and thumbs | Absent radii and thumbs |
| Genital | Undescended testes | Normal |
| Ears | Normal | Non‐patent external auditory meatus, low set left ear |
| Lung | Incomplete lobation | Not known |
| Cord vessels | Single umbilical artery | Not known |
| Growth | IUGR | IUGR |
| Chromosomes | 46,XY, abnormal breakage with diepoxybutane | Not known |
ASD, atrial septal defect; IUGR, intrauterine growth retardation; VSD, ventricular septal defect.
Methods
The chromosome breakage assay for Fanconi anaemia was carried out according to standard protocols. Cultured fibroblasts from the affected fetus were incubated in the presence of diepoxybutane at a final concentration of 0.01 μg/ml for 36 hours along with untreated cells and an identically treated control. The resulting chromosome preparations were analysed for chromosome instability by light microscopy and the results for the patient and control cultures compared with established laboratory ranges. X inactivation studies were undertaken on genomic DNA using polymerase chain reaction (PCR) primers that amplify a differentially methylated region and an adjacent polymorphic triplet repeat both within exon 1 of the human androgen receptor (HUMAR) gene at Xq12. PCR products were amplified from both undigested DNA and DNA digested with HpaII and CfoI. The amplified PCR products were analysed using the method described in Sharp et al.14
We screened for FANCB mutations by direct sequencing of PCR fragments amplified from genomic DNA from the affected fetus III‐1. The UCSC genome browser was used to identify intronic sequences flanking all exons of the full length FANCB gene (associated cDNAs AK091383, AX746948, and BC043596). We designed 11 primer sets to amplify the eight coding exons and corresponding splice sites. PCR products were amplified using standard techniques (primers and conditions available on request). cDNA from affected individual III‐1 was prepared from RNA isolated from fetal fibroblasts. Total RNA was reverse transcribed using the SuperScript First‐Strand Synthesis System for reverse transcriptase polymerase chain reaction (RT‐PCR) (Invitrogen, San Diego, California, USA) according to the manufacturer's protocol. The PCR product containing the abnormally spliced FANCB coding sequence was amplified using standard conditions with forward and reverse primers in exons 6 and 8, respectively.
Results
A fibroblast culture was established from the affected fetus III‐1 after the pregnancy was terminated. Chromosome analysis showed a normal 46,XY karyotype. Chromosome breakage studies with diepoxybutane showed an increased number of chromosome breaks within the affected range observed in Fanconi anaemia cells (table 2). The possibility that a mutation in FANCB was responsible for the observed clinical and cellular phenotype was considered. FANCB mutations have been shown to be associated with 100% skewed X inactivation in peripheral blood and other tissues in carrier females, the X chromosome carrying the mutant FANCB allele being preferentially inactivated.13 X inactivation studies in the proband (II‐1) and her mother (I‐1) were therefore undertaken. Both of these women were found to have 100% skewing of X inactivation at the HUMAR locus in peripheral blood leucocytes (fig 1C). The results show that these women have inactivated different HUMAR alleles. The HUMAR locus and FANCB map to Xq12 and Xp22.3, respectively. If, as inferred, both women have inactivated the mutant FANCB allele, the results can be explained by a single meiotic recombination event between these two loci, as previously described.13
Table 2 Percentage of spontaneous and diepoxybutane (DEB) induced chromosome breakage in patient and control cell lines.
| Cases | Per cent of breaks per cell | ||
|---|---|---|---|
| Spontaneous | DEB | ||
| Test 1 | III‐1 | 0.18 | 0.56 |
| Normal control | 0.04 | 0.16 | |
| Test 2 | III‐1 | 0.11 | 0.54 |
| Normal control | 0.02 | 0.04 | |
| Control range* | (0.00 to 0.14) | (0.00 to 0.20) | |
| Affected range* | (0.18 to 0.48) | (0.44 to 1.25) | |
*The quoted ranges for control and Fanconi anaemia cells are those established by the cytogenetics laboratory at Guy's Hospital.
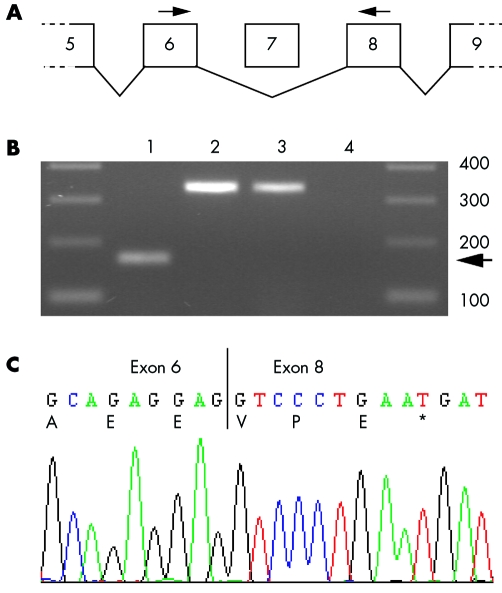
The eight coding exons of FANCB were screened for mutations by direct sequencing of genomic DNA.13 We identified a G→A substitution in intron 7 which mutates a highly conserved guanine residue at position +5 within the splice‐donor site (GTAAGT→GTAAAT, fig 1D). Sequencing of the mutant cDNA fragment from the affected male fetus showed that this causes skipping of exon 7 (fig 2). This causes a frameshift in the FANCB transcript which results in a stop codon at position 446 of the open reading frame (fig 2C). Genomic DNA from both obligate carrier females was sequenced and confirmed that they were heterozygous for the mutation (fig 1C).
Figure 2 The intron 7 splice site mutation in FANCB causes skipping of exon 7. This results in a frameshift and premature stop codon in exon 8. (A) The relative positions of primers used to amplify sequence from exons 6, 7, and 8 from FANCB cDNA. (B) Reverse transcriptase polymerase chain reaction of FANCB products. Amplification of the wild‐type transcript resulted in a 336 base pair (bp) product when control cDNA from human fibroblasts (lane 2) and fetal brain (lane 3) was used as template. Lane 1: the PCR product amplified from a fibroblast cell line derived from the affected fetus III‐1 was shorter (166 bp). Lane 4: water blank. The sizes of DNA markers are indicated in bp. (C) cDNA sequencing of the mutant FANCB cDNA confirmed that exon 7 had been deleted. This results in the addition of three novel amino acids and a premature stop codon in the open reading frame of exon 8.
Discussion
We have carried out mutation analysis on the FANCB gene in a pedigree with X linked VACTERL‐H. A mutation which causes abnormal splicing of the FANCB transcript was identified. This mutation causes a frameshift in the FANCB open reading frame which results in a premature stop codon and is likely to cause nonsense mediated decay of the abnormally spliced FANCB mRNA. This is the first mutation to be associated with X linked VACTERL‐H syndrome.
Our data, together with the reported clinical findings in four other unrelated affected males from different pedigrees, show that the growth retardation and bilateral radial ray defects associated with FANCB mutations appear to be highly penetrant (six of six affected).13 Head and kidney abnormalities, together with hypogonadism are also very common associations (five of six affected). However, owing to the small number of reported cases, ascertainment bias for these phenotypic associations cannot be excluded at the moment. Unfortunately, it is not possible to discern from published data on the other reported males with FANCB mutations whether hydrocephalus was part of the phenotype in these individuals.13 Three other VACTERL‐H pedigrees have been reported in which the phenotype is likely to be X linked.8,9,10 Symmetrical radial ray abnormalities and hydrocephalus were present in 10 of 10 affected males from these families, and renal agenesis and genital abnormalities are reported in all three pedigrees. Other congenital anomalies including vertebral defects, congenital heart disease, tracheo‐oesophageal fistula, and gut atresias have been described as part of the X linked VACTERL‐H syndrome in these reports. However, none of the affected males has survived, and necropsies were not always carried out. It is therefore difficult to establish whether these additional congenital anomalies occurred in affected males from all three families.
Interestingly, although hydrocephalus appears to be a highly penetrant trait, the anatomical causes appear to vary both within and between families. The affected fetus of the proband in our family had a patent aqueduct and his uncle was reported to have an Arnold‐Chiari malformation associated with a lower spina bifida occulta. Interestingly, in the two affected male cousins reported by Genuardi et al, one also had hydrocephalus associated with an Arnold‐Chiari malformation.8 In contrast, the affected males who underwent necropsy in the two families reported by Wang et al and Lomas et al were reported to have aqueduct stenosis.9,10 Thus, although the phenotypes observed in our family and the three other reported families are very similar, genetic heterogeneity cannot be ruled out at the moment. Amniocytes from an affected male reported by Lomas et al did not show increased chromosome breakage when challenged with mitomycin C, and chromosome breakage studies were not carried out in the family reported by Genuardi et al.8,10 However, chromosomes from affected males reported by Wang et al were found to have increased spontaneous breakages and sensitivity to mitomycin C, implicating a mutation in FANCB as the underlying cause of the VACTERL‐H phenotype.9
It is clinically important to discriminate between Fanconi anaemia and VACTERL association. Hydrocephalus and growth retardation are not taken to be features of the VACTERL association. However, VACTERL can be readily distinguished from Fanconi anaemia by the increased sensitivity of Fanconi anaemia cells to DNA cross linking agents such as diepoxybutane.5 Distinguishing cases of X linked Fanconi anaemia‐B from autosomal recessive forms expressing the VACTERL‐H phenotype may be less straightforward, particularly as the majority of affected males with Fanconi anaemia‐B are likely to occur as single cases.11,12,13 However, for genetic counselling it is important for this distinction to be made so that the parents and relatives of affected males can be given accurate information about the recurrence risks. The FANCB gene has been shown to undergo X inactivation, and the mutant allele to be preferentially inactivated in carrier females.13 X inactivation studies on suspected carrier females can therefore be helpful in suspected cases of Fanconi anaemia‐B: both carrier women in this report, and the three carrier females reported by Meetei et al, have 100% skewing of X inactivation in peripheral blood leucocytes.13 Selection against blood cells expressing mutations in X linked genes is a well documented feature of several severe X linked recessive conditions, and complete skewing of X inactivation in the general population is uncommon.14,15,16 Thus, if non‐random X inactivation is observed in the mothers of males with VACTERL‐H, this should raise the suspicion of Fanconi anaemia‐B.13 Detailed fetal ultrasonography to monitor for radial ray defects, hydrocephalus, and renal abnormalities may be offered to women at risk of having an affected male fetus as a means of non‐invasive prenatal diagnosis. However, until the full phenotypic spectrum of Fanconi anaemia‐B is defined, chromosome breakage studies and mutation analysis on male fetuses at risk of Fanconi anaemia‐B will be necessary for accurate prenatal diagnosis.
There appears to be strong selection against cells expressing the mutant FANCB allele in female carriers: 100% skewed X inactivation was observed in fibroblasts, blood cells, and urothelial cells from the carriers reported by Meetei et al, and these individuals and the carrier females reported in this study all have normal clinical phenotypes.13 X inactivation occurs randomly in early embryogenesis, and once inactivated, the inactive status of an X chromosome is clonally persistent.17 The absence of congenital abnormalities in FANCB carrier females suggests that selection against cells expressing the mutant FANCB allele occurs early in their development.13 If so, early selection against cells expressing the FANCB mutant allele may substantially reduce any additional cancer risks conferred by FANCB mutations. Although this cannot be fully excluded at the moment, none of the three female carriers reported by Meetei et al had been diagnosed with malignancy (ages 12, 29, and 43 years), and neither of the female carriers reported here have had cancer (ages 23 and 49 years, respectively).
In summary, we show that mutations in FANCB are a cause of X linked VACTERL‐H syndrome. Analysis of further cases and their family pedigrees will help to further define the clinical phenotype associated with FANCB mutations. The data reported here are of relevance to the genetic counselling of relatives of males with the VACTERL‐H phenotype and proven Fanconi anaemia‐B families.
Acknowledgements
We wish to thank the family for participating in this study and Dr Anita Whitehead for providing the x ray used in fig 1. Consent for publication of this image was obtained from the family. This work was approved by the institutional research ethics committee, and James Cox is supported by the Wellcome Trust.
Abbreviations
VACTERL - vertebral anomalies–anal atresia–cardiac abnormalities–tracheo‐oesophageal fistula–renal agenesis–limb defects
VACTERL‐H - VACTERL with hydrocephalus
Footnotes
Conflicts of interest: none declared
References
- 1.Faivre L, Guardiola P, Lewis C, Dokal I, Ebell W, Zatterale A, Altay C, Poole J, Stones D, Kwee M L, van Weel‐Sipman M, Havenga C, Morgan N, de Winter J, Digweed M, Savoia A, Pronk J, de Ravel T, Jansen S, Joenje H, Gluckman E, Mathew C G. Association of complementation group and mutation type with clinical outcome in Fanconi anemia. European Fanconi Anemia Research Group. Blood 2000964064–4070. [PubMed] [Google Scholar]
- 2.Tischkowitz M D, Hodgson S V. Fanconi anaemia. J Med Genet 2003401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levitus M, Waisfisz Q, Godthelp B C, de Vries Y, Hussain S, Wiegant W W, Elghalbzouri‐Maghrani E, Steltenpool J, Rooimans M A, Pals G, Arwert F, Mathew C G, Zdzienicka M Z, Hiom K, De Winter J P, Joenje H. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet 200537934–935. [DOI] [PubMed] [Google Scholar]
- 4.Meetei A R, Medhurst A L, Ling C, Xue Y, Singh T R, Bier P, Steltenpool J, Stone S, Dokal I, Mathew C G, Hoatlin M, Joenje H, de Winter J P, Wang W. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat Genet 200537958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faivre L, Portnoi M F, Pals G.et al Should chromosome breakage studies be performed in patients with VACTERL association? Am J Med Genet A 200513755–58. [DOI] [PubMed] [Google Scholar]
- 6.Evans J A, Stranc L C, Kaplan P, Hunter A G. VACTERL with hydrocephalus: further delineation of the syndrome(s). Am J Med Genet 198934177–182. [DOI] [PubMed] [Google Scholar]
- 7.Corsello G, Giuffre L. VACTERL with hydrocephalus: a further case with probable autosomal recessive inheritance. Am J Med Genet 199449137–138. [DOI] [PubMed] [Google Scholar]
- 8.Genuardi M, Chiurazzi P, Capelli A, Neri G. X‐linked VACTERL with hydrocephalus: the VACTERL‐H syndrome. Birth Defects Orig Artic Ser 199329235–241. [PubMed] [Google Scholar]
- 9.Wang H, Hunter A G, Clifford B, McLaughlin M, Thompson D. VACTERL with hydrocephalus: spontaneous chromosome breakage and rearrangement in a family showing apparent sex‐linked recessive inheritance. Am J Med Genet 199347114–117. [DOI] [PubMed] [Google Scholar]
- 10.Lomas F E, Dahlstrom J E, Ford J H. VACTERL with hydrocephalus: family with X‐linked VACTERL‐H. Am J Med Genet 19987674–78. [DOI] [PubMed] [Google Scholar]
- 11.Porteous M E, Cross I, Burn J. VACTERL with hydrocephalus: one end of the Fanconi anemia spectrum of anomalies? Am J Med Genet 1992431032–1034. [DOI] [PubMed] [Google Scholar]
- 12.Cox P M, Gibson R A, Morgan N, Brueton L A. VACTERL with hydrocephalus in twins due to Fanconi anemia (FA): mutation in the FAC gene. Am J Med Genet 19976886–90. [PubMed] [Google Scholar]
- 13.Meetei A R, Levitus M, Xue Y, Medhurst A L, Zwaan M, Ling C, Rooimans M A, Bier P, Hoatlin M, Pals G, de Winter J P, Wang W, Joenje H. X‐linked inheritance of Fanconi anemia complementation group B. Nat Genet 2004361219–1224. [DOI] [PubMed] [Google Scholar]
- 14.Sharp A, Robinson D, Jacobs P. Age‐ and tissue‐specific variation of X chromosome inactivation ratios in normal women. Hum Genet 2000107343–349. [DOI] [PubMed] [Google Scholar]
- 15.Schmucker B, Meindl A, Achatz H, Mittermuller J, Kruger G, Hergersberg M, Spiegel R, Schinzel A, Belohradsky B H, Murken J.et al A PCR based X‐chromosome inactivation assay for carrier detection in X‐linked immunodeficiencies using differential methylation of the androgen receptor gene. Immunodeficiency 19955187–192. [PubMed] [Google Scholar]
- 16.Ferraris A M, Forni G L, Mangerini R, Gaetani G F. Nonrandom X‐chromosome inactivation in hemopoietic cells from carriers of dyskeratosis congenita. Am J Hum Genet 199761458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puck J M, Willard H F. X inactivation in females with X‐linked disease. N Engl J Med 1998338325–328. [DOI] [PubMed] [Google Scholar]