Abstract
Background
X linked cone‐rod dystrophy (CORDX) is a recessive retinal disease characterised by progressive dysfunction of photoreceptors. It is genetically heterogeneous, showing linkage to three X chromosomal loci. CORDX1 is caused by mutations in the RPGR gene (Xp21.1), CORDX2 is located on Xq27.2‐28, and we recently localised CORDX3 to Xp11.4‐q13.1. We aimed to identify the causative gene behind the CORDX3 phenotype.
Methods
All 48 exons of the CACNA1F gene were screened for mutations by DNA sequencing. RNA from cultured lymphoblasts and peripheral blood activated T lymphocytes was analysed by RT‐PCR and sequencing.
Results
A novel CACNA1F mutation, IVS28‐1 GCGTC>TGG, in the splice acceptor site of intron 28 was identified. Messenger RNA studies indicated that the identified mutation leads to altered splicing of the CACNA1F transcript. Aberrant splice variants are predicted to result in premature termination and deletions of the encoded protein, Cav1.4 α1 subunit.
Conclusion
CACNA1F mutations cause the retinal disorder, incomplete congenital stationary night blindness (CSNB2), although mutations have also been detected in patients with divergent diagnoses. Our results indicate that yet another phenotype, CORDX3, is caused by a mutation in CACNA1F. Clinically, CORDX3 shares some features with CSNB2 but is distinguishable from CSNB2 in that it is progressive, can begin in adulthood, has no nystagmus or hyperopic refraction, has only low grade astigmatism, and in dark adaptation lacks cone threshold and has small or no elevation of rod threshold. Considering all features, CORDX3 is more similar to other X chromosomal cone‐rod dystrophies than to CSNB2.
Keywords: CACNA1F , CORDX3, CSNB2, mutation analysis, X linked cone‐rod dystrophy
X linked cone‐rod dystrophy (CORDX) is a progressive retinal disease primarily showing cone photoreceptor dysfunction. The disease is characterised by diminished visual acuity, photophobia, myopia, central scotomas in visual fields, impaired colour vision, and disturbed cone or cone‐rod responses in electroretinogram (ERG).1,2,3,4,5,6 Fundus findings vary from normal or subtle granularity of macula, to bull's eye maculopathy and central geographic atrophy of retinal pigment epithelium. In some patients tapetal‐like retinal sheen has also been observed.1,2,7 The disease begins usually in the first two decades of life and progresses gradually; however, there is intrafamilial variation with respect to age of onset and severity of symptoms.1,6 CORDX is genetically heterogeneous and three gene loci have been identified to date. CORDX1 (MIM 304020), located in Xp21.1, is caused by mutations in the ORF15 of the RPGR gene, which is also known to be the causative gene for RP3 type retinitis pigmentosa8,9,10 and atrophic macular degeneration.11CORDX2 (MIM 300085) is localised to the long arm of the X chromosome (Xq27.2–28), but the causative gene is still unknown.12 We recently mapped the third X linked cone‐rod dystrophy locus, CORDX3 (MIM 300476), to Xp11.4–q13.1.13
CORDX has some similarities with X linked congenital stationary night blindness (CSNBX). CSNBX is considered to be a non‐progressive retinal disorder characterised by a negative ERG, that is, b wave amplitude is smaller than the a wave in a mixed rod‐cone response.14 CSNBX is both clinically and genetically heterogeneous. The major clinical features are variable and may include life long impairment of night vision, myopia, nystagmus, strabismus, and reduced visual acuity; however, there is a wide intra‐ and interfamilial variation in the symptoms.15,16,17 According to the ERG findings, CSNBX can be subdivided into a complete type (type 1, CSNB1) and an incomplete type (type 2, CSNB2).18,19,20 The causative genes of CSNBX have recently been identified: CSNB1 (MIM 310500, Xp11.4) is caused by mutations in NYX,21,22 and CSNB2 (MIM 300071, Xp11.23) results from mutations in the calcium channel α1 subunit gene, CACNA1F.23,24
The CACNA1F gene (MIM 300110) consists of 48 exons spanning a region of 28 kb in Xp11.23.23,24 The CACNA1F protein product, Cav1.4/α1F, shows strong homology to α1 subunits of the voltage dependent L‐type calcium channels (VDCCs).23,24 VDCCs are hetero‐oligomeric complexes composed of pore forming α1 subunit accompanied by modulating subunits α2δ and β, and variably by γ.25 In neuroretina, three different VDCC α1 subunits have been detected: Cav1.2 (α1C, encoded by CACNA1C), Cav1.3 (α1D, CACNA1D), and Cav1.4 (α1F, CACNA1F).26,27
By immunofluorescent staining of rat retina sections, Cav1.4 has been localised to the outer plexiform layer, and in lesser amounts to the inner plexiform layer and the outer nuclear layer.26 In the outer plexiform layer, Cav1.4 localises to rod and possibly to cone active zones, implicating these channels in the release of glutamate from photoreceptor synaptic terminals. The biophysical properties of Cav1.4 channels make them especially suitable for mediating tonic neurotransmitter release in sensory cells.28,29 Besides its retinal expression,23,24CACNA1F is also expressed in bone marrow, thymus, adrenal gland, skeletal muscle,29 and T cells,30 suggesting a wider role of Cav1.4 in human physiology.
As CACNA1F maps within the Xp11.4‐q13.1 candidate gene region of CORDX3,13 we sequenced this gene in members of a large Finnish family with cone‐rod dystrophy, CORDX3.
Methods
Subjects
Blood samples were collected from family members of a large Finnish CORDX3 family.13 A complete pedigree and clinical studies of the family members have been published earlier.6 The research followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants in accordance with the requirements of the University of Kuopio Ethics Committee. DNA samples from 50 unrelated Canadian females and 100 Finnish male blood donors were used as normal controls.
DNA analysis
All 48 exons of the CACNA1F gene were PCR amplified from genomic DNA using published primer sequences.31 PCR products were run on agarose gels, gel purified, and then sequenced with either a forward or a reverse primer. Fnu4HI (New England Biolabs, Beverly, MA) restriction endonuclease site analysis was used to study the segregation of the identified sequence alteration in the CORDX3 family and normal controls.
RNA analysis
The effect of the mutation on CACNA1F mRNA was studied by RT‐PCR and cDNA sequencing. RNA was extracted from lymphoblastoid cell lines of two CORDX3 patients and one unrelated control using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Complementary DNA was synthesised using M‐MLV (H‐) RT enzyme and random 6‐mer primers (Promega, Madison WI) according to manufacturer's instructions. Exons surrounding the mutation were amplified from cDNA with forward primers located in exons 27 and 28, and reverse primers in exons 30, 31, and 34 (primer sequences and PCR conditions are available upon request). As these amplifications yielded multiple products, three different approaches were used to separate different sized PCR products for sequencing. First, amplified fragments were separated in 1–2% MetaPhor agarose (BioWhittaker Molecular Applications, Rockland, ME), excised, and column purified with the QIAquick Gel Extraction Kit (Qiagen). Second, amplified fragments were separated in a 6% polyacrylamide gel, after which silver stained32 bands were excised and dissolved in 10 mM Tris‐HCl pH 8.5 overnight on a shaker. Extracted bands were re‐amplified and column purified using the QIAquick PCR Purification Kit (Qiagen). Third, amplified PCR fragments were cloned using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA), and plasmids were extracted using the Plasmid Mini Kit (Qiagen). Purified fragments and plasmids were sequenced using the ABI PRISM BigDye Terminator v3.1 Cycle Sequencing Kit and the ABI310 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Peripheral activated T lymphocytes were isolated from 15 ml of blood from one affected male, one carrier female, and one unrelated control, and cultured using the method of Kotturi and co‐workers,30 briefly described here. The separated peripheral blood mononuclear cells (PBMCs) were washed in PBS and resuspended in RPMI, 10% fetal calf serum, 2 mM glutamine, 20 mM HEPES, and 1 mM sodium pyruvate, followed by 24 h stimulation with plate‐bound anti‐CD3 antibody. The PBMCs were washed and resuspended in RPMI supplemented with 5 ng/μl recombinant human interleukin‐2 for 14 days. Reverse transcription analysis was performed on total RNA extracted from activated peripheral blood T cells on days 11–14 using Trizol Reagent (Invitrogen, Burlington, ON). Isolated RNA was treated with the MessageClean Kit (GenHunter, Nashville, TN) to remove contaminating DNA, and then used as the template for first strand cDNA synthesis using the Omniscript RT Kit (Qiagen). A touchdown PCR protocol was used in RT‐PCR with sense primer (exon 28 TACCGTGTGGGATCTCAGTGT) and anti‐sense primer (exon 33 CGGATCCCTTCCCCTTACT). The resulting PCR products were separated on 1.5% agarose gels. Respective bands were excised, purified using the Gel Purification Kit (Qiagen), and sequenced with sense and anti‐sense primers, as described in the previous paragraph.
Results
Mutation analysis
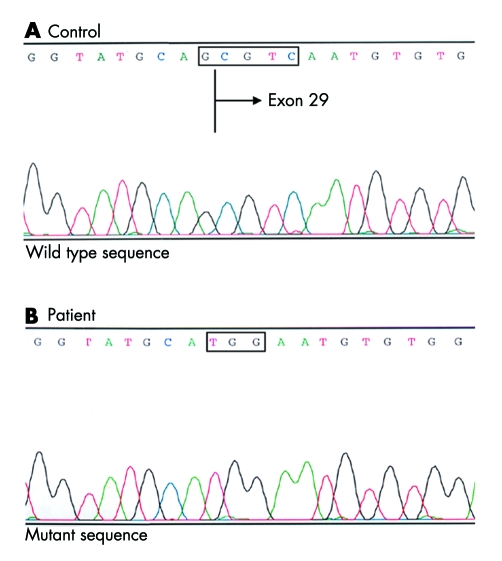
DNA sequence analysis of CACNA1F in a patient from our CORDX3 family revealed a novel mutation, IVS28‐1 GCGTC>TGG, in the splice acceptor site of intron 28 (fig 1). This CACNA1F mutation co‐segregated completely with the disease phenotype in the CORDX3 family (seven affected males, 10 carrier females, 33 non‐affected family members) but was not observed in 200 control chromosomes.
Figure 1 Electropherogram of the sense strand of an amplified genomic DNA fragment of CACNA1F from a CORDX3 patient. The change, IVS28‐1 GCGTC>TGG in CACNA1F, was present in the DNA of the patient (B) and absent in the normal control DNA (A).
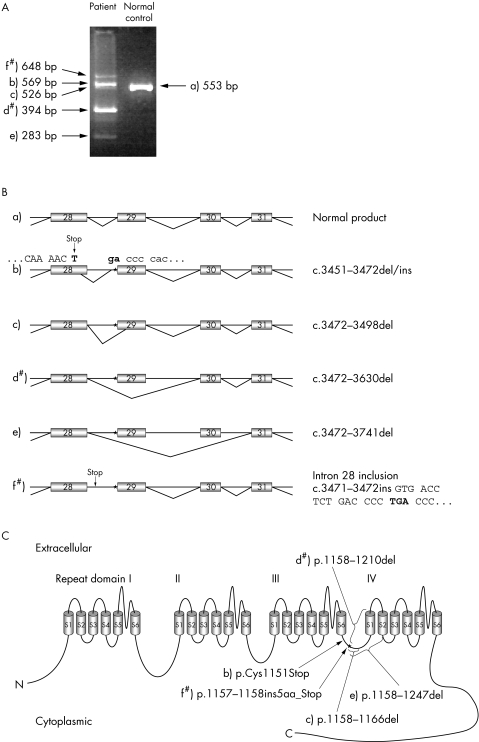
RNA studies in lymphoblastoid cells from CORDX3 patients revealed no normal transcripts but several aberrant CACNA1F splice variants. Using either an exon 27 or 28 forward primer and exon 30 or 31 reverse primer, five different splice variants were detected in patient samples (fig 2A,B), which were not observed in a control sample. With exon 28 forward primer and exon 34 reverse primer two additional splice variants were observed, namely the skipping of exon 32 and skipping of exons 31 and 32. Both were also identified in a control sample. Analysis of RNA from activated T cells of an affected male and a carrier female identified two of the aberrant splice variants (d and f, fig 2B), while the skipping of exon 32 was observed in RNA from both the patient and a control sample.
Figure 2 Analysis of transcripts involving the exon 28–31 region of CACNA1F. (A) RT‐PCR analysis of lymphoblast cells from a CORDX3 patient and unrelated control. (B) Normal splicing product (a), and five different outcomes of the CACNA1F splice site mutation, IVS28‐1 GCGTC>TGG, in CORDX3 patients: b) use of alternative splice donor site within exon 28 and alternate splice acceptor in intron 28, c) use of canonic intron 28 donor splice site and alternative acceptor splice site in exon 29, d) skipping of exon 29, e) skipping of exons 29 and 30, and f) no splicing, in which case intron 28 is retained in mRNA. The splice variants that were also seen in activated T cells are indicated by #. An asterisk denotes the location of the mutation. (C) The putative membrane topology of the human L‐type calcium channel Cav1.4 α1 subunit encoded by CACNA1F. The protein is composed of four repeat domains (I–IV), each containing six transmembrane segments (S1–S6), and connecting intra‐ and extracellular loops. The predicted consequences of the identified splice mutation, IVS28‐1 GCGTC>TGG, on the protein are shown. Mutation numbering is according to NCBI nucleotide and protein sequences, NM_005183 and NP_005174, respectively.
Predicted consequences of the identified CACNA1F splice site mutation on the Cav1.4 protein are illustrated in fig 2C. Two of the observed abnormal splice variants (b and f) contain different premature stop codons in the cytoplasmic linker region connecting the repeat domains III and IV, which result in truncation of the Cav1.4 protein. The other three aberrant transcript variants (c, d, and e) predict variably sized deletions in the domain III–IV linker region and the IVS1–S2 transmembrane region.
Discussion
In this study, we identified the causative gene for X linked cone‐rod dystrophy, CORDX3. The novel CACNA1F gene mutation, IVS28‐1 GCGTC>TGG, destroys the splice acceptor site of intron 28 leading to altered splicing of the CACNA1F transcript. We detected five aberrant splice variants in lymphoblast RNA from CORDX3 patients. These variants are predicted to cause variably sized deletions of the Cav1.4 protein or truncation of the C‐terminal part of the protein (fig 2) and are thought to underlie the pathophysiology in the patients from this Finnish family with CORDX3. A recent study of transient expression of CACNA1F mutants in human embryonic kidney cells demonstrated that truncation of the cytoplasmic C terminus of Cav1.4 leads to absence of the protein, indicating that the C‐terminal tail has an important role in protein processing and targeting.33 Moreover, some missense mutations have been shown to cause notable changes in channel gating, or to even completely prevent channel function.33,34 Based on these data, it is highly likely that the splice site mutation we observed in the Finnish CORDX3 patients results in total absence or significantly altered function of the Cav1.4 channel.
Boycott and co‐workers31 previously described alternative splicing for the exons 1, 2, and 9 of the CACNA1F gene. In this study we also identified mutation independent splice variants, in which exon 32 or exons 31 and 32 were excised. Exon 32 codes for a short sequence, NGGHLGE, constituting part of the loop between the domain IVS3 and S4 segments. Similar splice variants, where a short exon encoding part of this loop is spliced out, have also been described for other L‐type calcium channels, Cav1.1, Cav1.2, and Cav1.3.35,36,37,38 The splice variant missing both exons 31 and 32 leads to a deletion of the IVS3 transmembrane segment and part of the IVS3–S4 linker region of the protein, suggesting that the membrane topology of the C‐terminal part of the protein is altered. A similar splice variant has been identified for the Cav1.3 channel as well.36 Moreover, extensive splice variation has been detected within exons 31–34 that encode the IVS3–S4 region of the Cav1.2 protein.39 In the light of these observations, it seems that alternative splicing of L‐type calcium channels is a common phenomenon and results in increased channel variability, although the functional significance of such variation is not yet understood.
Over 60 unique CACNA1F mutations, including 10 in splice acceptor and donor sites, have been described to date. Most of the mutations have been identified in patients with a CSNB2 phenotype.23,24,31,40,41,42,43,44 However, some of the patients carrying a CACNA1F mutation have overlapping but sometimes divergent diagnoses: AIED‐like phenotype,42,45 CSNB2 with atypical retinal atrophy and visual field defects,46 retinal and optic disc atrophy with progressive decline of visual function,47 and severe CSNB2‐like phenotype associated with female carrier symptoms and intellectual disability.48 The present study indicates that yet another phenotype, CORDX3, is also caused by a mutation in the CACNA1F gene.
Clinically, CORDX3 and CSNB2 have some features in common, such as the range of visual acuities, myopic refraction, and the ERG abnormalities (table 1). In some features CORDX3 is different from CSNB2. The onset of CORDX3 varies from childhood (3 years) to adulthood (33 years), and the disease is progressive (visual acuity, refraction, colour vision and visual field), whereas CSNB2 is considered to be stationary and severe cases are usually observed early in life. Congenital nystagmus, hyperopic refraction, and astigmatism >1.5 D are not found in CORDX3 patients but are not infrequent in CSNB2. Ocular fundus changes, other than myopic changes, are not seen in CORDX3 patients, while both retinal and optic disk changes have been reported in several CSNB2 patients. In dark adaptation, CORDX3 patients have elevated or missing cone threshold and normal or only slightly elevated rod threshold. In CSNB2, the cone threshold is elevated but not missing, and the rod threshold is variably elevated, from 1 to 3 log units. CORDX3 has many clinical features similar to other X chromosomal cone‐rod dystrophies (table 1), but lacks their tendency to hyperopia and astigmatism >1.5 D and has different ocular fundus changes.
Table 1 Clinical characteristics of X chromosomal cone‐rod dystrophies and CSNB2.
| CORDX3 | CSNB2 | X chromosomal cone‐rod dystrophy (CORDX1, CORDX2) | |
|---|---|---|---|
| Mäntyjärvi et al6 | Pearce et al16 | Pinckers and Timmerman3 | |
| Tremblay et al18 | Jacobson et al2 | ||
| Bech‐Hansen et al23 | Kellner and Foerster50 | ||
| Boycott et al15 | Meire et al4 | ||
| Nakamura et al41 | Hong et al5 | ||
| Langrova et al49 | Bergen and Pinckers12 | ||
| Allen et al19 | Brown et al1 | ||
| Jacobi et al40 | |||
| 1. Onset | As child, adult | Congenital | As child, adult |
| 2. Progression | VA, refraction, VF, colour defect | Stationary | VA, refraction, VF, colour defect |
| 3. VA (range) | 20/300–20/40 | 20/400–20/25 | 20/800–20/15 |
| 4. Refraction (range) | −1 to −24 D | +8 to −18.25 D, mostly myopia | +3.25 to −20 D, mostly myopia |
| 5. Astigmatism >1.5 D (eyes) | 0/20 | 137/266, up to 5.5 D | 9/32, up to 3 D |
| 6. Congenital nystagmus (patients) | 0/10 | 67/139 | Rare |
| 7. Ocular fundus | Normal, | Normal, | Normal, |
| myopic changes | hypopigmentation, no foveal reflex, | macular atrophy, “bull's eye“, | |
| tilted disc, pale disc, | hypopigmentation, | ||
| myopic changes | myopic changes | ||
| 8. Colour vision | Normal, protan | Normal, tritan, non‐specific defect | Tritan, deutan, protan, achromatopsia |
| 9. VF | Normal, central scotoma, | Normal, | Normal, central scotoma, |
| central reduced sensitivity, | central scotoma | paracentral scotoma, | |
| concentric constriction | reduced central, paracentral sensitivity | ||
| 10. Dark adaptation | Cone threshold missing or elevated, | Cone threshold elevated, | Cone threshold missing or elevated, |
| rod threshold normal or elevated | rod threshold elevated | rod threshold normal or elevated | |
| <1 log unit | 0.5–3 log units | 1 log unit | |
| 11. ERG | Negative ERG, | Negative ERG, | Scotopic a and b wave decreased, |
| 30 Hz flicker decreased | 30 Hz flicker abnormal/decreased | photopic b wave decreased, | |
| with double‐peaked wave | 30 Hz flicker decreased, | ||
| negative ERG possible |
ERG, electroretinogram; D, diopter; VA, visual acuity; VF, visual field.
The phenotype described in a Maori family with a CACNA1F mutation shares several features with CSNB2, but is considered a distinct clinical entity due to the severity of the phenotype and the presence of intellectual impairment in several male patients.48 Moreover, all carrier females in this family had clinical and ERG abnormalities. Patients with a CACNA1F mutation and retinal and optic disk atrophy described by Nakamura et al47 also have some similarities with CSNB2. However, their phenotype included the distinctive features of retinal atrophy with attenuated vessels, optic disc atrophy, and progressive decline of visual function. A family described as having AIED‐like disease45 has upon re‐evaluation of the clinical features been rediagnosed as having CSNB2.42
Mutations in CACNA1F are evidently present in patients with different clinical diagnoses45,46,47,48 including (as we show here) cone‐rod dystrophy. Invariably, the phenotypic features in these conditions overlap those of CSNB2, the disorder in which CACNA1F mutations were originally identified.23,24 The observation that mutations in one gene can lead to different phenotypes is not uncommon among retinal diseases, as is seen, for example, in the case of the RPGR gene8,9,10,11 and peripherin/RDS gene (see Retina International's Mutation Databases: http://www.retina‐international.org/sci‐news/mutation.htm). The differences observed in the phenotypes resulting from CACNA1F mutations may reflect alterations in Cav1.4 channel function,34 together with the influence of modifying genes from the different genetic backgrounds of the patients, as previously suggested.15 The recent analysis of a Cacna1f knockout mouse has implicated a defect in the formation or maintenance of the synapses between photoreceptors and second order neurons of the retina as being central to the pathophysiology in this mouse model of CSNB2.51 Further genetic dissection of the molecular biology of synapse formation may assist us in understanding the clinical variability associated with mutations in CACNA1F.
In summary, our findings contribute to a better understanding of the phenotypic variability of retinal disorders and the underlying genetic defects. Such information is of potential help in both clinical and molecular genetic diagnostics, and the genetic counselling of patients and families affected by these eye diseases.
Acknowledgements
The authors thank the CORDX3 family members for co‐operation.
Electronic‐database information
Retina International's Mutation Databases can be found at http://www.retina‐international.org/ sci‐news/mutation.htm
Abbreviations
CORDX - X linked cone‐rod dystrophy
CSNBX - congenital stationary night blindness
ERG - electroretinogram
PBMC - peripheral blood mononuclear cell
VDCC - voltage dependent L‐type calcium channel
Footnotes
This research was funded by the Finnish State grant TYH1338, the Finnish Eye and Tissue Bank Foundation, the Foundation Fighting Blindness ‐ Canada, and Canadian Institutes of Health Research. NTBH is the Roy and Joan Allen Professor of Sight Research at the University of Calgary.
Competing interest: none declared
Retina International's Mutation Databases can be found at http://www.retina‐international.org/ sci‐news/mutation.htm
References
- 1.Brown J, Jr, Kimura A E, Gorin M B. Clinical and electroretinographic findings of female carriers and affected males in a progressive X‐linked cone‐rod dystrophy (COD‐1) pedigree. Ophthalmology 20001071104–1110. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson D M, Thompson H S, Bartley J A. X‐linked progressive cone dystrophy. Clinical characteristics of affected males and female carriers. Ophthalmology 198996885–895. [DOI] [PubMed] [Google Scholar]
- 3.Pinckers A, Timmerman G J M E N. Sex‐difference in progressive cone dystrophy I. Ophthalmic Paediatr Genet 1981117–23. [Google Scholar]
- 4.Meire F M, Bergen A A, De Rouck A, Leys M, Delleman J W. X linked progressive cone dystrophy. Localisation of the gene locus to Xp21‐p11.1 by linkage analysis. Br J Ophthalmol 199478103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong H K, Ferrell R E, Gorin M B. Clinical diversity and chromosomal localization of X‐linked cone dystrophy (COD1). Am J Hum Genet 1994551173–1181. [PMC free article] [PubMed] [Google Scholar]
- 6.Mäntyjärvi M, Nurmenniemi P, Partanen J, Myöhänen T, Peippo M, Alitalo T. Clinical features and a follow‐up study in a family with X‐linked progressive cone‐rod dystrophy. Acta Ophthalmol Scand 200179359–365. [DOI] [PubMed] [Google Scholar]
- 7.Heckenlively J R, Weleber R G. X‐linked recessive cone dystrophy with tapetal‐like sheen. A newly recognized entity with Mizuo‐Nakamura phenomenon. Arch Ophthalmol 19861041322–1328. [DOI] [PubMed] [Google Scholar]
- 8.Demirci F Y, Rigatti B W, Wen G, Radak A L, Mah T S, Baic C L, Traboulsi E I, Alitalo T, Ramser J, Gorin M B. X‐linked cone‐rod dystrophy (locus COD1): identification of mutations in RPGR exon ORF15. Am J Hum Genet 2002701049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meindl A, Dry K, Herrmann K, Manson F, Ciccodicola A, Edgar A, Carvalho M R, Achatz H, Hellebrand H, Lennon A, Migliaccio C, Porter K, Zrenner E, Bird A, Jay M, Lorenz B, Wittwer B, D'Urso M, Meitinger T, Wright A. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X‐linked retinitis pigmentosa (RP3). Nat Genet 19961335–42. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Peachey N S, Moshfeghi D M, Thirumalaichary S, Chorich L, Shugart Y Y, Fan K, Zhang K. Mutations in the RPGR gene cause X‐linked cone dystrophy. Hum Mol Genet 200211605–611. [DOI] [PubMed] [Google Scholar]
- 11.Ayyagari R, Demirci F Y, Liu J, Bingham E L, Stringham H, Kakuk L E, Boehnke M, Gorin M B, Richards J E, Sieving P A. X‐linked recessive atrophic macular degeneration from RPGR mutation. Genomics 200280166–171. [DOI] [PubMed] [Google Scholar]
- 12.Bergen A A, Pinckers A J. Localization of a novel X‐linked progressive cone dystrophy gene to Xq27: evidence for genetic heterogeneity. Am J Hum Genet 1997601468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalkanen R, Demirci F Y, Tyynismaa H, Bech‐Hansen T, Meindl A, Peippo M, Mäntyjärvi M, Gorin M B, Alitalo T. A new genetic locus for X linked progressive cone‐rod dystrophy. J Med Genet 200340418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake Y, Yagasaki K, Horiguchi M, Kawase Y, Kanda T. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol 19861041013–1020. [DOI] [PubMed] [Google Scholar]
- 15.Boycott K M, Pearce W G, Bech‐Hansen N T. Clinical variability among patients with incomplete X‐linked congenital stationary night blindness and a founder mutation in CACNA1F. Can J Ophthalmol 200035204–213. [DOI] [PubMed] [Google Scholar]
- 16.Pearce W G, Reedyk M, Coupland S G. Variable expressivity in X‐linked congenital stationary night blindness. Can J Ophthalmol 1990253–10. [PubMed] [Google Scholar]
- 17.Musarella M A, Weleber R G, Murphey W H, Young R S, Anson‐Cartwright L, Mets M, Kraft S P, Polemeno R, Litt M, Worton R G. Assignment of the gene for complete X‐linked congenital stationary night blindness (CSNB1) to Xp11.3. Genomics 19895727–737. [DOI] [PubMed] [Google Scholar]
- 18.Tremblay F, Laroche R G, De Becker I. The electroretinographic diagnosis of the incomplete form of congenital stationary night blindness. Vision Res 1995352383–2393. [DOI] [PubMed] [Google Scholar]
- 19.Allen L E, Zito I, Bradshaw K, Patel R J, Bird A C, Fitzke F, Yates J R, Trump D, Hardcastle A J, Moore A T. Genotype‐phenotype correlation in British families with X linked congenital stationary night blindness. Br J Ophthalmol 2003871413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake Y, Horiguchi M, Terasaki H, Kondo M. Scotopic threshold response in complete and incomplete types of congenital stationary night blindness. Invest Ophthalmol Vis Sci 1994353770–3775. [PubMed] [Google Scholar]
- 21.Bech‐Hansen N T, Naylor M J, Maybaum T A, Sparkes R L, Koop B, Birch D G, Bergen A A, Prinsen C F, Polomeno R C, Gal A, Drack A V, Musarella M A, Jacobson S G, Young R S, Weleber R G. Mutations in NYX, encoding the leucine‐rich proteoglycan nyctalopin, cause X‐linked complete congenital stationary night blindness. Nat Genet 200026319–323. [DOI] [PubMed] [Google Scholar]
- 22.Pusch C M, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, Scharfe C, Maurer J, Jacobi F K, Pinckers A, Andreasson S, Hardcastle A, Wissinger B, Berger W, Meindl A. The complete form of X‐linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine‐rich repeat protein. Nat Genet 200026324–327. [DOI] [PubMed] [Google Scholar]
- 23.Bech‐Hansen N T, Naylor M J, Maybaum T A, Pearce W G, Koop B, Fishman G A, Mets M, Musarella M A, Boycott K M. Loss‐of‐function mutations in a calcium‐channel alpha1‐subunit gene in Xp11.23 cause incomplete X‐linked congenital stationary night blindness. Nat Genet 199819264–267. [DOI] [PubMed] [Google Scholar]
- 24.Strom T M, Nyakatura G, Apfelstedt‐Sylla E, Hellebrand H, Lorenz B, Weber B H, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L‐type calcium‐channel gene mutated in incomplete X‐linked congenital stationary night blindness. Nat Genet 199819260–263. [DOI] [PubMed] [Google Scholar]
- 25.Catterall W A. Structure and regulation of voltage‐gated Ca2+ channels. Annu Rev Cell Dev Biol 200016521–555. [DOI] [PubMed] [Google Scholar]
- 26.Morgans C W. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci 2001422414–2418. [PubMed] [Google Scholar]
- 27.Xu H P, Zhao J W, Yang X L. Expression of voltage‐dependent calcium channel subunits in the rat retina. Neurosci Lett 2002329297–300. [DOI] [PubMed] [Google Scholar]
- 28.Koschak A, Reimer D, Walter D, Hoda J C, Heinzle T, Grabner M, Striessnig J. Cav1.4alpha1 subunits can form slowly inactivating dihydropyridine‐sensitive L‐type Ca2+ channels lacking Ca2+‐dependent inactivation. J Neurosci 2003236041–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McRory J E, Hamid J, Doering C J, Garcia E, Parker R, Hamming K, Chen L, Hildebrand M, Beedle A M, Feldcamp L, Zamponi G W, Snutch T P. The CACNA1F gene encodes an L‐type calcium channel with unique biophysical properties and tissue distribution. J Neurosci 2004241707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotturi M F, Carlow D A, Lee J C, Ziltener H J, Jefferies W A. Identification and functional characterization of voltage‐dependent calcium channels in T lymphocytes. J Biol Chem 200327846949–46960. [DOI] [PubMed] [Google Scholar]
- 31.Boycott K M, Maybaum T A, Naylor M J, Weleber R G, Robitaille J, Miyake Y, Bergen A A, Pierpont M E, Pearce W G, Bech‐Hansen N T. A summary of 20 CACNA1F mutations identified in 36 families with incomplete X‐linked congenital stationary night blindness, and characterization of splice variants. Hum Genet 200110891–97. [DOI] [PubMed] [Google Scholar]
- 32.Bassam B J, Caetano‐Anolles G, Gresshoff P M. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 199119680–83. [DOI] [PubMed] [Google Scholar]
- 33.Hoda J C, Zaghetto F, Koschak A, Striessnig J. Congenital stationary night blindness type 2 mutations S229P, G369D, L1068P, and W1440X alter channel gating or functional expression of Cav1.4 L‐type Ca2+ channels. J Neurosci 200525252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemara‐Wahanui A, Berjukow S, Hope C I, Dearden P K, Wu S B, Wilson‐Wheeler J, Sharp D M, Lundon‐Treweek P, Clover G M, Hoda J C, Striessnig J, Marksteiner R, Hering S, Maw M A. A CACNA1F mutation identified in an X‐linked retinal disorder shifts the voltage dependence of Cav1.4 channel activation. Proc Natl Acad Sci U S A 20051027553–7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu A S, Hebert S C, Brenner B M, Lytton J. Molecular characterization and nephron distribution of a family of transcripts encoding the pore‐forming subunit of Ca2+ channels in the kidney. Proc Natl Acad Sci U S A 19928910494–10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safa P, Boulter J, Hales T G. Functional properties of Cav1.3 (alpha1D) L‐type Ca2+ channel splice variants expressed by rat brain and neuroendocrine GH3 cells. J Biol Chem 200127638727–38737. [DOI] [PubMed] [Google Scholar]
- 37.Perez‐Reyes E, Wei X Y, Castellano A, Birnbaumer L. Molecular diversity of L‐type calcium channels. Evidence for alternative splicing of the transcripts of three non‐allelic genes. J Biol Chem 199026520430–20436. [PubMed] [Google Scholar]
- 38.Barry E L, Gesek F A, Froehner S C, Friedman P A. Multiple calcium channel transcripts in rat osteosarcoma cells: selective activation of alpha 1D isoform by parathyroid hormone. Proc Natl Acad Sci U S A 19959210914–10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Z Z, Liang M C, Lu S, Yu D, Yu C Y, Yue D T, Soong T W. Transcript scanning reveals novel and extensive splice variations in human l‐type voltage‐gated calcium channel, Cav1.2 alpha1 subunit. J Biol Chem 200427944335–44343. [DOI] [PubMed] [Google Scholar]
- 40.Jacobi F K, Hamel C P, Arnaud B, Blin N, Broghammer M, Jacobi P C, Apfelstedt‐Sylla E, Pusch C M. A novel CACNA1F mutation in a French family with the incomplete type of X‐linked congenital stationary night blindness. Am J Ophthalmol 2003135733–736. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura M, Ito S, Terasaki H, Miyake Y. Novel CACNA1F mutations in Japanese patients with incomplete congenital stationary night blindness. Invest Ophthalmol Vis Sci 2001421610–1616. [PubMed] [Google Scholar]
- 42.Wutz K, Sauer C, Zrenner E, Lorenz B, Alitalo T, Broghammer M, Hergersberg M, de la Chapelle A, Weber B H, Wissinger B, Meindl A, Pusch C M. Thirty distinct CACNA1F mutations in 33 families with incomplete type of XLCSNB and Cacna1f expression profiling in mouse retina. Eur J Hum Genet 200210449–456. [DOI] [PubMed] [Google Scholar]
- 43.Zeitz C, Minotti R, Feil S, Matyas G, Cremers F P, Hoyng C B, Berger W. Novel mutations in CACNA1F and NYX in Dutch families with X‐linked congenital stationary night blindness. Mol Vis 200511179–183. [PubMed] [Google Scholar]
- 44.Zito I, Allen L E, Patel R J, Meindl A, Bradshaw K, Yates J R, Bird A C, Erskine L, Cheetham M E, Webster A R, Poopalasundaram S, Moore A T, Trump D, Hardcastle A J. Mutations in the CACNA1F and NYX genes in British CSNBX families. Hum Mutat 200321169. [DOI] [PubMed] [Google Scholar]
- 45.Carlson S, Vesti E, Raitta C, Donner M, Eriksson A W, Forsius H. Clinical and electroretinographic comparison between Aland Island eye disease and a newly found related disease with X‐chromosomal inheritance. Acta Ophthalmol (Copenh) 199169703–710. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Ito S, Terasaki H, Miyake Y. Incomplete congenital stationary night blindness associated with symmetrical retinal atrophy. Am J Ophthalmol 2002134463–465. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura M, Ito S, Piao C H, Terasaki H, Miyake Y. Retinal and optic disc atrophy associated with a CACNA1F mutation in a Japanese family. Arch Ophthalmol 20031211028–1033. [DOI] [PubMed] [Google Scholar]
- 48.Hope C I, Sharp D M, Hemara‐Wahanui A, Sissingh J I, Lundon P, Mitchell E A, Maw M A, Clover G M. Clinical manifestations of a unique X‐linked retinal disorder in a large New Zealand family with a novel mutation in CACNA1F, the gene responsible for CSNB2. Clin Experiment Ophthalmol 200533129–136. [DOI] [PubMed] [Google Scholar]
- 49.Langrova H, Gamer D, Friedburg C, Besch D, Zrenner E, Apfelstedt‐Sylla E. Abnormalities of the long flash ERG in congenital stationary night blindness of the Schubert‐Bornschein type. Vision Res 2002421475–1483. [DOI] [PubMed] [Google Scholar]
- 50.Kellner U, Foerster M H. Cone dystrophies with negative photopic electroretinogram. Br J Ophthalmol 199377404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansergh F, Orton N C, Vessey J P, Lalonde M R, Stell W K, Tremblay F, Barnes S, Rancourt D E, Bech‐Hansen N T. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet 2005143035–3046. [DOI] [PubMed] [Google Scholar]