Abstract
iNKT cells are required for the induction of airway hyperreactivity (AHR), a cardinal feature of asthma, but how iNKT cells traffic to the lungs to induce AHR has not been previously studied. Using several models of asthma, we demonstrated that iNKT cells required the chemokine receptor CCR4 for pulmonary localization and for the induction of AHR. In both allergen-induced and glycolipid-induced models of AHR, wild-type but not CCR4−/− mice developed AHR. Furthermore, adoptive transfer of wild-type but not CCR4−/− iNKT cells reconstituted AHR in iNKT cell-deficient mice. Moreover, we specifically tracked CCR4−/− vs wild-type iNKT cells in CCR4−/−:wild-type mixed BM chimeric mice in the resting state, and when AHR was induced by protein allergen or glycolipid. Using this unique model, we showed that both iNKT cells and conventional T cells required CCR4 for competitive localization into the bronchoalveolar lavage/airways compartment. These results establish for the first time that the pulmonary localization of iNKT cells critical for the induction of AHR requires CCR4 expression by iNKT cells.
Bronchial asthma is a potentially life-threatening inflammatory disease of the lungs, characterized by the presence of large numbers of CD4+ T cells in the airways (1). In asthma, conventional allergen-specific CD4+ MHC class II-restricted T cells, which produce Th2 cytokines (IL-4, IL-5, and IL-13), are thought to orchestrate the inflammation in asthma (1, 2). In addition, in mouse models of asthma, iNKT cells are required for the development of airway hyperreactivity (AHR),4 a cardinal feature of asthma (3, 4). Furthermore, when directly activated by glycolipid Ags, iNKT cells induce the development of AHR, in the complete absence of conventional CD4+ T cells and adaptive immunity (5). Because CD4+ iNKT cells are also present in the lungs of patients with asthma (6–9), iNKT cells appear to play a critical role in the development of asthma, but the precise mechanisms of iNKT cell recruitment and homeostasis in the lungs have not been investigated.
NKT cells share features of classical T cells and NK cells, and most NKT cells express a highly conserved or invariant TCR repertoire consisting of Vα14-Jα18 (in mice) or Vα24-JαQ (in humans) (10). Through their invariant TCR, iNKT cells recognize bacterial and endogenous glycolipid Ags presented by the non-polymorphic MHC class I-like protein CD1d (11–14). When activated, iNKT cells rapidly produce large quantities of cytokines including IL-4 and IFN-γ, which allows these cells to critically amplify and regulate adaptive immune responses, and thus link innate and adaptive immunity and the development of autoimmune, antimicrobial, antitumor, antitransplant, and allergic immune responses (15–18).
Distinct functional subsets of iNKT cells present in the liver vs spleen (19) and likely express distinct chemokine receptor profiles (20, 21), suggesting that tissue localization and migration of functionally distinct iNKT cells could be controlled by the expression of specific chemokine receptors. Because chemokines regulate leukocyte trafficking, it is possible that a certain chemokine receptor expression profile defines airway homing of iNKT cells which can induce Th2 type responses and asthma. Although Th2 cells express CCR3, CCR4, and CCR8 (22, 23) and although the gut-homing chemokine receptor CCR9 has been suggested to play a role in the recruitment of iNKT to the airways (2, 5, 24), little is known about how iNKT cells home to the lungs. CCR3, while required for eosinophil recruitment, appears not to be required for the development of AHR (25). Evidence also suggests that CCR8 is not required for allergic lung inflammation (26, 27). Although CD4+ T cells use CCR4 for skin homing (28), the role of CCR4 in asthma remains controversial. CCR4−/− mice developed AHR in one mouse model of asthma (29), but developed attenuated AHR in another model (30). Indeed, both CCR4 ligands, CCL17 (thymus and activation-regulated chemokine (TARC)) and CCL22 (macrophage-derived chemokine (MDC)), are increased in the bronchoalveolar lavage (BAL) fluid of asthmatic patients (31, 32) and neutralization of CCL17 and CCL22 with mAbs blocks the development of AHR in mice (33, 34). Although CCR4+ T cells in the BAL fluid of mild asthmatics is comparable to controls, this number increases following segmental allergen challenge (33, 35–37). In any case, the specific mechanisms by which T cells enter the lung remain unclear and no studies have ever assessed localization of iNKT cells into the lung interstitium and airways (30, 38, 39).
Because the development of AHR requires the presence of iNKT cells and because asthma is characterized by the presence of iNKT cells in the lung tissue and airways, we sought to understand the pulmonary localization of iNKT cells in the development of AHR. We investigated the role of CCR4 in iNKT cell localization to the lungs and airways in several mouse models of asthma and found that CCR4 expression was required by iNKT cells to localize to the lungs and airways to induce the development of AHR.
Materials and Methods
Mice
Wild-type (WT) BALB/c ByJ, C57BL/6N, and CD45.1+ B6.SJL-Ptprca Pep3b/BoyJ mice were purchased from The Jackson Laboratory. CCR4−/− and Jα18−/− mice on the C57BL/6N background were a gift from GlaxoSmithKline Research and Development and M. Taniguchi (Chiba University, Chiba, Japan), respectively. CCR9−/− mice on the C57BL/6 background were generated by M.-A. Wurbel. Animals were used between 5 and 16 wk of age and were age and sex matched within each experiment. All animal protocols were approved by the Stanford University Committee on Animal Welfare and the Animal Care and Use Committee of Children’s Hospital, Boston.
Abs and reagents
α-Galactosylceramide (α-GalCer) and vehicle control were provided by P. B. Savage (Bringham Young University, Provo, UT). Neutralizing rat anti-CCL17 (clone 110904), rat anti-CCL22 (clone 158132), and isotype control (clone CAO06) mAbs were purchased from R&D Systems. Neutralizing rat anti-mouse CD1.1 (CD1d) mAb (hybridoma HB323; American Type Culture Collection) was used as described previously (6).
Induction of AHR and measurement of airway responsiveness
To assess glycolipid-induced AHR, α-GalCer or vehicle control was administered intranasally (i.n.; 1.5–2 μg) as previously described (6). Alternatively, AHR was induced by sensitization with 100 μg of OVA i.p. in alum and again 2 wk later along with one 50-μg OVA i.n. challenge. We sensitized other mice with a single i.n. administration of 50 μg of OVA in 100 of μg Aspergillus Ag (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). Twelve days after sensitization, mice were exposed to 50 μg/day of i.n. Ag for 3 consecutive days. AHR was measured at 24 h following i.n. challenge by methacholine-induced airflow obstruction in conscious mice placed in whole-body plethymographs (Buxo Electronics) as described elsewhere (4). In the invasive measurement of airway resistance, anesthetized and tracheotomized mice were mechanically ventilated as described (Buxo Electronics) (4).
Lymphocyte isolation and analysis of BAL fluid
Following measurement of AHR and sacrifice, mouse tracheas were cannulated and the lungs were lavaged twice with 1 ml of PBS and the fluid pooled as described (6). For some experiments, total BAL for each mouse or pooled BAL was stained and analyzed by flow cytometry. BAL iNKT cells numbers were quantified by multiplying hemocytometer cell counts excluding RBCs by percentage of iNKT within a cellular gate that included lymphocyte, monocyte, large epithelial cell, and granulocyte gate (by forward scatter (FSC) and side scatter (SSC)). Spleen, blood, and lung lymphocytes were isolated as described previously (6, 25).
Flow cytometry
Tissue homogenates were preincubated with anti-Fcγ blocking mAb (2.4G2; culture supernatant) and washed. iNKT cells were identified using TCRβ-CyChrome (clone H57-597; BD Pharmingen) or TCRβ PE-Cy5.5 (clone H57-597; eBioscience) and either PE-conjugated CD1d:tetramer (National Institutes of Health, National Institute of Allergy and Infectious Diseases major histocompatibility complex tetramer core facility, Atlanta GA) or CD1d:Ig dimer (BD Biosciences) loaded at a 10 M excess (α-α-GalCer or glycolipid vehicle to CD1d:Ig dimer per the manufacturer’s instructions) at 37°C overnight and subsequently labeled at a 2:1 mass ratio with PE- or allophycocyanin-conjugated anti-mouse IgG1 (eBioscience) by incubation for 1 h at 22°C. mAbs used were CD45.1-PE-Cy7 (clone A20), CD4-allophycocyanin-Cy7 (clone RM4-5; both eBioscience), and also CD45.2-FITC (clone 104), CD44-allophycocyanin (clone IM7), and CD45RB-PE (clone 16A; all BD Bioscience)s. Cells were analyzed on either a FACSCalibur flow cytometer (BD Biosciences) or a dual-laser MoFlo cytometer (DakoCytomation) configured for six colors with Summit 4.0 software (DakoCytomation).
Chemotaxis assay
Migration assays were conducted as described elsewhere (40). Briefly, 1–2 × 106 cells of spleen, liver, or lung single-cell suspensions were added to the upper wells of 5-μm pore, polycarbonate 24-well tissue culture inserts (Costar) in 100 μl, with 600 μl of diluted chemokine or medium in the bottom well. Murine chemokines were used at the following optimal concentrations: 50 nM CXCL12 and 100 nM CCL17 (R&D Systems). Cells were migrated in RPMI 1640 with 10% bovine serum at 37°C in 8% CO2 for 90 min and stained. Percentage of migration was calculated for each flow cytometry-defined subset by comparing its frequency in the input and migrated cell populations (32).
Lung chemokine assessment
Lung tissue was homogenized using a Tissue Tearor with the mechanical rotor (7–9000 rpm). Protease inhibitor was used at the vendor-recommended ratio, 10 μl of protease inhibitor:1 ml of cell lysate (1:100; Halt Protease Inhibitor Cocktail kit; Pierce Biotechnology). Levels of free CCL17 and CCL22 were measured by Quantikine ELISA kits according to the manufacturer’s instructions (R&D Systems).
Isolation of iNKT cells
iNKT cells were isolated, incubated in single-cell suspensions at 4°C with anti-FcγR blocking Ab 2.4G2 (1 μg/million cells), followed by a 1-h incubation at 4°C with CD1d:Ig dimer, previously loaded overnight at 10 M excess with α-GalCer per the manufacturer’s instructions (BD Biosciences). After washing, Cd1d:Ig dimer-labeled cells were incubated with anti-mouse IgG1 microbeads (Miltenyi Biotec) and magnetically sorted using the AutoMACS or the VarioMAX system (Miltenyi Biotec) according to the manufacturer’s instructions. Purity was obtained by staining cells with CD1d-tetramer and TCRβ. For some experiments, cells were further sorted to > 95% purity by FACS using a MoFlo cell sorter (DakoCytomation).
Restimulation of lymph node cells and sorted iNKT in vitro
Cells isolated from lymph nodes of OVA-primed mice were restimulated in vitro (7.5 × 105/well in 96-well plates) with 50 μg of OVA, and supernatants were collected for analysis of cytokines by ELISA at day 4. FACS-sorted iNKT (see above) were restimulated in vitro (5.0 × 104/well in 96-well plates) with 100 ng/ml α-GalCer, and supernatants were collected for analysis of cytokines by ELISA after 18 –24 h.
Competitive bone marrow (BM) reconstitution
BM chimeric mice were generated as described elsewhere (28). Equal numbers of BM cells (1 × 106 each) from CCR4−/− (CD45.2+/+) and WT (CD45.1+/+) donors were injected i.v. into lethally irradiated (1200-rad) 4-wk-old F1 WT mice (CD45.1+/−CD45.2+/−) to create CCR4−/−:WT chimeric mice. To control for any differences in reconstitution efficiency between CD45.1 and CD45.2 donor stem cells, control WT (CD45.1+/+) and WT (CD45.2+/+) chimeric mice, designated WT:WT chimeric mice, were created with WT (CD45.2+/+) and WT (CD45.2+/+) BM cells. At 8 –12 wk, the blood, lungs, and BAL fluid were harvested and the cells were analyzed by flow cytometry. To quantify competition between WT-derived and CCR4−/−-derived donor cells among iNKT cells, we normalized for the reconstitution ratio, which is the relative efficiency by which CD45.1 and CD45.2 BM cells engrafted in individual chimeric mice. We used CD4+ naive T cells as a normalizing population, because this population had previously been indicated to be unaffected by the presence or absence of CCR4. Thus, R = CD45.1:CD45.2) iNKT cell or conventional T cell population/(CD45.1:CD45.2) naive T cell population.
We calculated the relative disadvantage (D) for the CCR4−/− competing with WT BM cells as D = (RCCR4−/−:WT/RWT:WT).
Thus, D for any equally competitive control population is 1 and D > 1 implies that CCR4−/− -derived cells are disadvantaged in homing to the specific organ examined. Radioresistant host-derived cells were excluded from the analysis.
Statistical tests
Differences between groups with parametric distributions were analyzed by Student’s t test, otherwise the Mann-Whitney U test was used. Comparison of chemokine migrant vs input populations for relatedness was done by Williams corrected G test of independence. Significance for all statistical tests is shown in values for p < 0.05 (*) and p ≤ 0.01 (**).
Results
CCR4 is required for the development of AHR
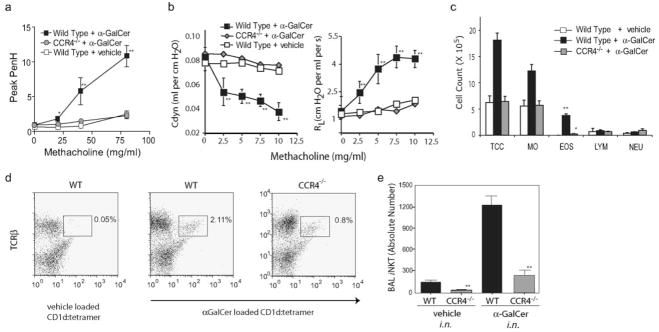
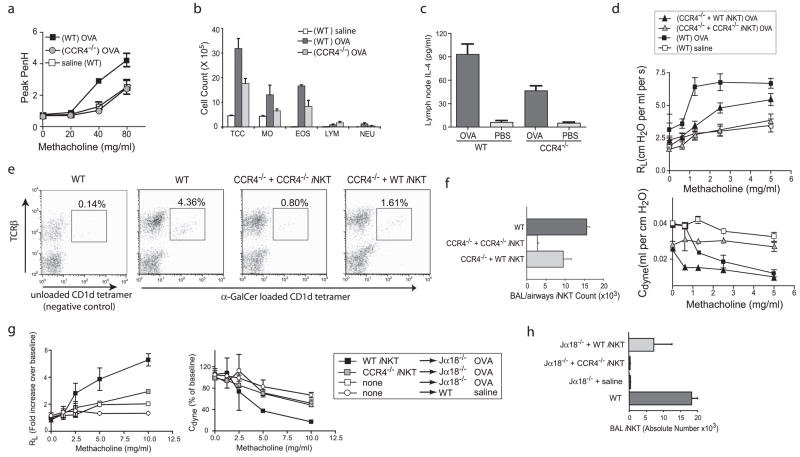
We examined AHR in CCR4−/− mice by directly activating pulmonary iNKT cells with intranasally administered α-GalCer. In this system, iNKT cells induce AHR without the need for class II MHC-restricted CD4+ T cells, B cells, or eosinophils (5). α-Gal-Cer (2 μg) failed to induce AHR in CCR4−/− mice, but induced severe AHR in WT mice, as measured by whole-body plethysmography (Fig. 1a) and confirmed by invasive measurement of airway resistance and dynamic compliance (Fig. 1b). α-GalCer-challenged CCR4−/− mice also failed to develop airway eosinophilia when compared with WT mice (Student’s t test, p < 0.01; Fig. 1c). Furthermore, the number of iNKT cells in the BAL fluid was greatly reduced in α-GalCer-challenged CCR4−/− mice compared with WT mice (Fig. 1, c and d), strongly suggesting that CCR4 contributes to iNKT cell localization to the airways. In contrast to CCR4−/− mice, CXCR6−/− mice and CCR9−/− mice challenged with α-GalCer developed severe AHR (M. J. Kan, M. A. Wurbel, E. H. Meyer, and E. C. Butcher, unpublished results). Therefore, the induction of AHR by direct activation of iNKT cells depends upon CCR4 and not CXCR6 or CCR9, as has been suggested previously (24).
FIGURE 1.
AHR induced with α-GalCer failed to occur in CCR4−/− mice. Statistical analysis is shown as the results of the pairwise Student t test between α-GalCer experimental and vehicle control groups with p ≤ 0.05 (*) and p ≤ 0.01 (**). a, Significant AHR assessed at 24 h following i.n. challenge with α-GalCer (2.0 μg/mouse) in WT mice did not occur in CCR4−/− mice. Data are the enhanced pause (Penh) values (mean ± SEM) of at least four mice per group, representative of four experiments. b, Invasive measurement of airway resistance confirmed CCR4−/− mice failed to develop AHR following α-GalCer at 24 h after 1.5 μg of i.n. challenge of α-GalCer, shown as dynamic compliance Cdyne (left, in ml per cm H2O) and airway resistance RL (right, in cm H2O per ml per s). Data represent the mean ± SEM from two experiments (n = 3–4). c, Airway eosinophilia in response to α-GalCer failed to occur in CCR4−/− mice. BAL fluid from the mice in a was analyzed 3 h after airway measurements. One representative experiment of four is shown as mean ± SEM of the number of cells per milliliter of BAL fluid. TCC, Total cell count; MO, monocytes; EOS, eosinophils; LYM, lymphocytes; NEU, neutrophils. d, iNKT cells in the BAL fluid of CCR4−/− mice sensitized were significantly reduced in percentage when compared with those of WT mice. Pooled BAL fluid collected from four mice in a were evaluated for iNKT cells using anti-TCRβ mAb and unloaded CD1d tetramer (negative control; left panel) or α-GalCer-loaded CD1d tetramer from WT (middle panel) vs CCR4−/− mice (right panel) or WT iNKT cells (rightmost panel), with percentage of lymphocytes indicated above the iNKT cell gate. e, iNKT cell counts in the BAL fluid was significantly reduced in CCR4−/− vs WT mice. BAL fluid was collected from individual mice (as in b) and stained for iNKT cells using anti-TCRβ mAb and α-GalCer-loaded CD1d tetramer, gated on lymphocytes by FSC and SSC. Absolute number of BAL iNKT was calculated and pooled across experiments, presented as mean ± SEM.
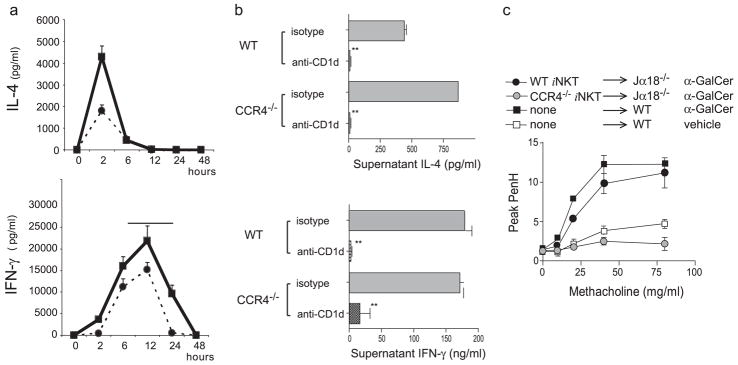
To specifically demonstrate that the failure of CCR4−/− mice to develop AHR was due to the inability of iNKT cells to migrate into the lungs, we adoptively transferred iNKT cells purified from WT and CCR4−/− mice (>95% purity) into iNKT cell-deficient Jα18−/− mice. Although CCR4−/− mice showed a 10 –20% reduction in the number of iNKT cells in the spleen and a reduction in serum IL-4 and IFN-γ produced after in vivo stimulation with α-GalCer compared with that in WT mice (Fig. 2a), iNKT cells isolated from CCR4−/− and WT mice produced comparable amounts of IL-4 and IFN-γ when activated in vitro with α-GalCer, in a CD1d-dependent manner (Fig. 2b). Moreover, when adoptively transferred into the Jα18−/− mice, iNKT cells from WT but not CCR4−/− mice reconstituted the development of AHR on challenge with α-GalCer (Fig. 2c). These results indicate that iNKT cells expressing CCR4 are required for α-GalCer-induced AHR.
FIGURE 2.
Although CCR4−/− mice showed reduced cytokine production in response to α-GalCer challenge in vivo, purified iNKT cells from CCR4−/− mice produced more IL-4 than wild-type iNKT cells when activated in vitro, but did not reconstitute AHR when transferred into glycolipid-challenged Jα18−/− mice. Statistical analysis is shown as the results of the pairwise Student t test between groups with p ≤ 0.05 (*) and p ≤ 0.01 (**). a, Either WT (solid line) or CCR4−/− (dashed lines) were given α-GalCer i.v. at time 0. Serum was collected over 48 h and analyzed for concentrations of IL-4 (upper panel) and IFN-γ (lower panel) by ELISA. b, iNKT cells from CCR4−/− mice produced both IL-4 and IFN-γ. Splenocytes were stained with CD1d tetramers (or CD1d:Ig dimers), magnetically enriched, and then purified by FACS, resulting in >95% purity. Sorted iNKT cells from CCR4−/− or WT mice were cultured for 24 h with α-GalCer (50 ng/ml) with or without blocking anti-CD1d mAb. Supernatants were analyzed for cytokine content by ELISA. Data are representative of three separate experiments shown as mean ± SEM of four replicates. c, iNKT cells from CCR4−/− mice failed to reconstitute the development of α-GalCer-induced AHR in Jα18−/− mice. Sorted iNKT cells from CCR4−/− or WT mice (1 × 106 cells/recipient) were obtained by selection over magnetic columns to > 70% purity and transferred into Jα18−/− recipients 30 min before i.n. challenge with α-GalCer. AHR was measured (as in Fig. 3a) 24 h later. Data are the mean ± SEM of enhanced pause (Penh) values from three to four mice per group representative of three experiments.
Pulmonary iNKT cells migrate preferentially to CCL17 (TARC)
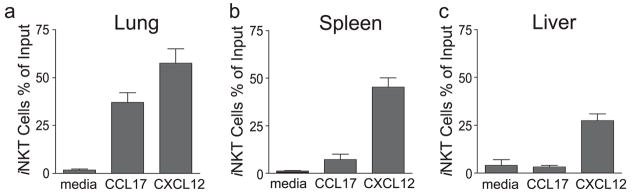
Although we were unable to stain iNKT cells for expression of CCR4 using any commercially available anti-CCR4 Abs, we demonstrated that a subset of iNKT cells expressed a functional CCR4 receptor, since this subset chemotaxed to the CCR4 ligand CCL17 (TARC) in a Transwell chemotaxis assay. Thus, 34% of the input resting iNKT cells isolated from the lungs of naive WT BALB/c mice migrated to CCL17 but not to medium (Mann-Whitney U test; p < 0.01; Fig. 3a). In contrast, only 8% of the input iNKT cells isolated from the spleen and none of the iNKT cells isolated from the liver migrated in response to CCL17 (Mann-Whitney U test; p < 0.05; Fig. 3, b and c). Comparison of total cell counts of chemokine migrant vs input populations for relatedness also assessed using Williams corrected G test of independence corroborated that iNKT cells counts were proportionately enriched in the CCL17 migrant when the cells were derived from the lungs ( p < 0.01) but not the liver ( p = 0.64). As expected, iNKT cells from all three sources migrated in response to the control chemokine CXCL12 (SOF-1α; stromal cell-derived factor 1α), which binds to a chemokine receptor, CXCR4, widely expressed on iNKT cells (21, 41). These results show that iNKT cells functionally CCR4 expressed and that a subset of iNKT cells that is preferentially present in the lung but not spleen or liver migrate to CCL17.
FIGURE 3.
iNKT cells that chemotaxed toward CCL17 were enriched in the lungs. Whole-cell suspensions from the lungs (a), spleen (b), or liver (c) of naive mice were allowed to migrate in Transwell inserts (5-μm pores) for 1.5 h to assess chemotaxis in response to medium, CCL17, or CXCL12 (as a positive chemotactic control). iNKT were identified by staining with α-GalCer-loaded CD1d:Ig dimer and anti-TCRβ mz Ab. Data are expressed as the fraction of the input iNKT cell population which migrate, with the mean ± SD reported for three pooled experiments. Statistical analysis is shown as the pairwise comparison of percent migrant values of CCL17 or CXCL12 vs medium control using the Mann-Whitney U test with p ≤ 0.05 (*) and p ≤ 0.01 (**).
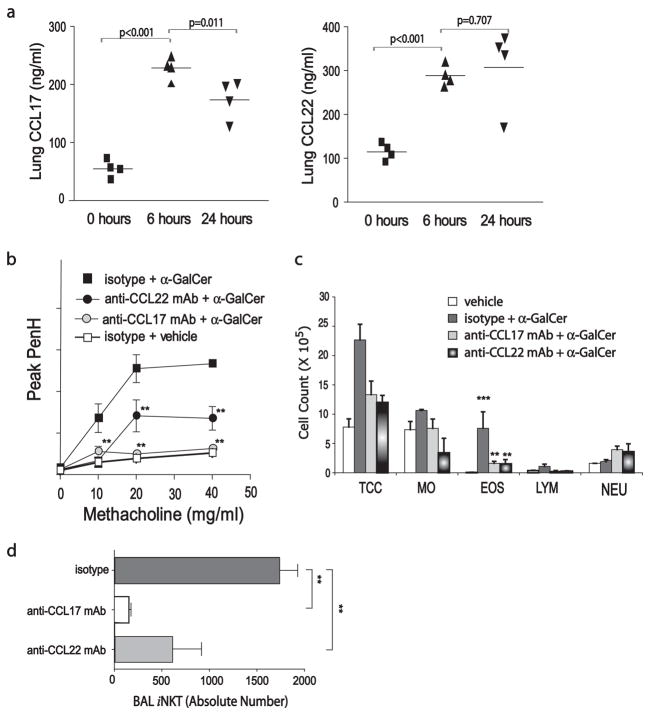
Induction of AHR requires CCL17
We next found that α-GalCer-induced AHR was accompanied by a 3- to 4-fold rise in pulmonary levels of the CCR4-binding chemokines CCL17 and CCL22, as measured by ELISA of whole lung lysates (Fig. 4a). Moreover, neutralization of CCL17 with an anti-CCL17 mAb (100 μg/mouse) abolished AHR induced with α-GalCer, while neutralization of CCL22 with an anti-CCL22 mAb (either 100 or 300 μg/mouse) greatly reduced but did not abolish α-GalCer-induced AHR (Fig. 4b). Treatment with either anti-CCL17 or anti-CCL22 mAb greatly reduced the number of eosinophils and lymphocytes in the BAL fluid (Fig. 4c) and reduced the number of iNKT cells in BAL fluid (Fig. 4d).
FIGURE 4.
CCL17 is required for α-GalCer-induced AHR. Statistical analysis is shown as the results of the pairwise Student t test between α-GalCer experimental and vehicle control groups with p ≤ 0.05 (*) and p ≤ 0.01 (**). a, CCL17 and CCL22 expression in the lungs increased significantly 6 and 24 h after i.n. challenge with α-GalCer (2 μg). Lung lysates were assayed for CCL17 and CCL22 by ELISA. Data are representative of three experiments with at least four mice per group. b, Neutralization of CCL22 reduced and neutralization of CCL17 blocked the development of α-GalCer-induced AHR. Anti-CCL17, anti-CCL22, or isotype control mAbs (100 μg) were administered 1 day before i.n. challenge with α-GalCer (1.5 μg). AHR was assessed as in Fig. 1a at 24 h after i.n. challenge. Data are the enhanced pause (Penh) values (mean ± SEM) from three to five mice per group representative of three experiments. c, Neutralization of CCL17 and CCL22 significantly reduced α-GalCer-induced airway eosinophilia. BAL fluid from the mice in b was analyzed at 3 h after airway measurements. Results of one representative experiment are shown as the mean ± SEM number of cells per milliliter of BAL fluid, TCC, Total cell count; MO, monocytes; EOS, eosinophils; LYM, lymphocytes; NEU, neutrophils. d, Neutralization of CCL17 and CCL22 significantly reduced the number of iNKT cells in the BAL fluid in mice challenged with α-GalCer. Individual BAL fluid collected from mice in (as in c) was stained for iNKT cells using anti-TCRβ mAb and α-GalCer-loaded CD1d tetramer, gated lymphocytes FSC and SSC. Absolute number of BAL iNKT was calculated (see Materials and Methods) and presented as mean ± SD.
CCR4 controls localization of iNKT and conventional T cells into the airways
To further examine localization of iNKT cells, we generated BM chimeric mice with CCR4−/− -derived and WT-derived BM as described previously (28). WT ((CD45.1 × CD45.20)F1 mice) recipients were lethally irradiated before reconstitution with equal numbers of BM cells from CCR4−/− mice expressing the congenic marker CD45.2 and from WT mice expressing the congenic marker CD45.1 (designated CCR4−/− WT chimeras). Control chimeras were generated with BM cells from WT mice expressing CD45.2 and from WT mice expressing CD45.1 (designated WT:WT chimeras). CCR4−/−:WT and WT:WT control chimeras were created and assessed in pairs. Engraftment efficiency of each donor BM for each chimeric mouse was determined by assessing the ratio of CD45.1 and CD45.2 expression by B cells and naive conventional CD4 T cells by flow cytometry. Because previous studies showed that CCR4 is not involved in the development of naive T cells, we normalized iNKT CD45.1:CD45.2 cell populations for each mouse by the engraftment ratio of naive conventional CD4 T cells (28).
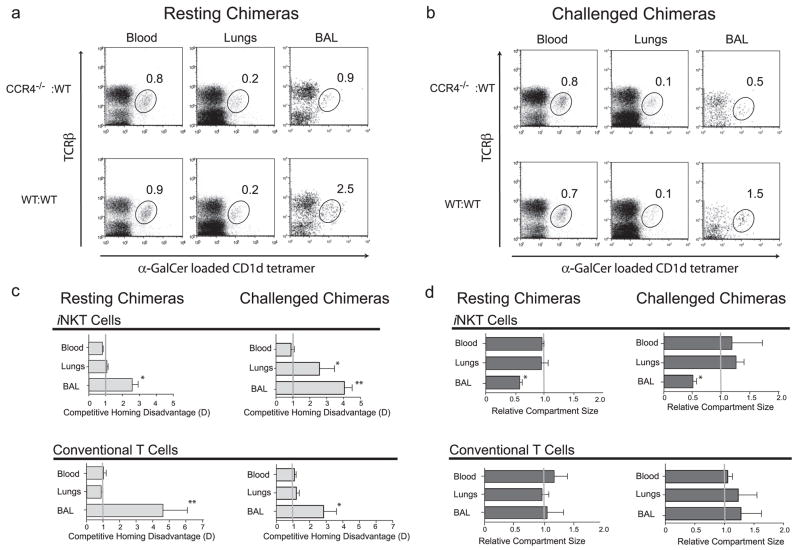
In resting chimeric mice, we found that both CCR4−/−:WT and control WT:WT chimeric mice had equivalent percentages of iNKT cells in the blood and lung tissue (after BAL fluid flush) (Fig. 5a). However, the CCR4−/−:WT chimeras had a lower frequency (and reduced total number) of iNKT cells in the BAL fluid/airway compartment (0.9% vs 2.5%; Fig. 5a). We also assessed homing of the iNKT cells 24 h after i.n. challenge with α-GalCer and found the same pattern in which the number of iNKT cells in the BAL fluid/airway compartment of CCR4−/−:WT chimeras was reduced (0.5% vs 1.5%; Fig. 5b) compared with the WT:WT control chimeras.
FIGURE 5.
Competitive localization of WT vs CCR4−/− iNKT in BM chimeric mice. Irradiated host mice were reconstituted with CD45.1+ WT and CD45.2+ CCR4−/− BM cells (WT: CCR4−/− chimera) or with CD45.1+ WT and CD45.2+ WT BM cells (WT:WT chimera). a, Resting CCR4−/−:WT chimeras had fewer iNKT cells only in the BAL fluid compartment. Six to 8 wk after BM reconstitution, iNKT cells were identified by staining with α-GalCer -loaded CD1d tetramer and anti- TCRβ mAb. The numbers in plots indicate the percentage of iNKT cells of total lymphocytes in the blood, lung digest (after BAL fluid harvest), or BAL fluid. Results are representative of five chimeric pairs. b, CCR4−/−:WT chimeras 24 h after i.n. challenge with α-GalCer (2 μg) have fewer iNKT cells only in the BAL fluid compartment. CCR4−/−:WT and WT:WT chimeras were assessed as in a and results are representative of six chimeric pairs. c, Upper left panel, analysis CCR4−/− BM-derived iNKT cell competitive homing disadvantage D. See Materials and Methods for calculation of disadvantage D. The relative disadvantage of CCR4−/− BM iNKT cells was 2- to 3-fold in resting mice in the BAL fluid compartment but not other tissue as calculated by flow cytometric assessment of CD45.1 and CD45.2+ cells in CCR4−/−:WT chimeras, compared with CD45.1+ and CD45.2+ WT cells in control WT:WT chimeras and represent the mean ± SD of pairwise comparisons normalized to engraftment ratios for five chimeric pairs. Statistical analysis shown as pairwise comparison of the D values derived from the BAL fluid or lungs to that of blood using the Mann-Whitney U test (p < 0.05 (*) and p ≤ 0.01 (**)). Upper right panel, The disadvantage of CCR4−/− BM-derived cells was greatest in the BAL fluid compartment of α-GalCer -challenged chimeric and also in the lung tissue but not blood from flow cytometric assessment as in b for six chimeric pairs. Lower left and lower right panels, Conventional T cells derived from CCR4−/− BM were disadvantaged only in the BAL fluid of CCR4−/−:WT resting chimeric mice as determined by flow cytometry and identification of anti-TCRβ mAb-positive cells excluding CD1d tetramer+, representative of the mean ± SD of pairwise comparisons above in resting (five pairs) and α-GalCer -challenged (six pairs) mice. d, CCR4 influenced the BAL/airway compartment size of iNKT but not conventional T cells. The relative compartment size is the ratio of the absolute number of iNKT cells in the indicated compartment of CCR4−/−:WT chimeras divided by that of WT:WT chimeras. Upper panels, Resting and α-GalCer -challenged CCR4−/−:WT chimeras had half the total iNKT cells in the BAL fluid/airways but not blood or lung tissue when compared with WT:WT chimeras. Lower panels, In contrast, CCR4−/−:WT chimeras had equal numbers of total conventional T cells in the BAL fluid/airways, lung tissue, and blood when compared with WT:WT chimeras. Data are presented as mean ± SEM ratios for pairwise comparisons (five resting and six challenged). Statistical analysis shown as pairwise comparison of the relative compartment size values for the BAL fluid or lungs to that of blood using the Mann-Whitney U test (p < 0.05 (*) and p ≤ 0.01 (**)).
Since in chimeric mice WT and CCR4−/− cells compete for access into various microenvironments (28, 42), by gating on iNKT cells and evaluating the expression of the congenic markers CD45.1 and CD45.2, we could evaluate the competitive relationship between CCR4−/− -derived vs WT-derived iNKT cells in pulmonary localization (see Materials and Methods for calculation homing disadvantage). In resting chimeric mice, we found a 2- to 3-fold disadvantage in the migration of CCR4−/− -derived iNKT cells into the BAL fluid/airway (Fig. 5c, upper left), but there was no disadvantage in the migration of CCR4−/− -derived iNKT cells into the blood or lung tissue. Thus, CCR4 is critical for competitive recruitment of iNKT cells into the BAL fluid/airway compartment in resting mice (Fig. 5c, upper left). By comparison, at 24 h following α-GalCer challenge, we found that there was a 3- to 4-fold disadvantage in the migration of CCR4−/− -derived iNKT cells into the BAL fluid/airway compartment and a 2- to 3-fold disadvantage in the migration of CCR4−/− -derived iNKT cells into the lung tissue, but no disadvantage in the blood (Fig. 5c, upper right). This indicates that, in the context of glycolipid-driven inflammation, iNKT cells become more dependent upon CCR4 for homing to the lungs and airways.
By gating on conventional T cells and expression of CD45.1 and CD45.2, we also evaluated the competitive homing of conventional T cells into the pulmonary compartments of both the resting and activated chimeric mice. Most conventional T cells in the airways (70 – 85%) expressed the CD4+ memory phenotype (CD4+CD44highCD45RBlow; data not shown). In both resting and α-GalCer-challenged chimeric mice, the CCR4−/− -derived CD4+ T cells were disadvantaged by 3- to 5-fold in the BAL fluid/airway compartment, but not in the blood or lung tissue itself (Fig. 5c, lower graphs). Taken together, these results show that iNKT cells require CCR4 for competitive localization to the lungs and airway compartments of challenged mice, whereas conventional T cells require CCR4 expression for localization only to the airway compartment.
iNKT cells require CCR4 for full population size in the airways of resting and glycolipid-challenged mice
To determine the effects of CCR4 on the pulmonary iNKT cell population size, we determined the relative compartment sizes of iNKT cells in the blood, lungs (after BAL fluid removal), and airways of CCR4−/−:WT vs WT:WT chimeras by pairwise division of the iNKT cell counts of each compartment in the CCR4−/−:WT chimera by that of the WT:WT chimeras, either in the resting state (Fig. 5d, upper left) or after α-GalCer challenge (Fig. 5d, upper right). We found that the iNKT cell population size in the blood and lungs of resting and challenged mice was equal between CCR4−/−:WT and WT:WT chimeras. However, the number of iNKT cells in the BAL fluid/airway compartment of both resting and challenged CCR4−/−:WT chimeras was half that of WT:WT chimeras. Thus, iNKT cells require CCR4 expression to fully populate the airways (BAL fluid) but not the lung tissue both in the resting and challenged state.
In the same way, we determined the relative compartment size of conventional T cells in the blood, lungs, and airways of the chimeras by pairwise division of T cell counts in the CCR4−/−:WT chimera by that of the WT:WT chimera, either in the resting state (Fig. 5d, lower left) or after α-GalCer challenge (Fig. 5d, lower right). We found that the conventional T cell population size was equal between CCR4−/−:WT and WT:WT chimeras in the blood, lungs, and airways of resting (Fig. 5d, lower left) and challenged mice (Fig. 5d, lower right). Thus, unlike iNKT cells, conventional T cells do not require CCR4 expression to fully populate the airways both in the naive resting state and in the context of immunological activation.
CCR4 expressing iNKT cells are required for OVA-induced AHR
We next examined the role of CCR4 in the development of protein allergen-induced AHR. CCR4−/− mice that were sensitized and challenged with OVA failed to develop significant AHR when compared with WT mice (Fig. 6a). Furthermore, OVA-sensitized and -challenged CCR4−/− mice had significantly decreased airway eosinophilia compared with similarly treated WT mice (Fig. 6b). In agreement with earlier reports, we verified that adaptive Th2-driven immunity occurred in CCR4−/− mice immunized with OVA, as shown by the production of IL-4 by bronchial lymphnode cells cultured in vitro for 4 days with OVA (Fig. 6c; IFN-γ was equally low in all samples). We confirmed our results using a second model of allergen-induced AHR, in which mice were sensitized i.n. with OVA plus Aspergillus fumigatus Ag. In this additional model, AHR was also significantly reduced in CCR4−/− vs WT mice, as assessed with both whole-body plethysmography and invasive measurement of airway resistance (data not shown).
FIGURE 6.
Adoptive transfer of WT but not CCR4−/− -derived iNKT cells induced the development of AHR in OVA-sensitized and challenged mice. Statistical analysis is shown as the results of the pairwise Student t test between experimental and negative control groups with p ≤ 0.05 (*) and p ≤ 0.01 (**). a, CCR4−/− mice failed to develop significant OVA-induced AHR. AHR was assessed in OVA-sensitized and -challenged mice (sensitized with alum or Aspergillus Ag (alum sensitized shown)). Data are Penh values (mean ± SEM) of at least four mice per group, representative of three experiments for OVA-sensitized groups (shown) and two for Aspergillus-sensitized groups. b, Airway eosinophilia was reduced in OVA-sensitized and -challenged CCR4−/− vs WT mice. BAL fluid from mice in a was taken 2 days after the final OVA i.n. challenge. Representative results are shown as the mean ± SEM number of cells per milliliter of BAL fluid. TCC, Total cell count; MO, monocytes; EOS, eosinophils; LYM, lymphocytes; NEU, neutrophils. c, IL-4 production by OVA-restimulated bronchial lymph node cells was measured in CCR4−/− and WT mice after sensitization and challenge with OVA (see Materials and Methods). d, Adoptive transfer of iNKT cells from WT but not CCR4−/− mice boosted AHR in CCR4−/− mice. Splenic iNKT cells were sorted from CCR4−/− or WT mice (1.5 × 106 cells/recipient) and transferred into OVA/alum-sensitized CCR4−/− mice 1 day before consecutive i.n. challenges with OVA. AHR was assessed by changes in airway resistance (RL) and dynamic compliance (Cdyne) in response to methacholine in anesthetized, tracheostomized, intubated, and mechanically ventilated mice. Data represent the mean ± SEM of three to four mice per group from three experiments. e, iNKT cell numbers in the BAL fluid of CCR4−/− mice sensitized and challenged with OVA were significantly reduced compared with those of WT mice. BAL fluid collected from each mouse in c was evaluated for iNKT cells using anti- TCRβ mAb and unloaded CD1d tetramer (negative control; leftmost panel) or α-GalCer -loaded CD1d tetramer from WT (second panel) vs CCR4−/− mice that were recipients to either CCR4−/− iNKT (third panel) or WT iNKT cells (rightmost panel), with percentage of lymphocytes indicated above the iNKT cell gate. f, Adoptive transfer of WT but not CCR4−/− increased the absolute number of iNKT cells in the BAL fluid of CCR4−/− mice sensitized and challenged with OVA. Flow cytometric analysis of mice similarly treated as in d were used to calculate the absolute number of iNKT cells in the BAL fluid (see Materials and Methods). g, Adoptive transfer of iNKT cells from WT but not CCR4−/− mice reconstituted AHR in Jα18−/− mice. iNKT cells were sorted from the spleens of CCR4−/− or WT mice (1 × 106 cells/recipient) and transferred into OVA/alum-sensitized Jα18−/− mice 1 day before consecutive i.n. challenges with OVA. AHR was assessed by changes in airway resistance (RL) and dynamic compliance (Cdyne, as in 6 days). Data are representative of the mean ± SEM of three to five mice per group from two experiments. h, Adoptively transferred iNKT cells from WT but not CCR4−/− mice localized into the BAL fluid of Jα18−/− mice after transfer. BAL fluid cells from individual mice treated as in a were collected 1 day after measurement of AHR and were stained with anti-TCRβ mAb and α-GalCer -loaded CD1d tetramer. One representative experiment of three is shown and data are the mean ± SD of calculated individual BAL iNKT cell numbers.
To further explore the CCR4 expression by iNKT cells in the development of allergen-induced AHR, we examined CCR4−/− mice reconstituted either with purified WT or CCR4−/− iNKT cells (1.5 × 106 cell/mouse) and sensitized and challenged with OVA. We found that adoptive transfer of WT but not CCR4−/− iNKT cells significantly increased AHR (Fig. 6d). The absence of allergen-induced AHR in CCR4−/− mice was associated with a significant reduction in the number of iNKT cells in the BAL fluid (Fig. 6, e and f ). When assessed by flow cytometry, the percentage of iNKT cells in the BAL fluid of OVA-sensitized and -challenged CCR4−/− mice was greatly reduced compared with WT mice, even with the adoptive transfer of CCR4−/− iNKT cells, but was increased in CCR4−/− mice receiving WT iNKT cells (Fig. 6e, center right and rightmost panels), as verified by the evaluation of the absolute number of iNKT cells in BAL fluid (Fig. 6f). Thus, CCR4−/− mice that received WT but not CCR4−/− iNKT cells showed an increase in the total number of iNKT cells in the BAL fluid/airway compartment. These results indicate that iNKT expressing CCR4 are required for localization to the airways where they serve as an effector cell population capable of modulating the development of OVA-induced AHR.
To obviate any confounding effects due to the absence of CCR4 in CCR4−/− mice on global T cell or immune development, we reconstituted iNKT cell-deficient Jα18−/− mice (which normally express CCR4 but lack iNKT cells and cannot develop AHR (4), with purified iNKT cells from WT or CCR4−/− mice. Using this model, we found that iNKT from WT but not from CCR4−/− mice reconstituted AHR in OVA-sensitized Jα18−/− mice (Fig. 6g). Moreover, only WT but not CCR4−/− iNKT cells were found in the BAL fluid of OVA-sensitized Jα18−/−-challenged mice (Fig. 6h), confirming that the expression of CCR4 by iNKT cells is required for the pulmonary localization of iNKT cells and development of OVA-induced AHR.
iNKT cells require CCR4 for full population size in the airways of allergen-challenged mice
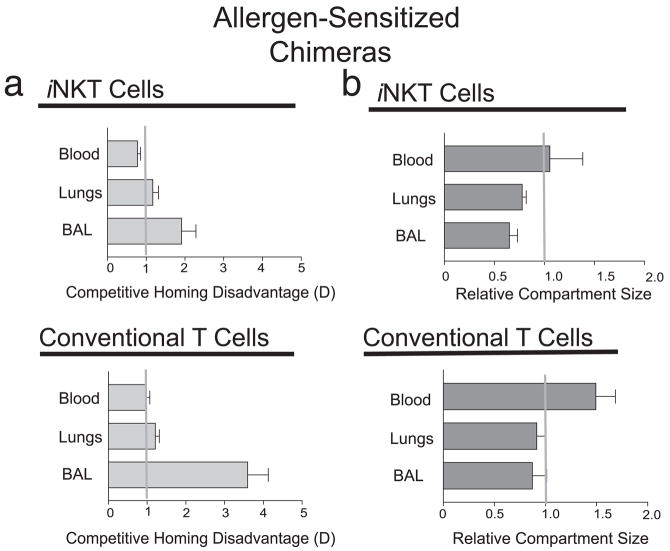
To further examine the localization of iNKT cells in allergen-induced AHR, we generated additional BM chimeric mice and examined the competitive localization of CCR4−/− vs WT iNKT cells when these mice were sensitized and challenged with OVA. Following OVA/alum sensitization and OVA i.n. challenge, we found a 2-fold disadvantage in the migration of CCR4−/− -derived iNKT cells into the BAL fluid/airway (Fig. 7a, upper panel), but no disadvantage in the migration of CCR4−/− -derived iNKT cells into the blood or lung tissue. Furthermore, the CCR4−/− -derived conventional CD4+ T cells were disadvantaged by 3- to 4-fold in the BAL fluid/airway compartment, but not in the blood or lung tissue itself (Fig. 7a, lower panel).
FIGURE 7.
Competitive localization of WT vs CCR4−/− iNKT and conventional T cells in allergen-sensitized BM chimeric mice. Irradiated host mice were reconstituted with CD45.1+ WT and CD45.2+ CCR4−/− BM cells (WT: CCR4−/− chimera) or with CD45.1+ WT and CD45.2+ WT BM cells (WT:WT chimera). The mice were sensitized to and challenged with OVA as described in Materials and Methods. a, Upper panel, Analysis of CCR4−/− BM-derived iNKT cell competitive homing disadvantage D in mice at 24 h after final i.n. challenge with OVA. See Materials and Methods for calculation of disadvantage D. The relative disadvantage of CCR4−/− BM iNKT cells was 2-fold in allergen-sensitized and -challenged mice in the BAL fluid compartment but not other tissue as calculated by flow cytometric assessment of CD45.1 and CD45.2+ cells in CCR4−/−:WT chimeras, compared with CD45.1+ and CD45.2+ WT cells in control WT:WT chimeras and represent the mean ± SD of pairwise comparisons normalized to engraftment ratios for four chimeric pairs. Statistical analysis shown as pairwise comparison of the D values derived from the BAL fluid or lungs to that of blood using the Mann-Whitney U test (p < 0.05 (*) and p ≤ 0.01 (**)). Lower panel, Conventional T cells derived from CCR4−/− BM are disadvantaged only in the BAL fluid of CCR4−/−:WT resting chimeric mice as determined by flow cytometry and identification of anti-TCRβ mAb-positive cells excluding CD1d tetramer+, represents the mean ± SD of pairwise comparisons (four pairs). b, CCR4 influences the BAL/airway compartment size of iNKT but not conventional T cells. The relative compartment size is the ratio of the absolute number of iNKT cells in the indicated compartment of CCR4−/−:WT chimeras divided by that of WT:WT chimeras. Upper panel, Allergen-challenged CCR4−/−:WT chimeras have three-fifths the total iNKT cells in the BAL fluid/airways but not blood or lung tissue when compared with WT:WT chimeras. Lower panel, In contrast, CCR4−/−:WT chimeras had slightly reduced numbers of total conventional T cells in the BAL fluid/airways, lung tissue, and blood when compared with WT:WT chimeras. Data are presented as mean ± SEM ratios for pairwise comparisons (four pairs). Statistical analysis shown as pairwise comparison of the relative compartment size values for the BAL fluid or lungs to that of blood using the Mann-Whitney U test (p < 0.05 (*) and p ≤ 0.01 (**)).
To determine the effects of CCR4 on the pulmonary iNKT cell population size, we examined the relative compartment sizes of iNKT cells in the blood, lungs (after BAL fluid removal), and airways of the CCR4−/−:WT vs WT:WT chimeras (Fig. 7b, upper panel). We found that the iNKT cell population size in the blood of allergen-sensitized mice was the same in CCR4−/−:WT and WT:WT chimeras (relative compartment size = 1). However, the number of iNKT cells was reduced in the lungs (0.78 ± 0.08-fold) and BAL fluid/airway compartment (0.65 ± 0.16-fold) in allergen-challenged CCR4−/−:WT chimeras when compared with that of WT:WT chimeras. Thus, iNKT cells require CCR4 expression to fully populate the airways (BAL fluid) and the lung tissue in OVA-challenged mice.
In the same way, we determined the relative compartment size of conventional T cells in the blood, lungs, and airways of the chimeras following allergen challenge (Fig. 7b, lower panel). We found that the conventional T cell population size was equal in the lungs and airways of allergen-challenged CCR4−/−:WT and WT:WT chimeric mice (although increased in the blood of the CCR4−/−:WT chimeras) (Fig. 7b, lower panel). Thus, unlike iNKT cells, conventional T cells do not require CCR4 expression to fully populate the airways in OVA-challenged mice.
Discussion
In these studies, we used multiple approaches to study the role of CCR4 in the development of AHR. In previous work, treatment of mice with anti-CCL17 and anti-CCL22 mAb blocked the development of allergen-induced AHR (33, 34), and AHR was reduced in CCR4−/− mice on days 7 and 30 after challenge with Aspergillus conida (30), suggesting that CCR4 was required for AHR. However, in another system, CCR4−/− mice developed mild AHR (29), although in this study whole-body plethysmography, which can be confounded by changes in the upper respiratory tract, was used exclusively to assess AHR. To resolve these conflicting results regarding CCR4 and AHR, we used three different mouse model systems of asthma and assessed AHR using both whole-body plethysmography as well as invasive measurement of airway resistance and compliance. Moreover, because iNKT cells are necessary and sufficient for the induction of AHR, unlike previous studies, we focused on iNKT cells rather than on conventional CD4 T cells in effecting AHR, e.g., by using α-GalCer to specifically activate iNKT cells and by examining models in which AHR in Jα18−/− mice was reconstituted with adoptively transferred iNKT. We compared CCR4−/− vs WT mice, administered anti-CCL17 and CCL22 mAb to WT mice, and adoptively transferred iNKT cells from CCR4−/− and WT mice into CCR4−/− mice. Importantly, we also directly compared the homing to the lungs and airways of WT and CCR4−/− NKT cells in mixed BM chimeric mice when AHR was induced by either α-GalCer or allergen. We found that CCR4 was required for the development of AHR in all of these systems, with both protein and glycolipid Ags, and that AHR was dependent on the localization of iNKT cells into the lungs and airways, which in turn required CCR4 function.
Our study is the first to examine the localization of iNKT cells into the lungs and airways and to identify a subset of murine iNKT cells that induces the development of AHR and that requires CCR4 for airway localization. This iNKT cell subset expresses CCR4, as defined by migration in vitro in response to the CCR4 ligand CCL17 (TARC) may be related to human CCR4+ iNKT cells expressing CD4 and producing both IL-4 and IFN-γ (41), but appears to be distinct from other iNKT cell subsets, such as those expressing CXCR6, which localize to the liver (20) and into allografts (43). In fact, CXCR6 mice show a reduction of iNKT cells in the liver and do not apparently have a defect in development or survival in other tissues (20), indicating that the use of chemotactic pathways by iNKT cells may be highly specialized. The CCR4 ligands CCL17 (TARC) and CCL22 (MDC) are produced in abundance by lung epithelial cells in a peribronchiolar fashion (**) and presumably attract CCR4+ iNKT cells to the lungs and airways. Our identification of a CCR4+ subset of murine iNKT cells that induces the development of AHR reinforces recent reports delineating functional subsets of mouse iNKT cells based upon their anatomical origins (19 –21, 44) and strongly suggests that discrete and separate functional iNKT cell populations may be identifiable by chemokine expression.
Conventional CD4+ T cells, which are not absolutely required for the development of AHR (5), are distinct from iNKT cells in their requirements for localizing into the lung tissue in asthma. We used mixed BM chimeric mice to clearly show that conventional CD4+ T cells derived from CCR4−/− BM were competitively disadvantaged in homing to the BAL fluid (airways) compartment, but not into the lung tissue. It is possible that conventional CD4+ T cells use multiple redundant chemokine receptors for localizing into the lung tissue, such as CCR3, CCR4, and CCR8, which are known to be expressed on Th2 cells.
Our study is the first to use mixed BM chimeric mice to directly compare homing of WT and CCD4−/− cells to the lungs and the first to evaluate and show that iNKT cells and conventional T cells are regulated differently in the BAL fluid (airways) compartment. Despite a competitive disadvantage in homing to the airways of conventional T cells and iNKT cells derived from CCR4−/− BM, the population size of conventional T cells (which are mostly CD4+ memory cells) in the airways of CCR4−/−:WT chimeric mice was of normal size, while that of iNKT cells was reduced. We believe that the number of conventional CD4+ memory T cells in the airways was maintained either by compensatory recruitment and/or by homeostatic control of CD4+ memory T cell expansion in response to niche intrinsic signals. In contrast, compensatory recruitment or homeostatic expansion of iNKT cells in the airways was ineffective in the CCR4−/−:WT chimeric mice. Thus, unlike conventional T cells, the establishment of iNKT cells in the BAL fluid (airways) compartment depends directly upon CCR4 expression. That iNKT cells depend upon CCR4 to populate the airway compartment implicates the CCR4 pathway as a potentially unique contributor to AHR and asthma. Furthermore, this work suggests that chemokine receptor expression may play a previously unrecognized but important role in establishment of iNKT cells and perhaps other innate immune cells in the periphery.
Until now, the study of homeostatic regulation of iNKT cell population size has been limited to the thymus, spleen, and liver, in which production of IL-15 controls iNKT cell population size and iNKT cells compete with NK cells and CD8 memory T cells (45, 46). Although IL-15 may be produced in lung tissue (47), IL-15 production in the airways may be limited, since neutralization of IL-15 with an anti-IL-15 mAb did not affect the development of AHR (E. H. Meyer, O. T. Umetsu, and T. Waldman, unpublished observations). The absence or restriction of IL-15 and/or other homeostatic signals in the airways may explain why homeostatic control of iNKT cells does not compensate for loss of CCR4 function and why iNKT cells in the airways are more sensitive to chemotactic control and may behave more like effector cell populations, which expand primarily in response to Ag.
In summary, we identified chemotaxis mediated by CCR4 as the mechanism by which iNKT cells localize into the airways where they must be present to induce the development of AHR in both glycolipid- and allergen-induced models of asthma in mice. Our studies indicate that iNKT cells in the lungs are enriched in their responsiveness to CCR4 ligands (CCL17) when compared with iNKT cells from the spleen or liver. Using WT and CCR4−/− mixed BM chimeric mice, we definitively showed that iNKT cells but not conventional CD4+ T cells require CCR4 function for competitive homing to the lung tissue. Furthermore, in the airways, the population size of iNKT but not conventional CD4+ T cells is reduced in CCR4−/−:WT chimeric mice. We conclude therefore that the function of iNKT cells in asthma depends on homing to the lungs and airways mediated by CCR4 and CCL17 (TARC). Thus, our studies provide important mechanistic insight into iNKT cell function and into potential therapeutic strategies for asthma.
Acknowledgments
We thank Dr. O. Akbari and H. Maxion for discussions; Dr. Christine Power and GlaxoSmithKline Research and Development for the CCR4−/− mice; Dr. Thomas Waldman (National Institutes of Health) for anti-IL-15 mAb; and the National Institute of Health Tetramer Facility (Bethesda, MD) for CD1d tetramers.
Footnotes
These studies were supported by National Institutes of Health Grants 2R01AI046784-07A1 (to J.J.C.), R01 HL62348 (to D.T.U.), and R01 AI26322 (to D.T.U.). M.A.W. was supported by the Crohn’s and Colitis Foundation of America Research Fellowship (New York, NY).
Abbreviations used in this paper: AHR, airway hyperreactivity; BAL, bronchoal-veolar lavage; α-GalCer, α-galactosylceramide; i.n., intranasal(ly); FSC, forward scatter; SSC, side scatter; WT, wild type; TARC, thymus and activation-regulated chemokine; MDC, macrophage-derived chemokine; BM, bone marrow.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Wills-Karp M. Immunological basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Umetsu DT, McIntire JJ, Akbari O, Macaubus C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 3.Lisbonne M, Diem S, Keller AD, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, et al. Cutting edge: invariant Vα14 NKT cells dare required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 4.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 5.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor plus natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 7.Spinozzi F, Porcelli SA. Recognition of lipids from pollens by CD1-restricted T cells. Immunol Allergy Clin North Am. 2007;27:79–91. doi: 10.1016/j.iac.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Sen Y, Yongyi B, Yuling H, Luokun X, Li H, Jie X, Tao D, Gang Z, Junyan L, Chunsong H, et al. Va24-invariant NKT cells from patients with allergic asthma express CCR9 at high frequency and induce Th2 bias of CD3+ T cells upon CD226 engagement. J Immunol. 2005;175:4914 – 4926. doi: 10.4049/jimmunol.175.8.4914. [DOI] [PubMed] [Google Scholar]
- 9.Hamzaoui A, Rouhou SC, Grairi H, Abid H, Ammar J, Chelbi H, Hamzaoui K. NKT cells in the induced sputum of severe asthmatics. Mediat Inflamm. 2006:71214–71217. doi: 10.1155/MI/2006/71214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 11.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 12.Kinjo Y, Wu D, Kim GS, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 13.Mattner J, DeBord KL, Ismail N, Goff RD, Cantu C, Zhou DP, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 14.Zhou BH, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 15.Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwenhuis EES, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, Corazza N, Colgan SP, Onderdonk AB, Blumberg RS. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan FS, Zeng DF, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003;9:355–363. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 19.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:650 – 661. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCRαβ NKT cell subsets. J Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez C, Coyle AJ, Gutierrez-Ramos JC. CC chemokine receptor (CCR) 3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med. 2000;191:265–273. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew A, Medoff BD, Carafone AD, Luster AD. Cutting edge: Th2 cell trafficking into the allergic lung is dependent on chemoattractant receptor signaling. J Immunol. 2002;169:651–655. doi: 10.4049/jimmunol.169.2.651. [DOI] [PubMed] [Google Scholar]
- 24.Sen Y, Yongyi B, Yuling H, Luokun X, Li H, Jie X, Tao D, Gang Z, Junyan L, Chunsong H, et al. Vα24-invariant NKT cells from patients with allergic asthma express CCR9 at high frequency and induce Th2 bias of CD3+ T cells upon CD226 engagement. J Immunol. 2005;175:4914 – 4926. doi: 10.4049/jimmunol.175.8.4914. [DOI] [PubMed] [Google Scholar]
- 25.Humbles A, Lu B, Friend D, Okinaga S, Lora J, Algarawi A, Martin T, Gerard N, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA. 2002;99:1479–1487. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goya I, Villares R, Zaballos A, Gutierrez J, Kremer L, Gonzalo JA, Varona R, Carramolino L, Serrano A, Pallares P, et al. Absence of CCR8 does not impair the response to ovalbumin-induced allergic airway disease. J Immunol. 2003;170:2138–2146. doi: 10.4049/jimmunol.170.4.2138. [DOI] [PubMed] [Google Scholar]
- 27.Chung C, Kuo F, Kumar J, Montani A, Lawrence C, Henderson W, Venkataraman C. CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J Immunol. 2003;170:581–587. doi: 10.4049/jimmunol.170.1.581. [DOI] [PubMed] [Google Scholar]
- 28.Baekkevold ES, Wurbel MA, Kivisakk P, Wain CM, Power CA, Haraldsen G, Campbell JJ. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J Exp Med. 2005;201:1045–1051. doi: 10.1084/jem.20041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, Conquet F, Proudfoot AEI, Wells TNC, Power CA. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1763. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuh J, Power C, Proudfoot A, Kunkel S, Lukacs N, Hogaboam C. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4−/− mice. FASEB J. 2002;16:A331. doi: 10.1096/fj.02-0193fje. [DOI] [PubMed] [Google Scholar]
- 31.Pilette C, Francis JN, Till SJ, Durham SR. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J. 2004;23:876 – 884. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- 32.Sekiya T, Yamada H, Yamaguchi M, Yamamoto K, Ishii A, Yoshie O, Sano Y, Morita A, Matsushima K, Hirai K. Increased levels of a TH2-type CC chemokine thymus and activation-regulated chemokine (TARC) in serum and induced sputum of asthmatics. Allergy. 2002;57:173–177. doi: 10.1034/j.1398-9995.2002.5720256.x. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki S, Takizawa H, Yoneyama H, Nakayama T, Fujisawa R, Izumizaki M, Imai T, Yoshie O, Homma I, Yamamoto K, Matsushima K. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J Immunol. 2001;166:2055–2062. doi: 10.4049/jimmunol.166.3.2055. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers CA, Proudfoot AEI, Coyle AJ, Gearing D, Gutierrez-Ramos JC. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- 35.Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, Andrew DP, Wu LJ, Butcher EC, Wardlaw AJ. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–2848. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- 36.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallinich T, Schmidt S, Hamelmann E, Fischer A, Qin S, Luttmann W, Virchow JC, Kroczek RA. Chemokine-receptor expression on T cells in lung compartments of challenged asthmatic patients. Clin Exp Allergy. 2005;35:26–33. doi: 10.1111/j.1365-2222.2004.02132.x. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd CM, Rankin SM. Chemokines in allergic airway disease. Curr Opin Pharmacol. 2003;3:443–448. doi: 10.1016/s1471-4892(03)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukacs NW, Miller AL, Hogaboam CM. Chemokine receptors in asthma: searching for the correct immune targets. J Immunol. 2003;171:11–15. doi: 10.4049/jimmunol.171.1.11. [DOI] [PubMed] [Google Scholar]
- 40.Campbell JJ, Bowman EP, Murphy K, Youngman KR, Siani MA, Thomspon DA, Wu L, Butcher EC. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for MIP-3B receptor CCR7. J Cell Biol. 1998;141:1053–1063. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim CH, Butcher EC, Johnston B. Distinct subsets of human Vα24-invariant NKT cells: cytokine responses and chemokine receptor expression. Trends Immunol. 2002;23:516–519. doi: 10.1016/s1471-4906(02)02323-2. [DOI] [PubMed] [Google Scholar]
- 42.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389 –395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 43.Seino K, Fukao K, Muramoto K, Yanagisawa K, Takada Y, Kakuta S, Iwakura Y, Van Kaer L, Takeda K, Nakayama T, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci USA. 2001;98:2577–2581. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JYT, Ceredig R, Surh CD, Kronenberg M. Homeostasis of Vα14i NKT cells. Nat Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 46.Ranson T, Vosshenrich CAJ, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci USA. 2003;100:2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito K, Yajima T, Kumabe S, Doi T, Yamada H, Sad S, Shen H, Yoshikai Y. Impaired protection against Mycobacterium bovis bacillus Calmette-Guérin infection in IL-15-deficient mice. J Immunol. 2006;176:2496–2504. doi: 10.4049/jimmunol.176.4.2496. [DOI] [PubMed] [Google Scholar]