Abstract
Background
Andersen‐Tawil syndrome (ATS) is a rare inherited disorder, characterised by periodic paralysis, cardiac dysarrhythmias, and dysmorphic features, and is caused by mutations in the gene KCNJ2, which encodes the inward rectifier potassium channel, Kir2.1. This study sought to analyse KCNJ2 in patients with familial ATS and to determine the functional characteristics of the mutated gene.
Methods and results
We screened a family with inherited ATS for the mutation in KCNJ2, using direct DNA sequencing. A missense mutation (T75R) of Kir2.1, located in the highly conserved cytoplasmic N‐terminal domain, was identified in three affected members of this family. Using the Xenopus oocyte expression system and whole cell voltage clamp analyses, we found that the T75R mutant was non‐functional and possessed a strong dominant negative effect when co‐expressed with the same amount of wild type Kir2.1. Transgenic (Tg) mice expressing the mutated form of Kir2.1 in the heart had prolonged QTc intervals compared with mice expressing the wild type protein. Ventricular tachyarrhythmias were observed in 5 of 14 T75R‐Tg mice compared with 1 of 7 Wt‐Tg and none of 6 non‐transgenic littermates. In three of five T75R‐Tg mice with ventricular tachycardia, their ECG disclosed bidirectional tachycardia as in our proband.
Conclusions
The in vitro studies revealed that the T75R mutant of Kir2.1 had a strong dominant negative effect in the Xenopus oocyte expression system. It still preserved the ability to co‐assemble and traffic to the cell membrane in mammalian cells. For in vivo studies, the T75R‐Tg mice had bidirectional ventricular tachycardia after induction and longer QT intervals.
Keywords: arrhythmia, genetics, ion channels
Kir2.1 is an inward rectifier potassium channel that plays an important role in determining and maintaining the resting membrane potential in many cell types, including skeletal muscle cells, cardiomyocytes, smooth muscle cells, neurones and osteoclasts.1 It also greatly contributes to the cardiac inward rectifier current (IK1), which serves as a repolarising current during the terminal phase of the cardiac action potential.2 Mutations in the gene KCNJ2, which encodes Kir2.1, have been identified in patients with Andersen‐Tawil syndrome (ATS).3 This is a rare familial disorder characterised by three different clinical manifestations: periodic paralysis; ventricular arrhythmias with prolongation of the QT interval; and skeletal and craniofacial abnormalities such as hypertelorism, low set ears, micrognathia, and clinodactyly.4,5 Recently, Yoon et al identified novel skeletal and dental findings and proposed additional diagnostic criteria for ATS, including syndactyly and microcephaly.6 These clinical features are highly dependent on the distribution of Kir 2.1 mutations, and further support the notion that ATS, like the other familial disorders causing QT prolongation and ventricular arrhythmias, is a type of channelopathy. Targeted deletion of the murine kcnj2 gene (Kir2.1−/−) has been shown to be capable of eliminating IK1 in newborn ventricular myocytes, resulting in a significantly broader and more spontaneous firing action of the potential compared with wild type (WT) myocytes.7 This finding may correlate with the QT prolongation and the development of spontaneous ventricular arrhythmias that are observed in patients with ATS. Previously, several mutations in KCNJ2 have been reported in patients with ATS phenotypes, and biophysical analysis has shown variable degrees of dominant negative suppression of Kir2.1 channel function when they are expressed together with WT Kir2.1.5,8,9,10,11,12
METHODS
Case histories
A 15 year old girl was referred because of episodic weakness and cardiac rhythm irregularities for 5 years. The symptoms either occurred spontaneously or with rest following exercise, and were not precipitated or worsened by cold. These attacks caused difficulties in walking and climbing steps, and lasted from several minutes to days. On physical examination, she had clinodactyly on the left side, scoliosis, a high arched palate, an asymmetrically placed lower mandible, and mild varus position of her left wrist. Her cardiac examination revealed frequent premature ventricular contractions (PVCs). The neurological examination was normal. Her serum potassium was always normal, including during the attacks. There was no electrical myotonia on EMG. Her ECG and Holter monitor demonstrated prominent U waves over the precordial leads, frequent PVCs, and non‐sustained bidirectional ventricular tachycardia (VT). The bidirectional VT had a right bundle branch block (RBBB) pattern; however, she never experienced palpitations or syncope. The corrected QT (QTc) interval on surface ECG was 0.38 s, and none of her family members showed a prolonged QTc interval. No structural anomaly was found by ECG. No dental abnormalities were recorded.
Her father also had a history of intermittent paralytic episodes, which had been present since he was 14 years of age. Such attacks, usually associated with resting after exercise or prolonged resting, similar to his daughter, resulted in the inability to walk and lasted from several hours to a few days. His serum potassium level was examined during each of these episodes. In all but one instance, the serum potassium was normal; in that situation, it was measured at 2.1 mEq/L. His history included surgery for a cleft palate and congenital facial deformities. In addition, bilateral clinodactyly, mild flexion deformities of his distal interphalangeal joints, an abnormal rib cage on the right and scoliosis were present. He also had a history of PVCs seen on an ECG, and had complained of palpitations 20 years previously.
The proband's 20 year old sister (patient II:1) was noted to have bilateral clinodactyly and occasional PVCs during her examination. However, she had no paralytic episodes or other subjective complaints. Their mother and 13 year old brother had normal resultss.
Mutation analysis
Genomic DNA was isolated from peripheral blood lymphocytes obtained after written informed consents. The coding region of KCNJ2 was amplified by PCR with four pairs of primers as follows: F1, 5′‐CCAAAGCAGAAGCACTGGAG‐3′; R1, 5′‐CCATGGAGCAGAGCTATCAA‐3′; F2, 5′‐ACGTGTGTGGACATTCGCTG‐3′; R2, 5′‐CAATCACGGCATTGTGACTG‐3′; F3, 5′‐CAGTCATGGCCAAGATGGCA‐3′; R3, 5′‐CACTGTGTCGTCATGGCAGT‐3′; F4, 5′‐GTGGTCATACTGGAAGGCAT‐3′, and R4, 5′‐CCAGAGAAGGAATCAGTCAG‐3′. The PCR products were purified with a purification kit (Qiagen, Valencia, CA, USA) and sequenced using a commercial kit and automated sequencer (BigDye Terminator cycle sequencing kit, version 3.1 and ABI Prism 3100; both Applied Biosystems, Foster City, CA, USA). The results were compared with the published sequences of the KCNJ2 (NM_000891). The absence of the mutation in 100 unrelated, ethnically matched unaffected individuals was confirmed by denaturing high performance liquid chromatography analysis.
Cloning of KCNJ2 cDNA and in vitro mutagenesis
The coding region of KCNJ2 was cloned by PCR from human genomic DNA, using primers F1 and R4 described above, into the plasmid pCR 2.1‐TOPO (Invitrogen, Carlsbad, CA, USA). In vitro mutagenesis was performed using a site directed mutagenesis kit (QuikChange; Stratagene, La Jolla, CA, USA) to create the mutant KCNJ2. WT and mutant KCNJ2 cDNAs were subcloned into pBluescript KS (+) and pBluescript SK (−) (Stratagene), using BamHI and NotI.
The WT and mutant KCNJ2 cDNAs were subcloned into pcDNA3.1/V5‐His TOPO vector (Invitrogen) by PCR and TOPO cloning. A construct encoding a C‐terminal Myc tagged KCNJ2 was prepared by subcloning the KCNJ2 cDNAs using BamHI and EcoRV from the pcDNA3.1/V5‐His constructs into the vector pCMV‐Myc‐tag 5A (Stratagene).
Expression of Kir2.1 in mammalian cells
Cells from the cardiac muscle cell line HL‐1, derived from the AT‐a subcutaneous mouse atrial cardiomyocyte tumour line,13 (obtained from Dr W C Claycomb, Louisiana State University Medical Center, USA) were maintained in Ex‐Cell 320 medium (JRH Biosciences, Lenexa, KS, USA) supplemented with 10% fetal bovine serum (Invitrogen), 10 µmol/l norepinephrine (Sigma‐Aldrich, St. Louis, MO, USA), 100 units/ml penicillin, 100 µg/ml streptomycin (Invitrogen), and 1× nonessential amino acids (Invitrogen). The cells were grown at 37°C in 5% CO2 and a relative humidity of approximately 95%. Cultured HL‐1 cells were plated at 5×104 cells per well on glass coverslips in two well slides. The following day, the cells were transfected with 1 μg of plasmid DNA (pcDNA3.1/V5‐His constructs) using Effectene (Qiagen), according to the manufacturer's protocol. After 48 hours, the cells were fixed and incubated with mouse anti‐V5 monoclonal antibody (Sigma‐Aldrich) diluted 1:1000 in CAS blocking solution (Zymed, South San Francisco, CA, USA). Immunoreactivity was detected by staining with FITC‐anti mouse (Molecular Probes, Eugene, OR, USA) and visualised by fluorescence microscopy using Olympus BX51 epifluorescence microscope equipped with a SPOT CCD camera.
Co‐immunoprecipitation studies were performed in HEK293 cells cultured in 100 mm dishes and transfected using Effectene with combinations of the vectors encoding WT or mutant KCNJ2 tagged with either V5 or c‐myc (4 μg total plasmid DNA per plate) (fig 1B). After 24 hours, the cell lysates were collected in RIPA buffer and incubated with beads pre‐coated anti‐Myc antibody (Protein G Plus‐Agarose beads; Santa Cruz Biotechnology, Carlsbad, CA, USA) or goat anti‐mouse IgG (Santa Cruz Biotechnology). Cellular lysates and immunoprecipitated proteins were separated on 4% to 12% Tris‐acetate polyacrylamide gels (NuPage; Invitrogen) at 100 V for 2 hours, and transferred to nitrocellulose membranes by electrotransfer at 21 V for 18 hours at 4°C. The lysate proteins were probed with anti‐Myc (Santa Cruz Biotechnology) antibody diluted 1:500, and the immunoprecipitated proteins were probed with anti‐Myc, as above, or anti‐V5 antibody (Sigma‐Aldrich) diluted 1:000. Signals were detected by staining with horseradish peroxidase labelled anti‐rabbit secondary anti‐goat antibody (Santa Cruz Biotechnology) followed by chemiluminescent detection using an enhanced chemiluminescence kit (Pierce Biotechnology, Rockford, IL, USA).
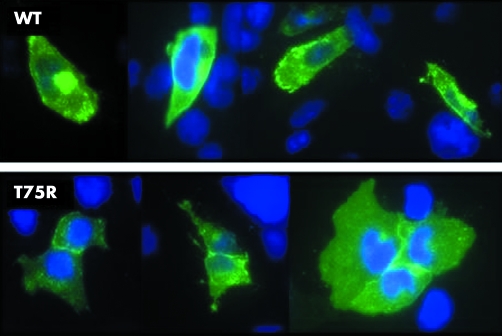
Figure 1 (A) Expression of Kir2.1 in HL1 cells transfected with plasmids expressing WT (top) or T75R Kir2.1 (bottom). Kir2.1 is stained green and nuclei are stained blue. (B) Co‐immunoprecipitation of Kir2.1 by itself. Cells were transfected with combinations of plasmids expressing WT or T75R (mut) Kir2.1. Myc tagged proteins were immunoprecipitated using anti‐Myc agarose and co‐immunoprecipitating proteins were detected by anti‐V5 antibodies (Co‐IP). The expression of the Myc tagged protein was confirmed by western blot analysis of whole cell lysates (WCL) using anti‐Myc.
Expression of Kir2.1 in Xenopus oocytes
The WT and mutant plasmids were linearised with BamHI and NotI, respectively (New England Biolabs, Beverly, MA), and run off cRNA transcripts were synthesised with commercial kits (T7 mMessage mMachine kit; Ambion, Austin, TX, USA), following the manufacturer's instructions. Defolliculated stage V and VI Xenopus oocytes were injected with 2–4 ng of cRNA using a nanoinjector (Drummond, Broomall, PA, USA) and incubated at 18°C for 2–3 days to allow channel expression. Whole oocyte currents were measured using a two electrode voltage oocyte clamp (Warner Instruments Inc., Hamden, CT, USA) while the oocytes were bathed in a modified ND96 solution (in mmol/L: NaCl 94, KCl 4, CaCl2 1.8, MgCl2 1, HEPES 5, adjusted to pH 7.4 with NaOH). Microelectrodes were pulled from borosilicate glass capillaries (TW100F‐4; World Precision Instruments, Inc., Sarasota, FL, USA) using a flaming/brown micropipette puller (Model P‐17; Sutter Instrument Co., Novato, CA) to feature tip resistance of <1 MΩ and filled with 3 mol/ LKCl. Voltage clamp data were acquired on a desktop PC using Clampex6 (Axon Instruments, Foster City, CA, USA), low pass filtered at 1 kHz (Frequency Devices, Haverhill, MA, USA), and digitised at 500 μs/point. The average voltage offset was <2 mV, and the average leak current during recording was <0.1 μA and subtracted offline by assuming ohmic leak.
Transgenic mouse models
The WT and mutant cDNAs were further subcloned from the pBluescript KS (+) vectors into an α‐myosin heavy chain promoter expression vector. The KCNJ2‐αMHC vectors were digested with BamH I, and after purification injected into fertilised oocyte (two cell blastocyst stage) derived from C57 and FVB mice, then the oocytes were transferred into the oviducts of pseudopregnant FVB mice. The genotype of offspring was determined by PCR and Southern blot analysis of genomic DNA.
Western analysis of transgene expression
The proteins from transgenic mouse hearts were insolated from modified RIPA buffer. The protein pellet was dissolved in 1% SDS. A 50 μg sample of protein diluted with Laemmli sample buffer (Bio‐Rad, Hercules, CA, USA) was heated to 100°C for 5 minutes, separated, and transferred to nitrocellulose membranes as described above. Kir2.1 was detected using a 1:100 dilution of a rabbit polyclonal anti‐Kir2.1 antibody (Santa Cruz Biotechnology), and staining as described above.
Histological assessment of mouse hearts
Hearts from transgenic and non‐transgenic mice were collected in 10% formalin buffered with phosphate buffered saline, dehydrated, and embedded in paraffin wax. Embedded hearts were sectioned at 2–5 μm, and stained with haematoxylin and eosin for routine histological examination or stained with Masson trichrome for collagen.
Echocardiography
Mice were anesthetised and subjected to echocardiography using a 15 MHz linear transducer (Acuson Sequoia Cardiac System, Siemens Medical Solutions, Malvern, PA, USA). Fractional shortening was calculated as previously described.14
Electrophysiology studies
Mice were anesthetised by intraperitoneal injection (15 mL/kg) of a mixture of sodium pentobarbital (6.61 mg/mL), sodium chloride (4.13 mg/mL), and ethanol (0.18 mL/mL). Electrophysiology studies were conducted on 9 normal (mean (SD) age 104 (41) days; weight 26 (5) g), 7 WT (age 187 (117) days; weight 31 (6) g), and 19 mutant mice (age 142 (68) days; weight 27 (4) g). Six limb leads were continuously recorded during electrophysiology study. Meanwhile, an eight electrode lumen catheter (1.1F gauge, model EPR‐801, Millar Instruments, Houston, TX, USA; or 2F gauge, model CIBer, NuMED, Denton, TX, USA) was inserted through the jugular vein and advanced into the right atrium and ventricle for pacing and recording. The ECG was filtered between 5 and 100 Hz, and intracardiac electrograms were filtered between 5 and 500 Hz, while the sampling rate was set at 1 kHz on all signals (CardioLab; GE Medical Systems, Waukesha, WI, USA). Electrophysiology methods similar to standard clinical electrophysiology studies were performed as previously described.15 Electrical stimulation was applied through the catheter electrodes using an external stimulator (model S8800; Astro‐Med, Inc., West Warwick, RI, USA) that delivered 2 ms wide current pulses. After a period of baseline rhythm, decremental atrial pacing was applied whereby the cycle length was repeatedly shortened by 10 ms, starting from a pacing cycle length 20 ms less than the intrinsic cycle length until 1:1 stimulus atrium conduction could not be maintained. After 10 seconds of pacing at each cycle length, sinus node recovery time was determined as the longest pause from the last paced atrial depolarisation to the first sinus return cycle. Atrial pacing cycle lengths resulting in Wenkebach and 2:1 AV conduction were also noted. Subsequently, refractory periods were determined by delivering an eight stimulus drive train (S1) at a cycle length of 100 ms, followed by a premature stimulus (S2) that was progressively decremented in 10 ms intervals. The effective refractory period was defined as the longest S1–S2 coupling interval that failed to generate a propagated beat with S2 in two separate attempts.
Arrhythmia inducibility was investigated in 6 normal, 7 WT, and 14 mutant mice. This was accomplished by delivering two extra stimuli (S2 and S3) that were programmed at progressively shorter intervals following a drive train (S1), with minimum coupling intervals of 40 ms. Pharmacological stimulation by epinephrine (adrenaline) (0.01 µg/g intravenously) was applied to further assess inducibility of arrhythmias.
Statistical analysis
One way analysis of variance with the Bonferroni multiple comparison procedure was conducted using the statistical software InStat (GraphPad Software, Inc., San Diego, CA, USA) to compare the mean currents between different groups. Statistical significance was set at p<0.05.
RESULTS
KCNJ2 mutation screening
We identified a heterozygous C→G224 substitution in the three affected members of this family. The mutation changed the threonine at codon 75 to arginine (T75R), which is located in the cytoplasmic N‐terminal portion of Kir2.1, close to the beginning of the first intramembrane domain (M1). It is a highly conserved amino acid residue among the other inward rectifier potassium channels. This mutation was not found in unaffected family members of the proband and in 100 unrelated, unaffected control individuals.
Functional consequences of T75R Kir2.1 mutation in vitro
To investigate the effect of the mutation on cellular localisation and homotetramer formation, we transfected mammalian cells with constructs expressing WT or mutant forms of Kir2.1. T75R Kir2.1 had a cellular distribution similar to that of wild type channels, with localisation in both the cytoplasm and cell membrane, with increased staining at the cell membrane at points of cell to cell contact (fig 1A), suggesting the T75R mutation does not affect protein trafficking. Further, by co‐immunoprecipitation studies, the mutation did not affect binding to WT or T75R subunits (fig 1B), suggesting that the mutation does not affect homotetramerisation.
To perform voltage clamp analyses of channel function we injected Xenopus oocytes with WT or mutant cRNA and found that expression of the mutant resulted in no current, identical to those oocytes injected with H2O (the mean (SD) currents at −150 mV were 0.018 (0.004) µA with T75R 4 ng/oocyte and 0.063 (0.004) µA with H2O; p = 1.0) and significantly lower than those injected with WT cRNA (p<0.001 at −150 mV; data not shown), similar to that reported previously for other Kir2.1 mutants.3 As ATS is an autosomal dominant disease, we co‐expressed T75R cRNA (2 ng/oocyte) and WT cRNA (2 ng/oocyte) at the same time. In contrast to those oocytes injected with half of the amount of WT cRNA alone, the K+ current was completely lost (mean current at −150 mV becoming 0.025 (0.005) µA; p<0.001), and was similar to those injected with H2O or 4 ng of T75R cRNA (p = 1.0). These findings suggested that the T75R mutant of Kir2.1 could cause complete loss of channel function when expressed alone, as well as possessing strong dominant negative effects when co‐expressed with identical amounts of WT Kir2.1.
Functional consequences of T75R Kir2.1 mutation in vivo
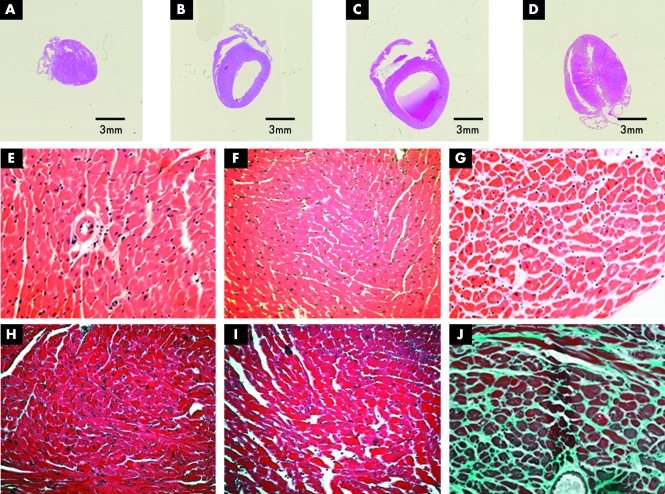
We developed lines of transgenic mice overexpressing either WT or T75R Kir2.1 (referred to as WT‐Tg and T75R‐Tg, respectively) in the heart (fig 2A–J). Lines expressing 2–3 times normal levels were analysed. WT‐Tg and T75R‐Tg lines showed cardiomegaly and most had dilated left ventricles at 7 months of age (fig 2B, C, respectively), although some T75R‐Tg mice had ventricular hypertrophy (fig 2D). However, histological assessment of cardiac samples showed that the T75R‐Tg transgenics also displayed oedema and increased fibrosis (fig 2G, J).
Figure 2 Morphology and histology of normal (NTg) and transgenic (Tg) mouse hearts. (A) Non‐Tg littermate control; (B) WT‐Tg , (C) and (D) T75R‐Tg. Staining with (E–G) haematoxylin and eosin, and (H–J) Masson's stain of sections from: (E, G) non‐TG, (F, I) WT‐Tg, and (G, J) T75R‐Tg.
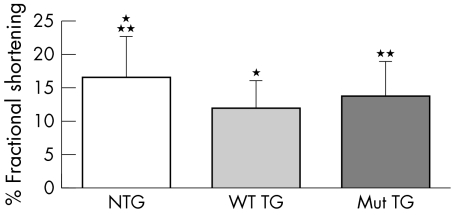
Echocardiography revealed significantly decreased function in both the WT‐Tg and T75R‐Tg mice with fractional shortening significantly reduced in both groups by comparison with the non‐TG littermate controls (fig 3). There was no statistical difference between the transgenic groups, although some of the WT transgenics displayed paradoxical movements indicative of right ventricular enlargement.
Figure 3 Mean fractional shortening of non‐Tg (n = 5), WT‐Tg (n = 4), and T75R‐Tg (n = 5). *p<0.01; non‐Tg vs WT‐Tg , **p<0.05; non‐Tg versus T75R‐Tg.
Electrophysiological evaluations of the transgenic mice are summarised in table 1. There was a tendency for slow heart rates in mutant mice, although the results did not reach statistical significance. The durations of P, PR, and QRS components of the ECG were similar among the three groups. Meanwhile, as illustrated in fig 4A, the QTc interval was significantly prolonged in T75R‐Tg mice compared with WT‐Tg mice (QT was defined from onset of QRS to return of T wave to baseline). No significant differences were observed between the three groups in the sinus node recovery time. The longest atrial pacing cycle length resulting in atrioventricular Wenkebach conduction and that resulting in 2:1 atrioventricular conduction tended to be longer in T75R‐Tg mice than in Wt‐Tg mice. Atrial and atrioventricular nodal effective refractory periods were similar among the three groups, whereas ventricular effective refractory period was significantly longer in T75R‐Tg mice than in WT‐Tg mice.
Table 1 Summary of ECG and EPS parameters.
| Normal (n = 9) | WT‐Tg (n = 7) | T75R‐Tg (n = 19) | ||||
|---|---|---|---|---|---|---|
| BCL | 148 (28) | 143 (34) | 153 (24) | |||
| P | 14 (2) | 13 (2) | 14 (3) | |||
| PR | 38 (9) | 38 (8) | 37 (50 | |||
| QRS | 11 (2) | 13 (1) | 12 (2) | |||
| QTc | 105 (12) | 91 (17)* | 117 (16)* | |||
| SNRTc | 64 (25) | 42 (21) | 49 (22) | |||
| AVW | 78 (9) | 70 (6) | 76 (10) | |||
| AV2:1 | 65 (5)* | 52 (5)*† | 59 (8)† | |||
| AERP | 36 (9) | 38 (10) | 39 (9) | |||
| AVERP | 54 (14) | 53 (6) | 55 (11) | |||
| VERP | 39 (15)* | 18 (4)*† | 37 (9)† |
*,†p<0.05 for multiple group comparison by one way analysis of variance per parameter. All parameters are expressed in ms and are presented as mean (SD). BCL, baseline cycle length; P, PR, and QRS, duration of ECG components; QTc, corrected QT interval (QTc = QT/RR1/3); SNRTc, longest sinus node recovery time corrected by baseline cycle length; AVW, longest atrial pacing cycle length resulting in Wenckebach AV conduction; AV2:1, longest atrial pacing cycle length resulting in 2:1 AV conduction; AERP, atrial effective refractory period; AVERP, AV node effective refractory period; VERP, ventricular effective refractory period.
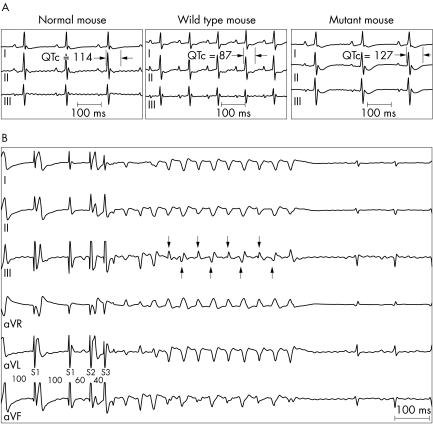
Figure 4 Electrophysiology of transgenic mouse models. (A) Representative intrinsic ECG (leads I, II, and III) acquired from normal (NTg), WT‐Tg, and T75R‐Tg mice. (B) Example of VT induced by electrical stimulation in a T75R‐Tg mouse. The VT was bidirectional as characterised by a beat to beat alteration of the QRS axis in ECG lead III (arrows). Note that S1 did not result in a propagated beat.
VT lasting four or more beats at a cycle length of 53 (14) ms was recorded in 5 of 14 T75R‐Tg mice; 1 mouse demonstrated spontaneous VT at baseline, 2 mice presented spontaneous VT following epinephrine administration, and 2 mice had VT induced by electrical stimulation (fig 4B). In three of the five T75R‐Tg mice with VT, the tachycardia was polymorphic of a particular type known as bidirectional that was characterised by a beat to beat alteration of the QRS axis in at least one ECG lead.16,17 The VT was monomorphic in two of five mutant mice. Meanwhile, only one of seven WT‐Tg mice presented monomorphic VT (cycle length, 61 ms) following provocation by epinephrine, and none of the six non‐Tg mice had VT. No PVCs were recorded in any mouse at baseline. However, epinephrine administration provoked PVCs in one normal mouse, four WT‐Tg mice, and five T75R‐Tg mice.
Spontaneous atrial tachycardia was recorded at baseline in two WT‐Tg mice, while none of the normal or T75R‐Tg mice had any atrial arrhythmias at baseline. Epinephrine administration induced atrial fibrillation/flutter in three T75R‐Tg mice, while no atrial arrhythmias were provoked in either non‐Tg or WT‐Tg mice. No atrial arrhythmias were induced by programmed electrical stimulation in any mouse.
DISCUSSION
Of the KCNJ2 mutations that have been reported to cause the phenotype of ATS and have been investigated, all have been found to result in complete loss of function when expressed alone. The reported mutations are distributed throughout different portions of Kir2.1, including the N‐terminal intracellular portion, M1 transmembrane portion, pore region, and C‐terminal intramembrane portion. However, if these mutants are co‐expressed with identical amounts of WT KCNJ2 in order to mimic the heterozygous mutations seen in the ATS patients, variable degrees of dominant negative effect are observed.5,8,9,10,11,12 The mechanisms that account for this variability have not been clarified. Previous studies revealed that the heterozygous mutations related to ATS may suppress not only the function of the Kir2.1 homotetramer, but also the function of co‐expressed Kir2.2 and Kir2.3 heteromers.18 Several of these mutations are located in a region implicated in binding membrane associated phosphatidylinositol 4,5‐bisphosphate (PIP2).12,19 These findings may also contribute to the extent of varied dominant negative effects between different mutants, in addition to the location of mutations. Although the T75R mutation was previously identified in a single proband as a de novo mutation,12 physiological characterisation of its effect on Kir2.1 function has not been reported.
In this study we show that the T75R mutant was non‐functional when it was expressed in Xenopus oocytes, and showed a very strong dominant negative effect when co‐expressed with identical amount of WT Kir2.1. Stockklausner et al reported that the N‐terminal domain of Kir2.1 is necessary for protein trafficking of this channel from the Golgi complex to the plasma membrane,20 thus it was possible that the T75R mutation affected the sequence required for the export of Kir2.1 from the Golgi complex. However, our data from expression of this mutant in mammalian cells showed that the T75R mutant still preserves the ability to localise to the membrane.
These data suggest that the mutant protein exerts a dominant negative effect by directly interfering with channel function. There are several possible mechanisms to account for this channel silencing, as follows. (a) Changes in the ion permeable pore region alter protein conformation, as has been reported for Kir2.3 mutations in weaver mice.21 (b) The C‐terminal domain is important for Kir2.1 assembly;22 however, our co‐immunoprecipitation data suggest that the T75R mutant does not affect its ability to bind itself or WT Kir2.1, suggesting that the mutant and WT proteins are able to form heterotetramers. (c) Mutations occurring in the N‐terminal region may disrupt the correct insertion of the M1 segment into the membrane;23 however, our data suggest that the mutation does not affect protein localisation. (d) The binding of Kir channels to PIP2 is critical for channel function, and mutations affecting this interaction have been reported in patients with ATS or Bartter syndrome.19 The T75R mutation is not in the PIP2 binding site, thus it appears that the T75R mutation affects Kir2.1 channel gating.
Electrocardiographic and electrophysiological studies of transgenic mice expressing either the WT or T75R Kir2.1 confirmed the cardiac phenotype of ATS by the following criteria. (a) Baseline ECG recordings exhibited a long QTc interval in T75R‐Tg mice compared with WT‐Tg mice, which was similar to previous findings in ATS patients.3 Similar ECG phenotypes were observed following IK1 suppression in the mouse heart.7,24 (b) Atrial electrical stimulation revealed evidence of AV nodal conduction abnormality in T75R‐Tg mice compared with WT‐Tg mice, as suggested by the prolongation in pacing cycle length, resulting in Wenkeback and 2:1 atrioventricular conduction. First degree heart block was reportedly described in ATS patients.9 (c) The ventricular effective refractory period was longer in mutant mice than in WT mice. Previous studies utilising computer, isolated cell, and heart models demonstrated that block of IK1 resulted in prolongation of action potential duration.25,26,27,28 On the other hand, similar to our WT‐Tg mice, upregulation of IK1 was recently shown to result in shortening of action potential duration and QTc interval.24 (d) T75R‐Tg mice developed VT more often than WT‐Tg or non‐Tg mice. Ventricular arrhythmias are common in ATS patients.29 (e) Bidirectional VT was a common tachycardia induced in T75R‐Tg mice. It was also documented in the proband of our family and in other reported families with ATS.30
Bidirectional VT is most commonly seen in cases of digitalis intoxication, but it has also been associated with hypokalaemic periodic paralysis, aconite poisoning, and catecholaminergic ventricular tachycardia with mutation of the cardiac ryanodine receptor.31 The pathogenesis of bidirectional VT is incompletely understood. Triggered activity due to delayed afterdepolarisation was considered the major mechanism underlying the digitalis induced bidirectional VT, but some clinical observations were consistent with an automatic, ectopic mechanism in cases with channelopathy.32 To our knowledge, our T75R overexpressing transgenic mice are the first gene modified mouse model of bidirectional VT. The mechanism of bidirectional VT in ATS may require further investigation.
The observation that overexpression of either WT or T75R Kir2.1 resulted in significant ventricular dilatation, with an associated decrease in cardiac function, suggests that the induction of dilation was related to properties of the channel protein unaffected by the mutation, such as increased hydrolysis of PIP2, leading to hypertrophic signalling pathways,33 or may simply reflect a non‐specific poison‐peptide phenomenon.
The severity of clinical symptoms seems unrelated to the extent of dominant negative effects among different mutations of KCNJ2.9 In addition, carriers of non‐penetrant mutations have also been reported.3 In the three individuals that carry the heterozygous T75R mutation in this family, ventricular arrhythmias and dysmorphic features can be observed in all; however, only two of these subjects presented with periodic paralysis. None of the family members had a prolonged QTc interval, but prominent U waves could be observed in all the three affected members. These ECG findings were similar to the previously reported R67W mutation in Kir2.1,10 but no sex specific cardiac and skeletal muscle phenotype could be observed in our patients. However, in the individual with the T75R mutation previously reported,12 there was long QTc prolongation, as well as clinodactyly, micrognathia, low set ears, hypertelorism, and short stature. These observations confirm the variability of clinical expression of mutations in Kir2.1.
A study using in vivo viral gene transfer of a dominant negative Kir2.1 to the left ventricle of guinea pigs showed significantly reduced IK1 activities in ventricular myocytes.26 That further led to prolonged action potentials, decelerated phase III repolarisation, and depolarisation of the resting membrane potential. These findings corresponded to the previously described mechanism of U wave formation‐prolongation of the repolarisation phase of the action potential by after‐potential.34 They also account for the polymorphic ventricular arrhythmias observed in ATS patients, which may be triggered by the early after‐depolarisation resulting from action potential prolongation. Further, transgenic mice expressing a dominant negative Kir2.128 had significant changes in key parameters of excitability, including prolongation of QRS complexes and QT intervals, and IK1 was significantly reduced in isolated ventricular myocytes.
In summary, we identified a mutation of Kir2.1 (T75R) with a variable clinical expression of the ATS phenotype, which was non‐functional when expressed in Xenopus oocytes, and showed a very strong dominant negative effect when co‐expressed with identical amount of WT Kir2.1.
ACKNOWLEDGEMENTS
Chun‐Wei Lu was sponsored by the foundation of National Science Council in Taiwan, R.O.C. This work was funded by NIH grants HL62570 and HL 67155 (J A Towbin), and the John Patrick Albright Foundation (J A Towbin). J A Towbin is also supported by the Texas Children's Hospital Foundation Chair in Pediatric Cardiovascular Research, the Abercrombie Pediatric Cardiology Fund of Texas Children's Hospital, and contributions from the Powers family.
Abbreviations
ATS - Andersen‐Tawil syndrome
IK1 - inward rectifier current
PIP2 - phosphatidylinositol 4,5‐bisphosphate
PVC - premature ventricular contraction
QTc - corrected QT interval
RBBB - right bundle branch block
Tg - transgenic
VT - ventricular tachycardia
WT - wild type
Footnotes
Competing interests: there are no competing interests.
References
- 1.Jongsma H J, Wilders R. Channelopathies: Kir2.1 mutations jeopardize many cell functions. Curr Biol 200111R747–R750. [DOI] [PubMed] [Google Scholar]
- 2.Lopatin A N, Nichols C G. Inward rectifiers in the heart: an update on I(K1). J Mol Cell Cardiol 200133625–638. [DOI] [PubMed] [Google Scholar]
- 3.Plaster N M, Tawil R, Tristani‐Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson M R, Iannaccone S T, Brunt E, Barohn R, Clark J, Deymeer F, George A L, Jr, Fish F A, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony S H, Wolfe G, Fu Y H, Ptacek L J. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 2001105511–519. [DOI] [PubMed] [Google Scholar]
- 4.Andersen E D, Krasilnikoff P A, Overvad H. Intermittent muscular weakness, extrasystoles, and multiple developmental anomalies. A new syndrome? Acta Paediatr Scand 197160559–564. [DOI] [PubMed] [Google Scholar]
- 5.Tawil R, Ptacek L J, Pavlakis S G, DeVivo D C, Penn A S, Ozdemir C, Griggs R C. Andersen's syndrome: potassium‐sensitive periodic paralysis, ventricular ectopy, and dysmorphic features. Ann Neurol 199435326–330. [DOI] [PubMed] [Google Scholar]
- 6.Yoon G, Oberoi S, Tristani‐Firouzi M, Etheridge S P, Quitania L, Kramer J H, Miller B L, Fu Y H, Ptácek L J. Andersen‐Tawil syndrome: Prospective cohort analysis and expansion of the phenotype. Am J Med Genet A 2006140A312–321. [DOI] [PubMed] [Google Scholar]
- 7.Zaritsky J J, Redell J B, Tempel B L, Schwarz T L. The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol 2001533697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ai T, Fujiwara Y, Tsuji K, Otani H, Nakano S, Kubo Y, Horie M. Novel KCNJ2 mutation in familial periodic paralysis with ventricular dysrhythmia. Circulation 20021052592–2594. [DOI] [PubMed] [Google Scholar]
- 9.Tristani‐Firouzi M, Jensen J L, Donaldson M R, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu Y H, Ptacek L J, Tawil R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J Clin Invest 2002110381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. KCNJ2 mutation results in Andersen syndrome with sex‐specific cardiac and skeletal muscle phenotypes. Am J Hum Genet 200271663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosaka Y, Hanawa H, Washizuka T, Chinushi M, Yamashita F, Yoshida T, Komura S, Watanabe H, Aizawa Y. Function, subcellular localization and assembly of a novel mutation of KCNJ2 in Andersen's syndrome. J Mol Cell Cardiol 200335409–415. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson M R, Jensen J L, Tristani‐Firouzi M, Tawil R, Bendahhou S, Suarez W A, Cobo A M, Poza J J, Behr E, Wagstaff J, Szepetowski P, Pereira S, Mozaffar T, Escolar D M, Fu Y H, Ptacek L J. PIP2 binding residues of Kir2.1 are common targets of mutations causing Andersen syndrome. Neurology 2003601811–1816. [DOI] [PubMed] [Google Scholar]
- 13.Claycomb W C, Lanson N A, Jr, Stallworth B S, Egeland D B, Delcarpio J B, Bahinski A, Izzo N J., Jr HL‐1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 1998952979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemoto S, DeFreitas G, Mann D L, Carabello B A. Effects of changes in left ventricular contractility on indexes of contractility in mice. Am J Physiol Heart Circ Physiol 2002283H2504–H2510. [DOI] [PubMed] [Google Scholar]
- 15.Appleton G O, Li Y, Taffet G E, Hartley C J, Michael L H, Entman M L, Roberts R, Khoury D S. Determinants of cardiac electrophysiological properties in mice. J Interv Card Electrophysiol 2004115–14. [DOI] [PubMed] [Google Scholar]
- 16.Priori S G, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho F E, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 200210669–74. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum M B, Elizari M V, Lazzari J O. The mechanism of bidirectional tachycardia. Am Heart J 1969784–12. [DOI] [PubMed] [Google Scholar]
- 18.Preisig‐Muller R, Schlichthorl G, Goerge T, Heinen S, Bruggemann A, Rajan S, Derst C, Veh R W, Daut J. Heteromerization of Kir2.x potassium channels contributes to the phenotype of Andersen's syndrome. Proc Natl Acad Sci USA 2002997774–7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes C M, Zhang H, Rohacs T, Jin T, Yang J, Logothetis D E. Alterations in conserved Kir channel‐PIP2 interactions underlie channelopathies. Neuron 200234933–944. [DOI] [PubMed] [Google Scholar]
- 20.Stockklausner C, Klocker N. Surface expression of inward rectifier potassium channels is controlled by selective Golgi export. J Biol Chem 200327817000–17005. [DOI] [PubMed] [Google Scholar]
- 21.Patil N, Cox D R, Bhat D, Faham M, Myers R M, Peterson A S. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet 199511126–129. [DOI] [PubMed] [Google Scholar]
- 22.Tinker A, Jan Y N, Jan L Y. Regions responsible for the assembly of inwardly rectifying potassium channels. Cell 199687857–868. [DOI] [PubMed] [Google Scholar]
- 23.Umigai N, Sato Y, Mizutani A, Utsumi T, Sakaguchi M, Uozumi N. Topogenesis of two transmembrane type K+ channels, Kir 2.1 and KcsA. J Biol Chem 200327840373–40384. [DOI] [PubMed] [Google Scholar]
- 24.Li J, McLerie M, Lopatin A N. Transgenic up‐regulation of IK1 in the mouse heart leads to multiple abnormalities of cardiac excitability. Am J Physiol Heart Circ Physiol 2004 [DOI] [PubMed]
- 25.Imoto Y, Ehara T, Matsuura H. Voltage‐ and time‐dependent block of iK1 underlying Ba2+‐induced ventricular automaticity. Am J Physiol 1987252H325–H333. [DOI] [PubMed] [Google Scholar]
- 26.Miake J, Marban E, Nuss H B. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant‐negative suppression. J Clin Invest 20031111529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren M, Guha P K, Berenfeld O, Zaitsev A, Anumonwo J M, Dhamoon A S, Bagwe S, Taffet S M, Jalife J. Blockade of the inward rectifying potassium current terminates ventricular fibrillation in the guinea pig heart. J Cardiovasc Electrophysiol 200314621–631. [DOI] [PubMed] [Google Scholar]
- 28.McLerie M, Lopatin A. Dominant‐negative suppression of I(K1) in the mouse heart leads to altered cardiac excitability. J Mol Cell Cardiol 200335367–378. [DOI] [PubMed] [Google Scholar]
- 29.Sansone V, Griggs R C, Meola G, Ptacek L J, Barohn R, Iannaccone S, Bryan W, Baker N, Janas S J, Scott W, Ririe D, Tawil R. Andersen's syndrome: a distinct periodic paralysis. Ann Neurol 199742305–312. [DOI] [PubMed] [Google Scholar]
- 30.Kannankeril P J, Roden D M, Fish F A. Suppression of bidirectional ventricular tachycardia and unmasking of prolonged QT interval with verapamil in Andersen's syndrome. J Cardiovasc Electrophysiol 200415119. [DOI] [PubMed] [Google Scholar]
- 31.Grimm W, Marchlinski F E. Accelerated idioventricular rhythm and bidirectional ventricular tachycardia. In: Zipes DP, Jalife J, eds. Cardiac electrophysiology.From cell to bedside. 4th ed. Philadelphia, Pennsylvania: Saunders, 2004700–704.
- 32.Laohakunakorn P, Benson D W, Yang P, Yang T, Roden D M, Kugler J D. Bidirectional ventricular tachycardia and channelopathy. Am J Cardiol 200392991–995. [DOI] [PubMed] [Google Scholar]
- 33.Lamers J M, De Jonge H W, Panagia V, Van Heugten H A. Receptor‐mediated signalling pathways acting through hydrolysis of membrane phospholipids in cardiomyocytes. Cardioscience 19934121–131. [PubMed] [Google Scholar]
- 34.di Bernardo D, Murray A. Origin on the electrocardiogram of U‐waves and abnormal U‐wave inversion. Cardiovasc Res 200253202–208. [DOI] [PubMed] [Google Scholar]