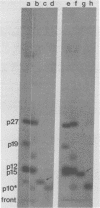
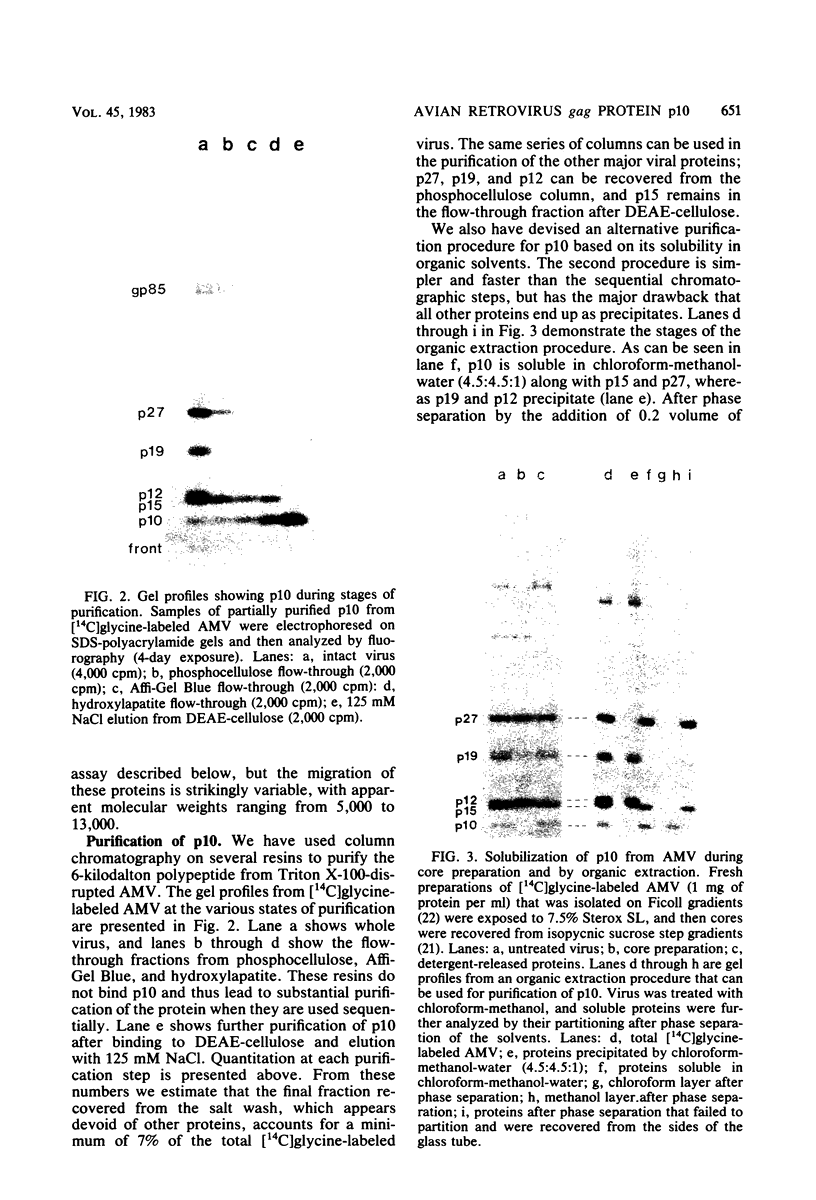
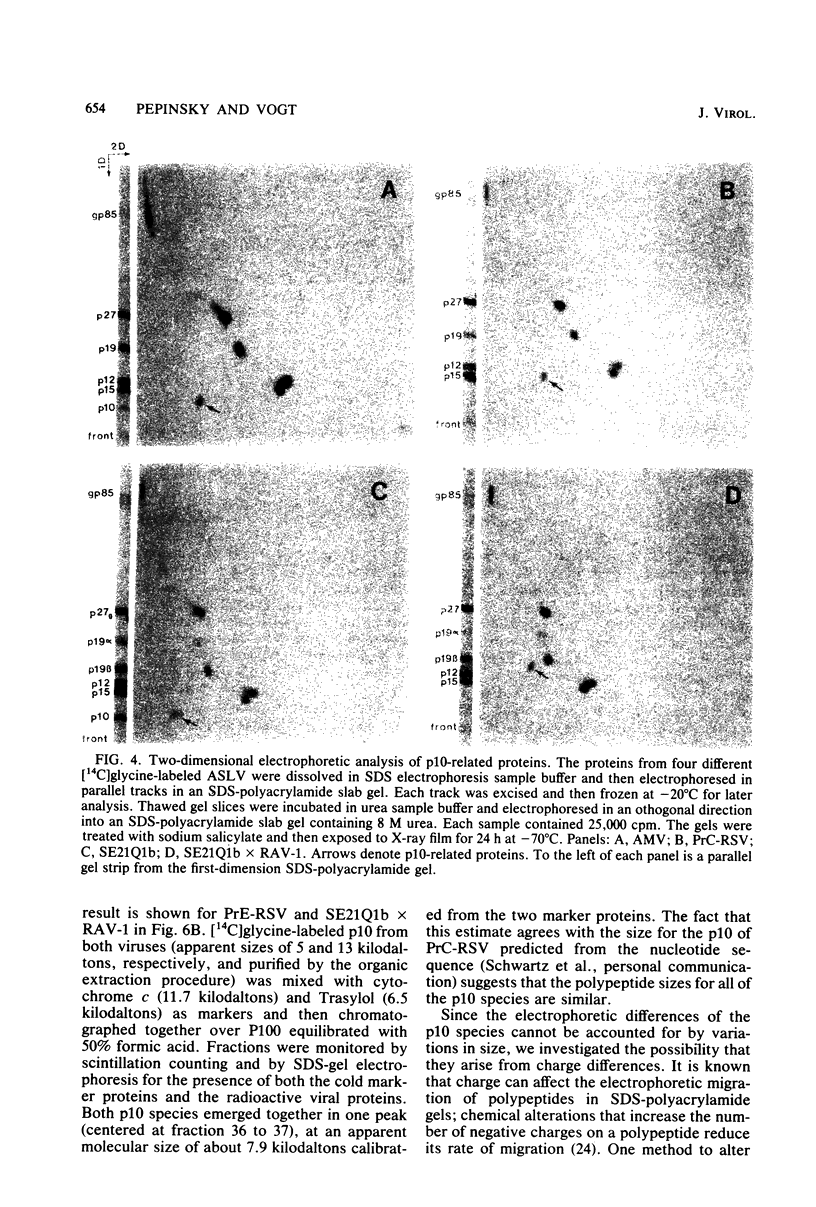
Abstract
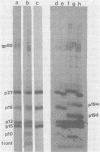
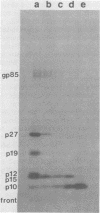
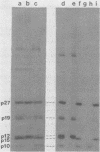
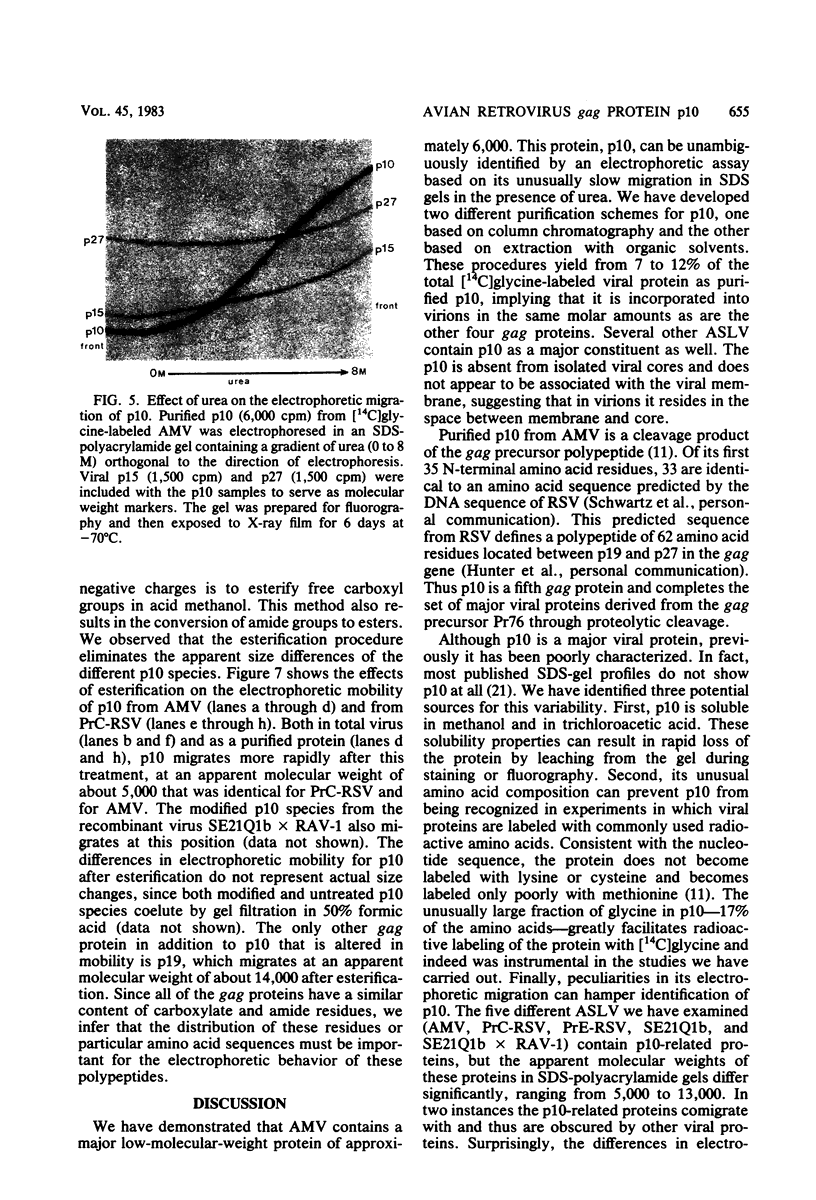
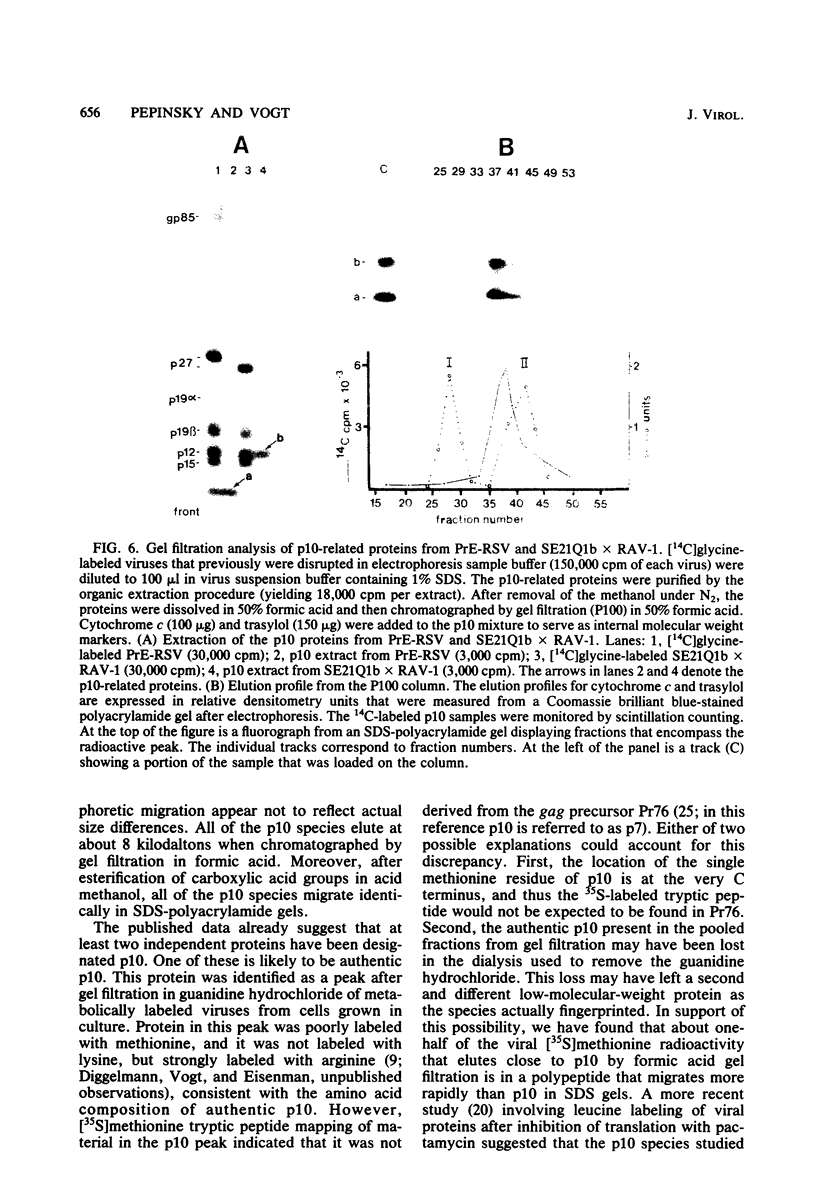
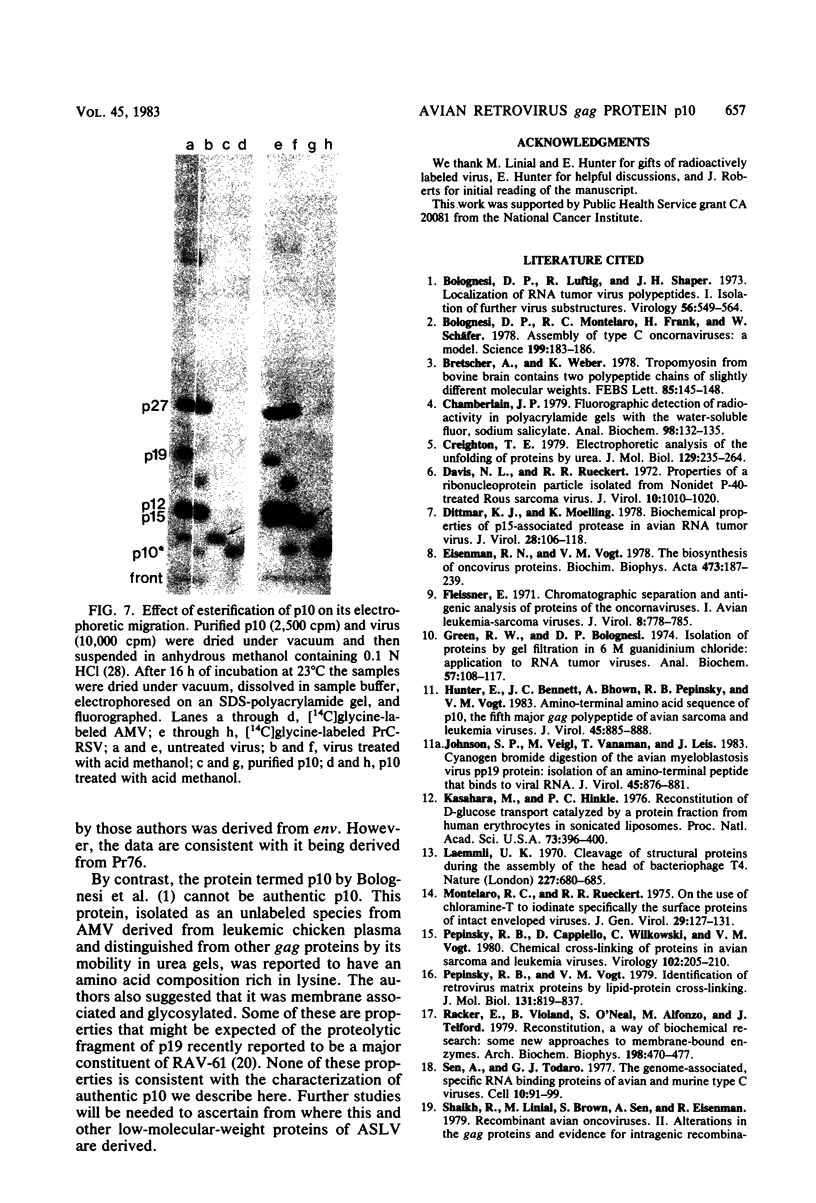
We have developed procedures for the purification of a 6,000-dalton protein from avian myeloblastosis virus. This protein is a major component of avian myeloblastosis virus, accounting for over 7% of total protein, and thus is equimolar with the other internal structural proteins in virions. As described in the accompanying paper (Hunter et al., J. Virol. 45:885-888, 1983), the results of N-terminal amino acid sequence analysis identify the protein as a product of the gag gene. We suggest denoting this protein as p10, according to nomenclature that is already in use for a previously identified but poorly defined low-molecular-weight protein or proteins of avian sarcoma and leukemia viruses. In virions p10 appears to be located between the core and the membrane. Several of its properties may explain why p10 has not been characterized previously. Among these are its abnormal amino acid composition, its solubility under conditions where most proteins are fixed into sodium dodecyl sulfate-polyacrylamide gels, and the variability in its electrophoretic migration in different avian sarcoma viruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi D. P., Luftig R., Shaper J. H. Localization of RNA tumor virus polypeptides. I. Isolation of further virus substructures. Virology. 1973 Dec;56(2):549–564. doi: 10.1016/0042-6822(73)90057-3. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Tropomyosin from bovine brain contains two polypeptide chains of slightly different molecular weights. FEBS Lett. 1978 Jan 1;85(1):145–148. doi: 10.1016/0014-5793(78)81267-8. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Electrophoretic analysis of the unfolding of proteins by urea. J Mol Biol. 1979 Apr 5;129(2):235–264. doi: 10.1016/0022-2836(79)90279-1. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P. Isolation of proteins by gel filtration in 6M guanidinium chloride: application to RNA tumor viruses. Anal Biochem. 1974 Jan;57(1):108–117. doi: 10.1016/0003-2697(74)90057-8. [DOI] [PubMed] [Google Scholar]
- Hunter E., Bennett J. C., Bhown A., Pepinsky R. B., Vogt V. M. Amino-terminal amino acid sequence of p10, the fifth major gag polypeptide of avian sarcoma and leukemia viruses. J Virol. 1983 Feb;45(2):885–888. doi: 10.1128/jvi.45.2.885-888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. P., Veigl M., Vanaman T., Leis J. Cyanogen bromide digestion of the avian myeloblastosis virus pp19 protein: isolation of an amino-terminal peptide that binds to viral RNA. J Virol. 1983 Feb;45(2):876–881. doi: 10.1128/jvi.45.2.876-881.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution of D-glucose transport catalyzed by a protein fraction from human erythrocytes in sonicated liposomes. Proc Natl Acad Sci U S A. 1976 Feb;73(2):396–400. doi: 10.1073/pnas.73.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Rueckert R. R. On the use of chloramine-T to iodinate specifically the surface proteins of intact enveloped viruses. J Gen Virol. 1975 Oct;29(1):127–131. doi: 10.1099/0022-1317-29-1-127. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Cappiello D., Wilkowski C., Vogt V. M. Chemical crosslinking of proteins in avian sarcoma and leukemia viruses. Virology. 1980 Apr 15;102(1):205–210. doi: 10.1016/0042-6822(80)90081-1. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Racker E., Violand B., O'Neal S., Alfonzo M., Telford J. Reconstitution, a way of biochemical research; some new approaches to membrane-bound enzymes. Arch Biochem Biophys. 1979 Dec;198(2):470–477. doi: 10.1016/0003-9861(79)90521-6. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977 Jan;10(1):91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Shealy D. J., Mosser A. G., Rueckert R. R. Novel p19-related protein in Rous-associated virus type 61: implications for avian gag gene order. J Virol. 1980 May;34(2):431–437. doi: 10.1128/jvi.34.2.431-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg K., Hurley N. E., Davis N. L., Rueckert R. R., Fleissner E. Structural studies of avian myeloblastosis virus: comparison of polypeptides in virion and core component by dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):513–528. doi: 10.1128/jvi.13.2.513-528.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg K., Litwack M. D., Desmukes B. Structural studies on avian myeloblastosis virus: rapid purification and quantitation. Proc Soc Exp Biol Med. 1972 Oct;141(1):215–221. doi: 10.3181/00379727-141-36745. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tung J. S., Knight C. A. Relative importance of some factors affecting the electrophoretic migration of proteins in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1972 Jul;48(1):153–163. doi: 10.1016/0003-2697(72)90179-0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]