Abstract
Attempts were made to increase the efficiency of infectious particle formation during the in vitro assembly of bacteriophage T7 from procapsids and DNA. It was found that dextrans and some smaller, related compounds (sucrose and sorbitol) increase this efficiency by a factor of 8 to 50. Dextrans also inhibited elevated temperature-induced emptying of DNA from bacteriophages T7, P22, and T4, suggesting that the stimulation of assembly is caused, at least in part, by the stabilization of packaged DNA in capsids. The data indicated that the sugars and polyols can slow DNA emptying from bacteriophages at elevated temperature whether they permeate the bacteriophage capsid or not. In contrast, the data suggested that permeation of some particle, probably a capsid, results in inhibition of in vitro T7 assembly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F., RAPPAPORT C., MUSCATINE N. A. On the structure and osmotic properties of phage particles. Ann Inst Pasteur (Paris) 1953 Jan;84(1):5–14. [PubMed] [Google Scholar]
- ANDERSON T. F. The morphology and osmotic properties of bacteriophage systems. Cold Spring Harb Symp Quant Biol. 1953;18:197–203. doi: 10.1101/sqb.1953.018.01.030. [DOI] [PubMed] [Google Scholar]
- Benchimol S., Lucko H., Becker A. Bacteriophage lambda DNA packaging in vitro. The involvement of the lambda FI gene product, single-strand DNA, and a novel lambda-directed protein in the packaging reaction. J Biol Chem. 1982 May 10;257(9):5201–5210. [PubMed] [Google Scholar]
- Bjornsti M. A., Reilly B. E., Anderson D. L. Morphogenesis of bacteriophage phi 29 of Bacillus subtilis: DNA-gp3 intermediate in in vivo and in vitro assembly. J Virol. 1982 Feb;41(2):508–517. doi: 10.1128/jvi.41.2.508-517.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L. W. In vitro packaging of bacteriophage T4 DNA. Virology. 1981 Aug;113(1):336–344. doi: 10.1016/0042-6822(81)90160-4. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Gekko K., Timasheff S. N. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry. 1981 Aug 4;20(16):4667–4676. doi: 10.1021/bi00519a023. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Packaging and maturation of DNA of bacteriophage T7 in vitro. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3545–3549. doi: 10.1073/pnas.71.9.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemmerle N. B., Masker W. E. In vitro packaging of UV radiation-damaged DNA from bacteriophage T7. J Virol. 1977 Sep;23(3):509–516. doi: 10.1128/jvi.23.3.509-516.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P., Parsegian V. A. Measurement of forces between lecithin bilayers. Nature. 1976 Feb 19;259(5544):601–603. doi: 10.1038/259601a0. [DOI] [PubMed] [Google Scholar]
- Leibo S. P., Kellenberger E., Kellenberger-van der Kamp C., Frey T. G., Steinberg C. M. Gene 24-controlled osmotic shock resistance in bacteriophage T4: probable multiple gene functions. J Virol. 1979 Apr;30(1):327–338. doi: 10.1128/jvi.30.1.327-338.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibo S. P., Mazur P. Effect of osmotic shock and low salt concentration on survival and density of bacteriophages T4B and T4Bo1. Biophys J. 1966 Nov;6(6):747–772. doi: 10.1016/S0006-3495(66)86693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Serwer P. DNA packaging in vitro by an isolated bacteriophage T7 procapsid. J Virol. 1982 Sep;43(3):1138–1142. doi: 10.1128/jvi.43.3.1138-1142.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman B. M., Nickel B. G. Electrostatic forces in muscle and cylindrical gel systems. Biophys J. 1980 Oct;32(1):49–63. doi: 10.1016/S0006-3495(80)84915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J. I., Fujisawa H., Minagawa T. Biological activity of purified bacteriophage T3 prohead and proheadlike structures as precursors for in vitro head assembly. Virology. 1978 Dec;91(2):283–290. doi: 10.1016/0042-6822(78)90376-8. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., Jarvik V., Botstein D. Encapsulation of phage P22 DNA in vitro. Virology. 1979 Jun;95(2):550–564. doi: 10.1016/0042-6822(79)90508-7. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Wang J. C., Calendar R. In vitro packaging of covalently closed circular monomers of bacteriophage DNA. J Mol Biol. 1975 Nov 5;98(3):465–478. doi: 10.1016/s0022-2836(75)80080-5. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
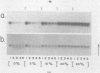
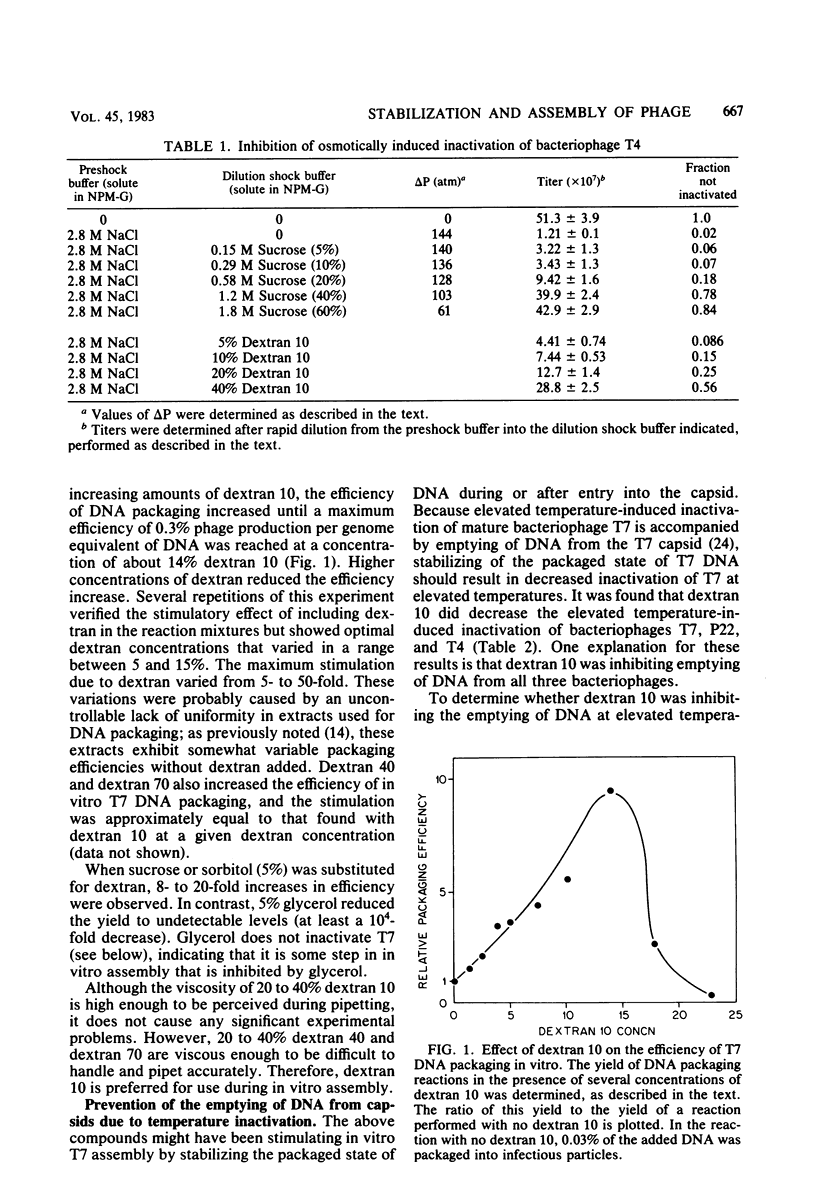
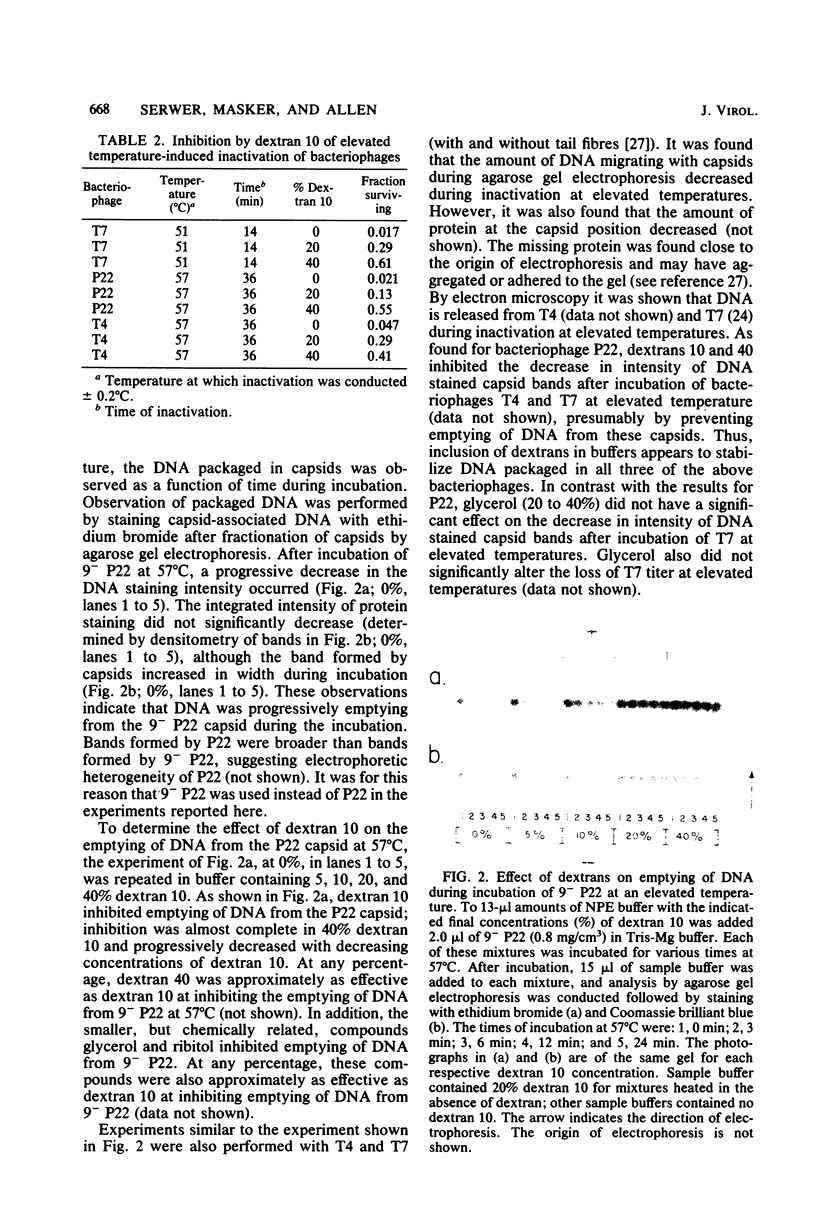
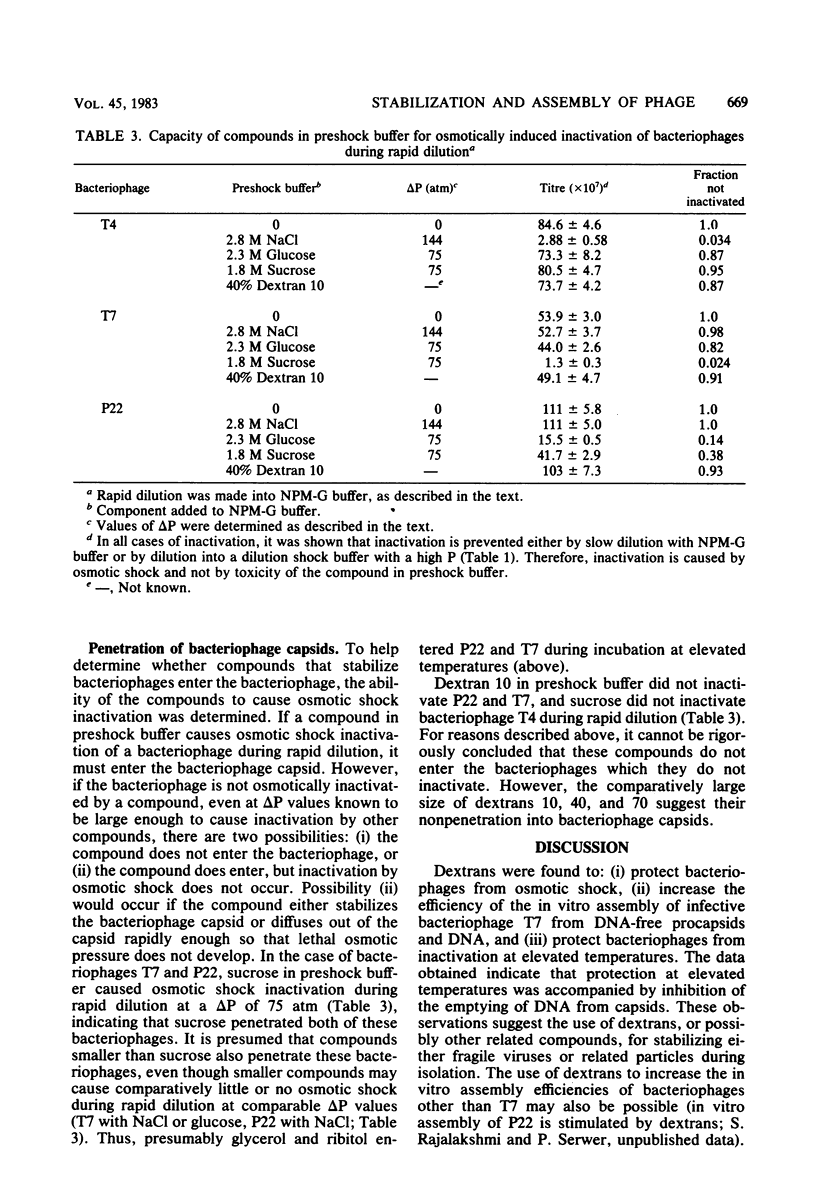
- Serwer P. A metrizamide-impermeable capsid in the DNA packaging pathway of bacteriophage T7. J Mol Biol. 1980 Mar 25;138(1):65–91. doi: 10.1016/s0022-2836(80)80005-2. [DOI] [PubMed] [Google Scholar]
- Serwer P. Buoyant density sedimentation of macromolecules in sodium iothalamate density gradients. J Mol Biol. 1975 Mar 5;92(3):433–448. doi: 10.1016/0022-2836(75)90290-9. [DOI] [PubMed] [Google Scholar]
- Serwer P., Graef P. R., Garrison P. N. Use of ethidium bromide fluorescence enhancement to detect duplex DNA and DNA bacteriophages during zone sedimentation in sucrose gradients: molecular weight of DNA as a function of sedimentation rate. Biochemistry. 1978 Apr 4;17(7):1166–1170. doi: 10.1021/bi00600a005. [DOI] [PubMed] [Google Scholar]
- Serwer P. Internal proteins of bacteriophage T7. J Mol Biol. 1976 Nov 5;107(3):271–291. doi: 10.1016/s0022-2836(76)80005-8. [DOI] [PubMed] [Google Scholar]
- Serwer P., Pichler M. E. Electrophoresis of bacteriophage T7 and T7 capsids in agarose gels. J Virol. 1978 Dec;28(3):917–928. doi: 10.1128/jvi.28.3.917-928.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin S., Parrott C. L. Influence of glycerol and other polyhydric alcohol on the quaternary structure of an oligometric protein. Arch Biochem Biophys. 1975 Feb;166(2):426–432. doi: 10.1016/0003-9861(75)90405-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]