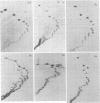
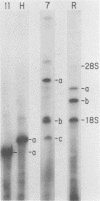
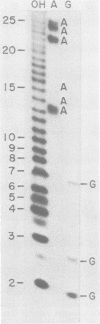
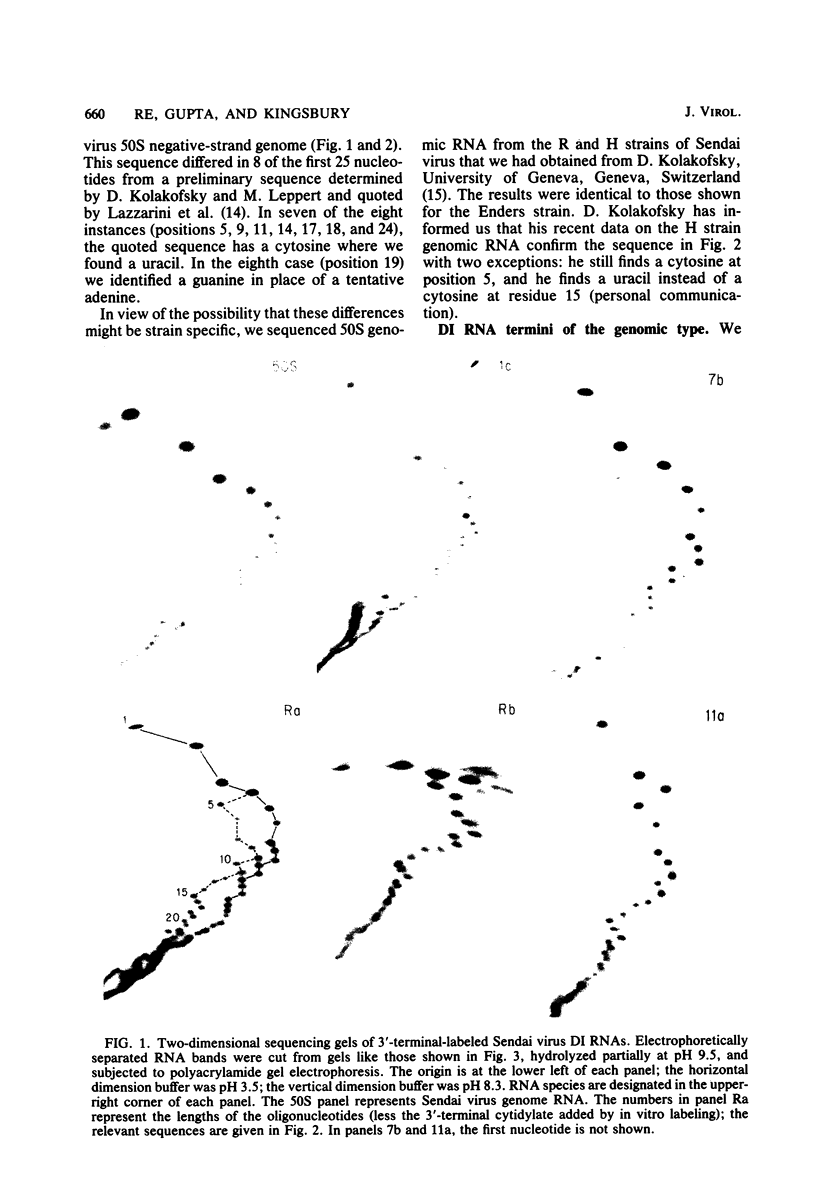
Abstract
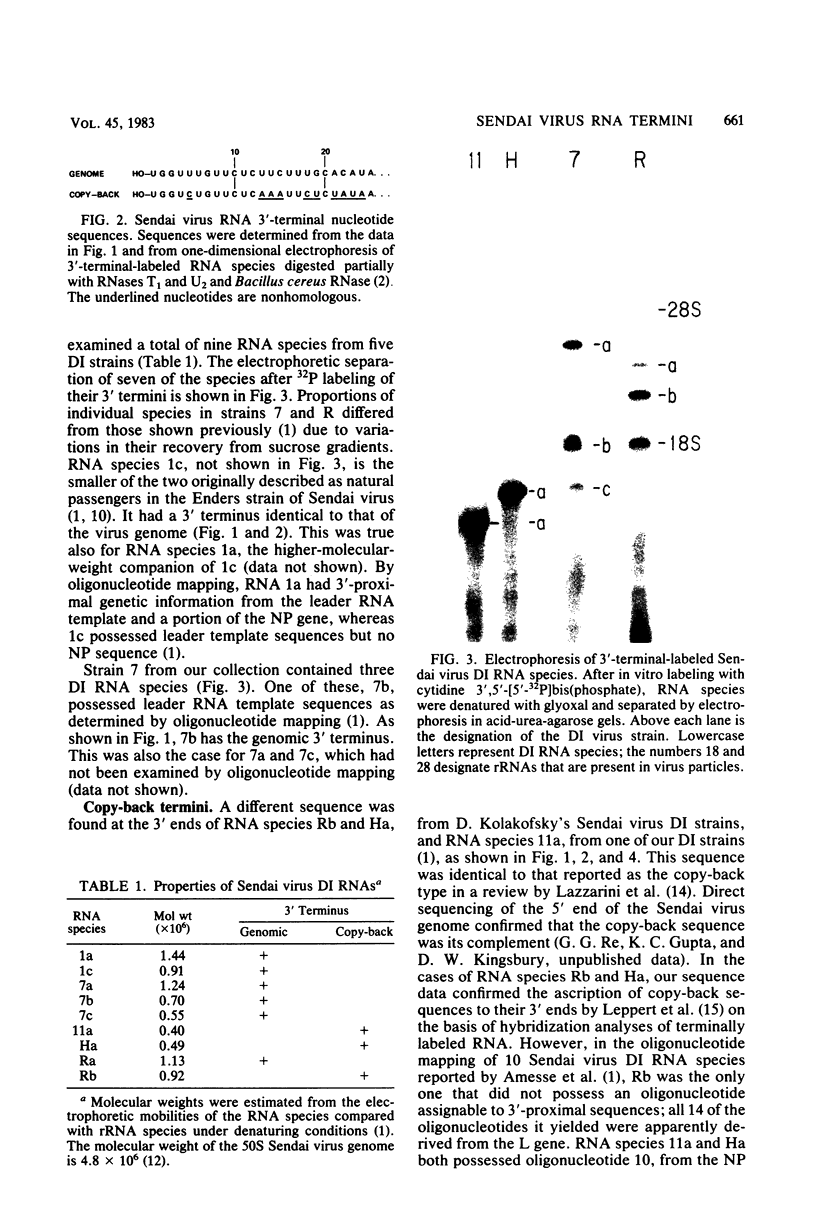
Direct sequencing of nine Sendai virus defective interfering RNA species revealed two kinds of 3'-terminal sequences. Six RNA species had 3' termini identical to the virus genome (negative strand), confirming that internal deletions are a frequent cause of Sendai virus defectiveness. The other three RNA species had 3'-terminal sequences identical to that described as the complement of the 5' terminus of the virus genome (R. A. Lazzarini, J. D. Keene, and M. Schubert, Cell 26:145-154, 1981), indicating that they are of the copy-back type. Extensive homology between these two types of 3' sequences evidently accounts for the ability of the copy-back sequence to function as an initiation signal for viral RNA replication. There may not be a selective advantage of one type of terminus over the other, since one defective interfering strain possessed two RNA species, one of which had the genomic 3' terminus and the other copy-back type.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesse L. S., Pridgen C. L., Kingsbury D. W. Sendai virus DI RNA species with conserved virus genome termini and extensive internal deletions. Virology. 1982 Apr 15;118(1):17–27. doi: 10.1016/0042-6822(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Rao D. D., Huang A. S. RNA synthesis of vesicular stomatitis virus. VIII. Oligonucleotides of the structural genes and mRNA. Gene. 1979 Feb;5(2):141–157. doi: 10.1016/0378-1119(79)90099-4. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Conserved polyadenylation signals in two negative-strand RNA virus families. Virology. 1982 Jul 30;120(2):518–523. doi: 10.1016/0042-6822(82)90055-1. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Chien I. M., Lazzarini R. A. Vesicular stomatitis virus defective interfering particle containing a muted internal leader RNA gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2090–2094. doi: 10.1073/pnas.78.4.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Bruschi A. Self-annealing of Sendai virus RNA. J Virol. 1974 Jul;14(1):33–39. doi: 10.1128/jvi.14.1.33-39.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Bruschi A. Antigenomes in Sendai virions and Sendai virus-infected cells. Virology. 1975 Jul;66(1):185–191. doi: 10.1016/0042-6822(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D. Transfer ribonucleic acid nucleotidyltransferase and transfer ribonucleic acid in Sendai virions. J Virol. 1972 Sep;10(3):555–559. doi: 10.1128/jvi.10.3.555-559.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J. Origin and replication of defective interfering particles. Curr Top Microbiol Immunol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- Tracy S. Improved rapid methodology for the isolation of nucleic acids from agarose gels. Prep Biochem. 1981;11(3):251–268. doi: 10.1080/00327488108061767. [DOI] [PubMed] [Google Scholar]