Abstract
Bitter taste–sensing G protein–coupled receptors (type 2 taste receptors [T2Rs]) are expressed in taste receptor cells of the tongue, where they play an important role in limiting ingestion of bitter-tasting, potentially toxic compounds. T2Rs are also expressed in gut-derived enteroendocrine cells, where they have also been hypothesized to play a role in limiting toxin absorption. In this study, we have shown that T2R gene expression in both cultured mouse enteroendocrine cells and mouse intestine is regulated by the cholesterol-sensitive SREBP-2. In addition, T2R stimulation of cholecystokinin (CCK) secretion was enhanced directly by SREBP-2 in cultured cells and in mice fed chow supplemented with lovastatin and ezetimibe (L/E) to decrease dietary sterol absorption and increase nuclear activity of SREBP-2. Low-cholesterol diets are naturally composed of high amounts of plant matter that is likely to contain dietary toxins, and CCK is known to improve dietary absorption of fats, slow gastric emptying, and decrease food intake. Thus, these studies suggest that SREBP-2 activation of bitter signaling receptors in the intestine may sensitize the gut to a low-fat diet and to potential accompanying food-borne toxins that make it past the initial aversive response in the mouth.
Introduction
In mammals, the sense of taste has been divided into 5 different modalities: bitter, sweet, sour, salty, and umami. Of these, bitter perception has a particularly important role in host defense, as it functions as a warning signal against the ingestion of toxic substances through direct taste aversion, induction of the pharyngeal gag reflex, and nausea (1, 2). The recognition of bitter components in the diet is mediated through a family of single-exon GPCRs called type 2 receptors (T2Rs) (2–4). Different mammalian species have varying numbers of functional T2Rs and pseudogenes, and this family is subject to high rates of genetic variation (1, 2). Current genome annotation records suggest there are more than 30 putative functional T2Rs in the mouse genome, while there are somewhat fewer in humans, and most have close homologs in the mouse (5, 6). As predicted by their roles in dietary sensing, T2Rs are prominently expressed in the taste receptor cells (TRCs) of the tongue and surrounding lingual epithelia of the oral cavity (4). Individual T2Rs respond to different bitter tasting agonists, which initiate a signal in the TRC that is relayed through a classic G protein–coupled cascade. The signal relay utilizes a specific Gα subunit called α-gustducin that ultimately activates the gustatory nerve (7–9). In contrast, there is a single heterodimeric GPCR for sweet sensing made up of T1R2 and T1R3 receptor monomers that is also expressed in TRCs (10, 11).
Interestingly, mRNAs for both the sweet and bitter receptors along with α-gustducin are also expressed in the intestinal mucosa, enteroendocrine cells, and pancreas in both humans and rodents (6, 12). Additional studies of cultured lines of enteroendocrine cells indicate T2Rs are expressed and respond to receptor agonists by increasing secretion of cholecystokinin (CCK) (13, 14). Sweet taste receptors are also expressed in enteroendocrine cells, and their expression in the gut has recently been linked to elevated glucagon like peptide–1 (GLP-1) secretion and enhanced glucose uptake (15, 16). These findings suggest that a conserved taste receptor–sensing mechanism exists in the digestive tract that might play important roles in regulation of gastrointestinal peptide secretion, with significant influences on dietary absorption, satiety, and intestinal physiology. One hypothesis suggests that T2Rs in the intestinal tract may stimulate the secretion of gut peptides to limit the absorption of potentially toxic compounds that reach the small intestine despite the initial aversion response through T2R action in the TRCs of the tongue.
SREBPs are basic helix-loop-helix transcription factors and are recognized as key regulators of mammalian lipid homeostasis (17, 18). The 3 known SREBP isoforms are encoded by 2 genes, SREBF1 and SREBF2. SREBP-1a and SREBP-1c are encoded by the SREBF1 gene through the use of different promoters that give rise to alternate first coding exons (18). SREBP-1 preferentially activates genes involved in fatty acid and glucose/insulin metabolism, whereas SREBP-2, encoded by a distinct gene, is more specific for genes of cholesterol metabolism (19).
We recently found that in mice fed a chow diet supplemented with lovastatin and ezetimibe (L/E) to limit dietary sterol absorption and decrease endogenous HMG-CoA reductase activity, nuclear levels of hepatic SREBP-2 are upregulated, while nuclear SREBP-1 expression declines (20). ChIP studies revealed gene-specific binding of SREBP-1 and SREBP-2 isoforms to known SREBP-responsive genes (20). In the current study, we performed a genomic promoter-wide ChIP-chip analysis for SREBP-2 binding to chromatin from livers of L/E-fed mice, which revealed that a number of the T2R promoters were bound by SREBP-2. Further studies showed that T2R gene expression and CCK secretion from both cultured enteroendocrine cells and mouse intestine are under control of SREBP-2. These results suggest that cholesterol regulation and SREBP-2 might be associated with dietary sensing of bitter, potentially toxic compounds. Based on the known roles of CCK in stimulating gall bladder contraction and limiting both food intake and gastric motility, we suggest that SREBPs have evolved as important environmental sensors to coordinate physiological responses to dietary constituents as diverse as essential nutrients and toxins.
Results
T2Rs as novel SREBP-2–responsive genes.
In order to identify novel SREBP-2 target genes, we performed a whole genome promoter ChIP-chip assay with chromatin isolated from livers of mice fed a diet supplemented with L/E. The lovastatin inhibits HMG-CoA reductase, and the ezetimibe blocks dietary cholesterol absorption. The mice respond as though they are cholesterol starved, and nuclear levels of hepatic SREBP-2 increase significantly (20). Using DNA enriched with an SREBP-2 antibody, we interrogated a promoter array containing tiled oligonucleotide probes from more than 25,000 mouse promoters. Interestingly, we found that SREBP-2 bound to several promoters for the T2R family of bitter taste receptor genes. An example of this is presented in Figure 1A, showing that SREBP-2 bound to several adjacent tiled probes from the mT2R138 promoter (top graph). In contrast, use of a control IgG provided no significant enrichment (middle graph). We also used T2R138 promoter–specific primers in a gene-specific quantitative PCR (qPCR) to confirm the microarray results (Figure 1B).
Figure 1. Identification of mT2R138 as novel SREBP-2 target gene.
SREBP-2 ChIP-chip analysis was performed in triplicate using 1.5 kb mouse whole genome promoter arrays from NimbleGen/Roche. (A) Representation of the hybridization of ChIP-enriched DNA to the genomic locus for T2R138 analyzed in the Signal Map software program. The top graph in light blue shows the log2 ratios of hybridization signals for tiled probes for SREBP-2 antibody–enriched ChIP DNA divided by nonenriched input DNA. The middle panel, with green symbols, shows a similar analysis when a control IgG fraction was used as antibody. The T2R138 gene coding sequence is indicated by the dark blue area in the bottom graph. chr6, chromosome 6. (B) Oligonucleotides were designed to amplify the binding region of the T2R138 promoter and used in a gene-specific qPCR to confirm the ChIP-chip result using equal amounts of DNA from the input, SREBP-2 antibody–enriched, or control IgG-enriched samples directly as indicated. Data are mean ± SEM; n = 3 for 3 separate experiments.
SREBP-2 regulates mT2R138 expression in STC-1 cells.
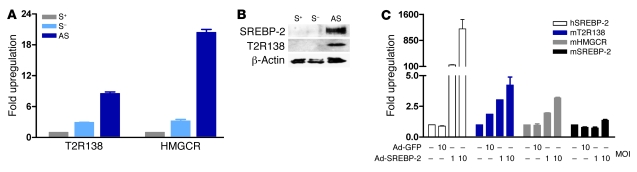
T2Rs are expressed in the taste-sensing tissues of the tongue and oral cavity and have recently been shown to be expressed in the enteroendocrine cells of the gastrointestinal tract (1, 2, 21). To evaluate whether T2R gene expression is modulated in cells and tissues known to express T2Rs, we next turned to analyze whether SREBP-2 might regulate T2R expression in the enteroendocrine STC-1 cell line (Figure 2). Sterol depletion, a standard culture manipulation resulting in increased nuclear accumulation of SREBPs and an increase in SREBP target gene expression (17, 18), specifically increased mRNA levels of SREBP-2 (data not shown) and its well-known target gene, HMG-CoA reductase, in STC-1 cells (Figure 2A). mT2R138 mRNA levels were also increased to a comparable level. In addition, both mT2R138 mRNA and protein levels and the nuclear form of SREBP-2 were dramatically elevated by addition of atorvastatin as well as sterol depletion to limit cholesterol synthesis in the STC-1 cells (Figure 2, A and B). Furthermore, when STC-1 cells were infected with a recombinant adenovirus expressing human SREBP-2, mT2R138 mRNA expression was increased in a dose-dependent manner (Figure 2C).
Figure 2. SREBP-2 induces T2R138 expression in STC-1 cells.
STC-1 cells were plated and incubated for 24 hours in medium containing or lacking additional sterols (12 μg/ml cholesterol and 1 μg/ml 25-hydroxycholesterol) or lacking sterols but with atorvastatin (10 μM) as described in Methods. (A) mRNA levels for SREBP-2 and T2R138 were analyzed by RT-qPCR. (B) Whole cell lysates were analyzed for SREBP-2 or T2R138 protein levels by immunoblotting. Data are representative of 3 separate experiments. (C) STC-1 cells were infected with Ad-hSREBP-2 or Ad-GFP for 24 hours, and mRNA levels of human (virus-encoded) or endogenous mouse SREBP-2, HMG Co-A reductase (HMGCR), and T2R138 were analyzed by RT-qPCR. Data are mean ± SEM; n = 3 for 3 separate experiments. S+, sterols+; S–, sterols–; AS, atorvastatin.
SREBP-2 activates mT2R138 promoter through binding to SRE2.
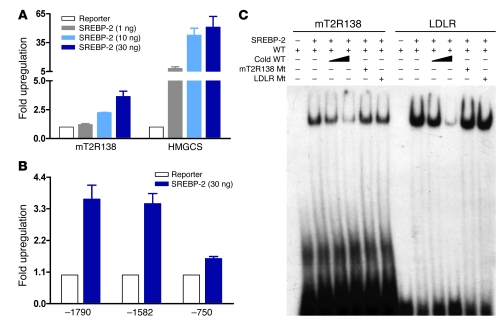
To determine whether there is a direct effect of SREBP-2 on the mT2R138 gene and its promoter, we fused the T2R138 gene 5′ flanking sequence (–1,790 to +119) to luciferase and performed cotransfection assays in 293T cells with an expression vector for SREBP-2. Inclusion of the SREBP-2–expressing construct resulted in a 4-fold increase in T2R138 promoter activity (Figure 3A), and by analyzing a series of promoter deletion constructs, an SREBP-2–responsive region was identified between –1,582 and –750 (Figure 3B). This region harbors sequences with strong homology to the consensus SRE motif (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI36461DS1), and we showed that purified recombinant SREBP-2 bound specifically to a small oligonucleotide probe encompassing this region (Figure 3C).
Figure 3. SREBP-2 activates mT2R138 promoter in transient assays.
(A) 293T cells in 24-well microplates were cotransfected with –1,790 mT2R138 reporter and increasing amounts of the empty vector (pcDNA 3.1) or SREBP-2 expression construct as described in Methods, and the luciferase activities were normalized to β-gal activity. (B) 293T cells were cotransfected with a deletion series of T2R138 promoter constructs (–1,580 and –750) along with the empty vector or SREBP-2 expression construct (30 ng). Data are mean ± SEM; n = 3 for 5 separate experiments. (C) Purified recombinant SREBP-2 was used in an EMSA assay with 32P-labeled probe containing the putative SRE2 of mT2R138 promoter. Data are representative of 3 separate experiments. HMGCS, HMG Co-A synthase; LDLR, LDL receptor; Mt, mutant.
SREBP-2 regulates CCK secretion through bitter taste signaling.
Treatment of STC-1 cells with T2R agonists results in a rise in intracellular Ca2+, followed by an increase in gut-derived peptide secretion (13, 21). Based on our studies so far, we hypothesized that SREBP-2 might regulate signaling through T2Rs, and because we have focused so far on mT2R138, we chose to evaluate signaling through its known agonist, phenylthiocarbamide (PTC). As shown in Figure 4, PTC stimulates the secretion of CCK in STC-1 cells, and this is enhanced when the cells are cultured in sterol-free medium to induce SREBP-2. The sterol-dependent increase in secretion was blunted when EGTA was added to prevent the increase in intracellular Ca2+ (Figure 4A). We also showed that sterol depletion resulted in an increase in the PTC-dependent secretion of GLP-1, another gut-derived peptide, from STC-1 cells (Figure 4B).
Figure 4. Bitter taste agonist signaling stimulates secretion of gut peptides CCK (A) and glucagon-like peptide–1 (GLP-1) (B) from STC-1 cells is SREBP-2 responsive.
STC-1 cells were cultured in the absence or presence of sterols (12 μg/ml cholesterol and 1 μg/ml 25-hydroxycholesterol), and after 24-hour incubation, medium was removed and cultures were rinsed with HBSS. Cells were pretreated in the absence or presence of 1.25 mM EGTA (for block of calcium flux) for 2 minutes as indicated, and then PTC was added to the cultured STC-1 cells at different concentrations for 45 minutes at 37°C. Secreted levels of CCK and GLP-1 were measured in the culture media by an EIA assay. Data are mean ± SEM; n = 5 for 6 separate experiments.
To more directly evaluate T2R138 and SREBP-2 in the PTC stimulation of CCK secretion in STC-1 cells, we treated cells with an siRNA that targets mT2R138 mRNA or a dominant-negative (DN) SREBP adenovirus that decreases SREBP activity (Figure 5). In this experiment, sterol depletion increased mT2R138 mRNA levels and stimulated PTC-dependent secretion of CCK as in Figure 4, and both were significantly blunted by either an siRNA directly targeting T2R138 expression or the DN-SREBP adenovirus (Figure 5).
Figure 5. CCK secretion in STC-1 cells is regulated by T2R138 agonist through SREBP-2.
(A) STC-1 cells in 12-well microplates were transfected with an siRNA targeting mT2R138 or control siRNA (10 nM). Forty-eight hours after transfection, medium was removed and cultures were rinsed with PBS, and cells were cultured in the absence or presence of sterols (12 μg/ml cholesterol and 1 μg/ml 25-hydroxycholesterol); where indicated, atorvastatin (1 μM) was included. After 24-hour incubation, cells were treated with PTC (10 mM final concentration) for 45 minutes. The media and cells were collected and analyzed for CCK by EIA or RNA levels by RT-qPCR. (B) STC-1 cells in 6-well microplates were infected with Ad-DN-SREBP or Ad-GFP (10 MOI) for 24 hours and analyzed as in A. Data are mean ± SEM; n = 5 for 5 separate experiments. §P < 0.05, †P < 0.01, #P < 0.001, *P = NS versus control. NCi, negative control siRNA.
Localization of mT2R138 and regulation of mT2Rs in mouse small intestine.
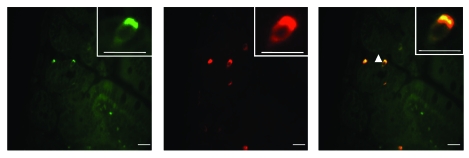
T2R gene expression has been detected in RNA isolated from the small intestine, but the localization of T2R protein has not been reported; however, its presumed location is enteroendocrine cells. To evaluate the localization of mT2R138 receptor in the mouse small intestine, tissue sections were prepared from the mouse proximal intestine and incubated with antibodies against T2R138 and chromogranin A, a well-established marker for enteroendocrine cells (22, 23). Consistent with previously published results (15, 24, 25), chromogranin A was detected by immunofluorescence in a small fraction of cells with morphology expected for enteroendocrine cells, and this was coincident with cells expressing T2R138 (Figure 6).
Figure 6. Colocalization of T2R138 and chromogranin A in the mouse small intestine.
Tissue sections from mouse proximal intestine were embedded in paraffin and analyzed for expression of the T2R138 taste receptor protein (green) and chromogranin A protein (red) by immunofluorescence (scale bars: 10 μm). T2R138 colocalized with chromogranin A as shown in the merged image (yellow), and the two proteins localized at the apical membrane (triangle) in a small fraction of total cells.
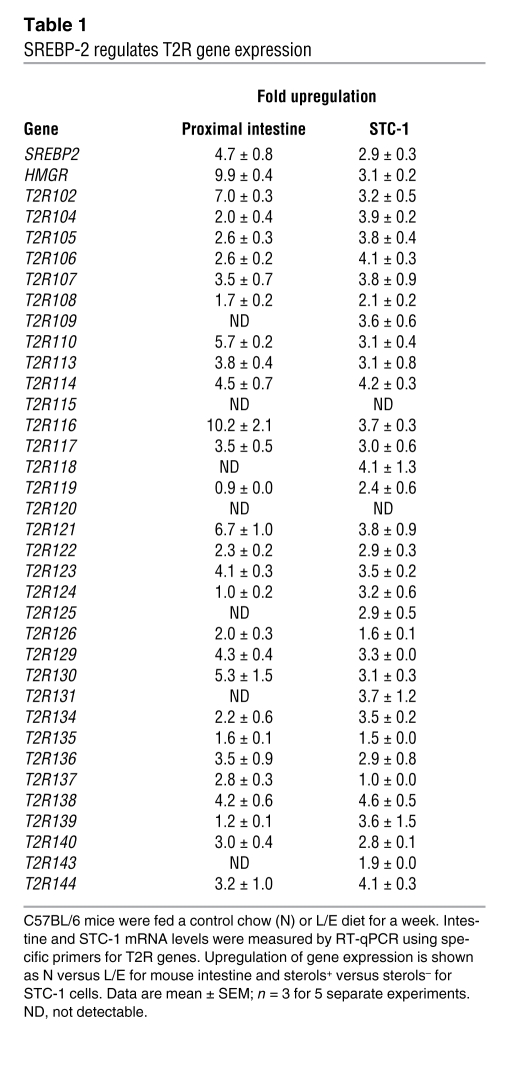
We next evaluated whether SREBP-2 regulates mT2R gene expression and activity in the intact animal. For this analysis, we measured mRNA levels for 34 identified mouse T2R genes in both STC-1 cells and RNA prepared from tissues of mice fed either a control or L/E-supplemented diet. As shown in Table 1, expression of most T2R genes was upregulated when SREBP-2 levels were increased in both the sterol-depleted STC-1 cells and the proximal small intestine from L/E-treated versus control mice. In contrast, T2R gene expression in the tongue was not induced by the L/E feeding, and most T2Rs were either not expressed or displayed only minor variation in response to the L/E treatment in the distal small intestine (Supplemental Table 1).
Table 1 .
SREBP-2 regulates T2R gene expression
SREBP-2 regulates bitter tastant–elicited plasma CCK secretion.
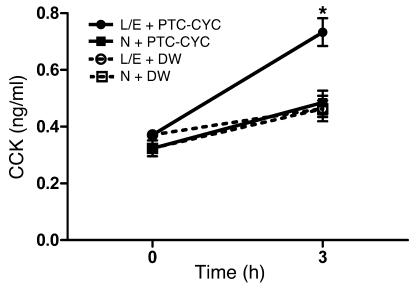
To determine whether the T2R signaling mechanism we studied above in STC-1 cells is also functional and possibly regulated by SREBP-2 in the mouse gastrointestinal tract, a mixture of PTC and cycloheximide (CYC), T2R agonists that target 2 different T2Rs, was introduced into control and L/E-fed mice through oral gavage, and plasma levels of CCK were measured 3 hours later. As shown in Figure 7, plasma levels of CCK were significantly elevated in the agonist-treated animals of the L/E-fed group.
Figure 7. Stimulation of CCK secretion in response to oral gavage with T2R agonists is increased by dietary upregulation of SREBP-2.
C57BL/6 mice were fed a control chow diet (N) or a chow diet supplemented with L/E as described in Methods, and then a mixture of T2R agonists (PTC at 30 mg/kg BW and cycloheximide [CYC] at 10 mg/kg BW) was introduced through oral gavage. Plasma CCK levels were measured 3 hours later. Data are mean ± SEM; for n = 5 for 5 separate experiments. *P < 0.05 relative to N-fed mice (3 hours). DW, distilled water.
Discussion
Bitter tasting agonist signaling through the T2Rs in the tongue represents an evolutionarily conserved aversive response to prevent the ingestion of bitter-tasting dietary toxins. T2Rs are also expressed in endocrine cells of the mammalian gut (6, 21), where they have been hypothesized to stimulate gut hormone secretion to prevent the absorption of potentially toxic compounds that are swallowed despite their bitter taste in the mouth. The T2Rs are unique single-exon GPCRs; there are 25 predicted functional receptors in the human genome, and while the mouse has homolog of all 25, there are approximately 10 additional functional T2Rs in the mouse genome (1).
Bitter tasting agonist binding to T2Rs activates a signaling cascade through a selective Gα subunit, α-gustducin, leading to the stimulation of phospholipase Cβ2 (PLCβ2) (26). In the tongue, activation of PLCβ2 leads to production of the 2 intracellular messengers inositol-1,4,5-trisphosphate and diacylglycerol and ultimately leads to the gating of the transient receptor potential protein and activation of the gustatory nerve (26–28). In enteroendocrine cell lines, bitter tasting agonist signaling increases intracellular Ca2+ concentrations and stimulated CCK secretion (13, 21). Moreover, gastric infusion of the bitter tasting agonist denatonium benzoate delayed gastric emptying and elicited aversion to a flavored drink in rats (29), and because CCK delays gastric emptying and decreases food intake, it could play a direct role in the gut sensing mechanism to help limit the absorption of diet-derived bitter tasting toxins (30).
In this study, we identified promoters for T2R genes as novel binding targets of the cholesterol-sensitive SREBP-2 transcription factor. At first, the physiological relevance of this binding was unclear, as our ChIP-chip analysis was performed on liver tissue where expression of T2Rs was undetectable. However, we showed that SREBP-2 activates T2R gene expression in the enteroendocrine cell line STC-1, and agonist-responsive signaling through one receptor, T2R138, was enhanced in an SREBP-2–dependent fashion. We also demonstrated that T2R138 localized to enteroendocrine cells in the proximal intestine, where mRNA levels for many of the individual T2Rs were increased when mice were fed a diet supplemented with L/E, which increases nuclear SREBP-2 activity. Additionally, when a mixture of T2R agonists was introduced into the gastrointestinal tract of mice directly by oral gavage, plasma levels of CCK were elevated in the L/E treatment group.
Taken together with other published observations, our data indicate SREBP-2 might be associated with dietary sensing of bitter-tasting constituents of food that are potentially toxic. We suggest that the increased SREBP-2 activity associated with a low-cholesterol diet may be important to increase the sensitivity of the intestinal T2R signaling system. This would make the gut more responsive to the presence of potentially toxic compounds that escape the initial aversive response in the mouth.
In considering this possibility, it is interesting to note that a naturally low-cholesterol diet is high in plant material, which is inherently richer in bitter and potentially toxic compounds relative to a high-cholesterol diet composed of significant amounts of animal flesh. Thus, on a low-cholesterol diet, there is an increased need to absorb the relatively small amounts of essential fats and fat-soluble vitamins that are present and to prevent the absorption of potentially toxic/bitter substances in plant-derived foods (31–33).
The well-described hormonal actions of CCK are consistent with a role in limiting absorption of dietary toxins while maximizing absorption of essential lipid components that are only provided in limited amounts when ingesting a plant-enriched diet (30, 34). CCK both reduces appetite and slows gastric emptying, which would on the one hand reduce further consumption of the toxic meal and, on the other, ensure that the food that does make it into the stomach has more time to be expelled through emesis, a protective response that is also induced by bitter receptor signaling. Additionally, CCK stimulates gall bladder contraction, and the associated increase in bile acids entering the intestine may aid in the breakdown and absorption of complex carbohydrates and other plant matter that is difficult to digest. This would also facilitate the absorption of low levels of essential fats and fat-soluble vitamins. Both the T2Rs and SREBP pathway are conserved in humans, so it is likely that the mechanism described here is relevant to human physiology as well. Since the combination drug Vytorin, which is a mixture of ezetimibe and a statin, is being widely used to treat hypercholesterolemia in humans, it might be possible to evaluate the link between SREBP-2 and T2Rs in humans.
SREBPs were first identified as key regulators of cholesterol and fatty acid metabolism in mammals (35, 36). Subsequent studies indicate that they are more globally involved in cell-environment interactions, including apoptosis in response to bacterial toxin (37) and phagocytosis (38). In fission yeast, SREBPs have evolved to sense oxygen levels (39) and Cph2, a bHLH protein closely related to SREBPs, is involved in pseudohyphal growth in Candida albicans (40), which occurs in response to environmental cues. Taken together with our current studies, these observations indicate that SREBPs have evolved to play a fundamental role across the Eukarya in the interactions between organisms and their environment.
Methods
ChIP-chip promoter array.
ChIP assays from mouse liver were performed as previously described (20). To prepare samples for the ChIP-chip array, after reversing the crosslinking and isolating the ChIP-enriched DNA, gene-specific enrichment for HMG-CoA reductase and LDL receptor promoters in the L/E chromatin relative to chow control chromatin was confirmed, and the SREBP-2, IgG control, and input DNA were prepared for hybridization to the 1.5-kb mouse promoter array (NimbleGen/Roche) using a random PCR amplification protocol (41). The hybridization was analyzed by the Signal Map software program from NimbleGen/Roche.
The qPCR primers for the mouse promoters were as follows: HMG-CoA reductase, sense, 5′-GCTCGGAGACCAATAGGA-3′, antisense, 5′-CCGCCAATAAGGAAGGAT-3′; LDL receptor, sense 5′-GAACTTCCCACTGCTGC-3′, antisense, 5′-CACGCCCAGAGTCATTC-3′; T2R138, sense, 5′-TGTCAGCAAGTGCTTCTTGG-3′, antisense, 5′-GGGCTACCCACTCATTCAAA-3′.
Plasmids.
The mouse mT2R138 promoter constructs (–1,780 to +119 and 5′ deletions) were cloned by PCR amplification using mouse genomic DNA as template, followed by recombination with the pDONR201 vector according to Gateway technology (Invitrogen). The mT2R138 construct was transferred by Gateway technology (Invitrogen) into the luciferase reporter vector p-LUC-GW kindly provided by J. Imbert (Institut Paoli-Calmettes, Marseille, France). All constructs were verified by DNA sequencing. The plasmids, 2×flag pCDNA3.1+ SREBP-2 and pSynSRE-positive control SREBP reporter, have been previously described (42).
Transient transfection in 293T cells.
293T cells (2 × 105 cells/well) seeded in 24-well plates were transfected with luciferase reporter and expression plasmids using Lipofectamine 2000 reagent (Invitrogen). A pCMV–β-gal expression construct was included in every transfection as a normalization control. Twenty-four hours after transfection, cells were harvested and assayed for luciferase and β-gal activities.
siRNA transfection and adenovirus infection in STC-1 cells.
STC-1 cells (ATCC) were maintained in DMEM supplemented with 10% FBS and antibiotics (100 U/ml penicillin plus 50 mmol/l streptomycin) in an atmosphere of 5% CO2 at 37°C. The siRNA targeting mT2R138 (GenBank accession number NM_001001451) and nontargeting siRNA control were purchased from Dharmacon (siGENOME ON-TARGETplus SMART pool L-067135-01-0005 for T2R138). The cells (2 × 105 cells/well) seeded in 12-well plates were transfected for 48 hours with 10 nM of each siRNA using RNAiMAX reagent (Invitrogen) according to the manufacturer’s instructions, and dishes were washed and refed with media with or without sterols (12 μg/ml cholesterol and 1 μg/ml 25-hydroxycholesterol) and with or without atorvastatin, as described previously (43). Cells were harvested 24 hours later. For adenovirus infection, STC-1 cells (1 × 106 cells/well) seeded in 6-well plates were infected with Ad-hSREBP-2 (I. Shechter, Uniformed Services University, Bethesda, Maryland, USA), Ad-DN-SREBP (J.B. Kim, Seoul National University, Seoul, Republic of Korea), or Ad-GFP (10 MOI) for 24 hours and collected for analysis after a further 24-hour incubation.
RNA analysis.
Total RNA was extracted from STC-1 cells or mouse tongue or intestine with TRIzol (Invitrogen) and QIAGEN RNeasy isolation kits (QIAGEN) and used for RT-qPCR with an iQ5 Real-Time PCR Detection System (Bio-Rad). mRNA levels were normalized for expression of ribosomal protein L32 mRNA as control and calculated by the comparative threshold cycle method.
Immunoblotting.
Cells were washed PBS and centrifuged at 1,000 g for 5 minutes at 4°C. Pellets were resuspended in lysis buffer (50 mM HEPES-KOH at pH 7.6, 100 mM NaCl, 1.5 mM MgCl2, 1% Nonidet P-40, and 10 μg/ml leupeptin, 5 μg/ml pepstatin, 25 μg/ml N-acetyl-leucinal-leucinal-norleucinal, and 2 μg/ml PMSF), rotated at 4°C for 30 minutes, and centrifuged at 20,000 g for 20 minutes at 4°C. The supernatants were separated by 10% (v/v) SDS-PAGE and then transferred to nitrocellulose membranes and subjected to immunoblotting using the polyclonal goat anti-mT2R138 (F-14; Santa Cruz Biotechnology Inc.), a polyclonal rabbit anti-mouse SREBP-2, and β-actin (Sigma-Aldrich). The blots were visualized using ECL detection reagents (GE Healthcare).
CCK secretion from STC-1 cells.
Cells were rinsed with HBSS (Invitrogen) supplemented with 20 mM HEPES, and 0.1% BSA and PTC (Sigma-Aldrich) or EDTA dissolved in HBSS were then added to the culture. Cells were then incubated at 37°C for 45 minutes. The medium was collected and centrifuged at 4°C for 5 minutes at 1,000 g to remove cell debris, and the supernatants were stored at –20°C. CCK was measured by ELISA (Phoenix Pharmaceuticals Inc.).
Animals and gavage administration of T2Rs agonists.
Six-week-old male C57BL/6 mice were purchased from The Jackson Laboratory and fed a normal rodent chow diet and allowed to adapt for 2 weeks to a 12-hour light/12-hour dark cycle. Mice were separated into 2 groups of 4–6 animals per group. One was maintained on a normal chow diet (N) and the other group (L/E) was fed normal chow supplemented with a mixture of lovastatin (100 mg lovastatin [2.5 tablet equivalents]/100 g chow, w/w; Mylan) and ezetimibe (from MERCK/Schering-Plough Pharmaceuticals; 21 mg ezetimibe [2.1 tablet equivalents]/100 g chow, w/w). After 7 days on this diet, a mixture of PTC and CYC (Sigma-Aldrich) in sterilized distilled water was administered by oral gavage to one group of mice on each diet, and distilled water was delivered to control groups. All manipulations were staggered so that animals were all sacrificed at the same time by CO2 asphyxiation at the end of the dark cycle (8 a.m.). For RNA isolation, tongue, liver, and intestine were collected. Blood samples were collected from the heart before (0 hours) and after gavage (3 hours), and plasma CCK concentration was measured by ELISA (Phoenix Pharmaceuticals Inc.). All results reported here were from experiments repeated at least twice, and 2 independent feeding studies were performed, with similar results across all experimental measurements. Animal experiments were approved by the University of California, Irvine IACUC (protocol 97-1545).
Immunohistochemistry.
Sections of paraffin-embedded jejunum (10 μm thick) were dewaxed in xylene and rehydrated, and antigen retrieval was performed by heating of sections with 10 mM citrate buffer, pH 6, for 15 minutes in a microwave. Sections were washed in PBS and blocked for 30 minutes in 1% BSA, 0.02% Triton X-100, and 10% goat serum. Serial sections were incubated with both primary antibodies against goat polyclonal T2R138 (1:100; Santa Cruz Biotechnology Inc.) and rabbit polyclonal chromogranin A (1:100; Abcam), washed in PBS 3 times for 15 minutes, and incubated with Alexa Fluor 488–conjugated donkey anti-goat secondary antibody (Invitrogen) and Cy3-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories Inc.). The sections were washed in PBS 3 times for 15 minutes and mounted by VECTASHIELD (Vector laboratories). Images from serial sections were acquired by an inverted microscope Axioskop using the AxioVision camera and software (Zeiss).
Statistics.
The data are presented as mean ± SEM. Differences between the means of the individual groups were assessed by 1-way ANOVA with Duncan’s multiple range tests for the results shown in Figure 5; differences were considered significant at P < 0.05. Student’s t test was used to compare the chow (N) versus L/E groups; significance was determined at P < 0.05. The statistical software package Prism 5.0 (GraphPad Software) was used for these analyses.
Supplementary Material
Acknowledgments
We thank Y.K. Seo for assistance and Seo and other laboratory members for helpful discussions. We also thank Jean Imbert for the p-LUC-GW construct, Jae-Bum Kim for the DN adenovirus, Ishiahu Shechter for the Ad-SREBP-2 vector, and Naomi Morrissette and her laboratory members for assistance with microscopy. This work was supported by a grant from the NIH to T.F. Osborne (HL48044).
Footnotes
Nonstandard abbreviations used: CCK, cholecystokinin; DN, dominant-negative; L/E, lovastatin and ezetimibe; PTC, phenylthiocarbamide; qPCR, quantitative PCR; T2R, type 2 taste receptor; TRC, taste receptor cell.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3693–3700 (2008). doi:10.1172/JCI36461
Bing Zhu’s present address is: Department of Pathology, University of Texas Medical Branch, Galveston, Texas, USA.
References
- 1.Bachmanov A.A., Beauchamp G.K. Taste receptor genes. Annu. Rev. Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrashekar J., Hoon M.A., Ryba N.J., Zuker C.S. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 3.Adler E., et al. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/S0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 4.Chandrashekar J., et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/S0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 5.Matsunami H., Montmayeur J.P., Buck L.B. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 6.Wu S.V., Chen M.C., Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol. Genomics. 2005;22:139–149. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 7.Hoon M.A., Northup J.K., Margolskee R.F., Ryba N.J. Functional expression of the taste specific G-protein, alpha-gustducin. Biochem. J. 1995;309:629–636. doi: 10.1042/bj3090629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin S.K., McKinnon P.J., Margolskee R.F. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 9.Wong G.T., Gannon K.S., Margolskee R.F. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 10.Li X., et al. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson G., et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/S0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 12.Hofer D., Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem. Cell Biol. 1998;110:303–309. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 13.Chen M.C., Wu S.V., Reeve J.R., Jr., Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am. J. Physiol. Cell Physiol. 2006;291:C726–C739. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- 14.Sternini C., Anselmi L., Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang H.J., et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mace O.J., Affleck J., Patel N., Kellett G.L. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J. Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown M.S., Goldstein J.L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 18.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. . J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborne T.F. Sterol regulatory element-binding proteins (SREBPs): key regulators of nutritional homeostasis and insulin action. J. Biol. Chem. 2000;275:32379–32382. doi: 10.1074/jbc.R000017200. [DOI] [PubMed] [Google Scholar]
- 20.Bennett M.K., Seo Y.K., Datta S., Shin D.J., Osborne T.F. Selective Binding of sterol regulatory element-binding protein isoforms and co-regulatory proteins to promoters for lipid metabolic genes in liver. J. Biol. Chem. 2008;283:15628–15637. doi: 10.1074/jbc.M800391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S.V., et al. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facer P., et al. Chromogranin: a newly recognized marker for endocrine cells of the human gastrointestinal tract. Gastroenterology. 1985;89:1366–1373. doi: 10.1016/0016-5085(85)90657-2. [DOI] [PubMed] [Google Scholar]
- 23.Portela-Gomes G.M., Stridsberg M., Johansson H., Grimelius L. Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J. Histochem. Cytochem. 1997;45:815–822. doi: 10.1177/002215549704500606. [DOI] [PubMed] [Google Scholar]
- 24.Margolskee R.F., et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozengurt N., et al. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- 26.Rossler P., Kroner C., Freitag J., Noe J., Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur. J. Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 27.Huang L., et al. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat. Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 28.Perez C.A., et al. A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 29.Glendinning J.I., Yiin Y.M., Ackroff K., Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol. Behav. 2008;93:757–765. doi: 10.1016/j.physbeh.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liddle R.A. Cholecystokinin cells. Annu. Rev. Physiol. 1997;59:221–242. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- 31.Bazzano L.A., Serdula M.K., Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr. Atheroscler. Rep. 2003;5:492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 32.Dauchet L., Amouyel P., Hercberg S., Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J. Nutr. 2006;136:2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 33.Drewnowski A., Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr. 2000;72:1424–1435. doi: 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- 34.Chandra R., Liddle R.A. Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 2007;14:63–67. doi: 10.1097/MED.0b013e3280122850. [DOI] [PubMed] [Google Scholar]
- 35.Tontonoz P., Kim J.B., Graves R.A., Spiegelman B.M. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama C., et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 37.Gurcel L., Abrami L., Girardin S., Tschopp J., van der Goot F.G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Castoreno A.B., et al. Transcriptional regulation of phagocytosis-induced membrane biogenesis by sterol regulatory element binding proteins. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13129–13134. doi: 10.1073/pnas.0506716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todd B.L., Stewart E.V., Burg J.S., Hughes A.L., Espenshade P.J. Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Mol. Cell. Biol. 2006;26:2817–2831. doi: 10.1128/MCB.26.7.2817-2831.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane S., Zhou S., Pan T., Dai Q., Liu H. The basic helix-loop-helix transcription factor Cph2 regulates hyphal development in Candida albicans partly via TEC1. Mol. Cell. Biol. 2001;21:6418–6428. doi: 10.1128/MCB.21.19.6418-6428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robyr D., Kurdistani S.K., Grunstein M. Analysis of genome-wide histone acetylation state and enzyme binding using DNA microarrays. Methods Enzymol. 2004;376:289–304. doi: 10.1016/S0076-6879(03)76019-4. [DOI] [PubMed] [Google Scholar]
- 42.Toth J.I., Datta S., Athanikar J.N., Freedman L.P., Osborne T.F.2004Selective coactivator interactions in gene activation by SREBP-1a and -1c. Mol. Cell. Biol. 248288–8300. . 10.1128/MCB.24.18.8288-8300.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenfeld J.M., Osborne T.F. HLH106, a Drosophila sterol regulatory element-binding protein in a natural cholesterol auxotroph. J. Biol. Chem. 1998;273:16112–16121. doi: 10.1074/jbc.273.26.16112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.