Abstract
Expressions of activation markers have been described on the surface of T cells in the blood and the lung in both health and disease. We have studied the distribution of activation markers on human lung T cells and have found that only certain populations exist. Importantly, the presence or absence of some markers appears to predict those of others, in particular cells which express CD103 also express CD49a and CD69, whereas cells which do not express CD69 also do not express CD49a or CD103.
In view of the paucity of activation marker expression in the peripheral blood, we have hypothesised that these CD69+, CD49a+, and CD103+ (triple positive) cells are retained in the lung, possess effector function (IFNγ secretion) and express particular chemokine receptors which allow them to be maintained in this environment.
We have found that the ability of the triple negative cells to secrete IFNγ is significantly less than the triple positive cells, suggesting that the expression of activation markers can highlight a highly specialised effector cell. We have studied the expression of 14 chemokine receptors and have found that the most striking difference between the triple negative cells and the triple positive cells is the expression of CXCR6 with 12.8±9.8% of triple negative cells expressing CXCR6 compared to 89.5±5.5% of triple positive cells.
We propose therefore that CXCR6 may play an important role in the retention of T cells within the lung.
Keywords: Cell trafficking, Chemokines, Human, Lung, T lymphocytes
Introduction
Human memory T cells re-circulate from blood to tissue via the lymphatic system as part of a surveillance mechanism to increase the chance of encountering the antigen to which they have been sensitised. Memory T cells preferentially return to the organ in whose associated lymph nodes they first encountered antigen, a process known as T cell homing (Butcher et al., 1999). This process is directed by organ specific expression of adhesion receptors and chemokines. Specific pathways have been described for secondary lymphoid tissue, skin and small intestine (Forster et al., 1999; Kunkel et al., 2000; von Andrian and Mackay, 2000; Reiss et al., 2001). As yet, markers which identify cells programmed to return to the lung have not been identified, although these cells express a distinct phenotype of adhesion and chemokine receptors (Campbell et al., 2001).
The lung is an important tertiary lymphoid organ which contains large numbers of T cells distributed between various intercommunicating compartments (Wardlaw, 2002). Lung CD3+ T cells are >95% CD45RO+ and a variable but high proportion of both CD4+ and CD8+ T cells express activation markers. Activation markers were initially described on lymphocytes stimulated in vitro with mitogens and antigen. These have been divided into three groups according to their appearance on stimulated cells. Early activation markers such as CD69 and CD25 appear within hours of stimulation in contrast to the very late activation markers (CD49a), which appear only after weeks of stimulation; other markers fall in between these two extremes (e.g. CD103).
In contrast to tissue T cells, there is little expression of activation markers on peripheral blood T cells in either health or disease (Corrigan et al., 1988; Ancochea et al., 1993). The kinetics of migration of T cells from the lung mucosa to the draining lymph nodes is not known with any certainty but based on other tissues is probably in the order of 24 h (Pabst and Binns, 1995). However, the delayed up-regulation of some activation markers suggests that those cells which express activation markers cannot be re-circulating very rapidly and therefore must be retained within the lung for significant periods (Saltini et al., 1988). Evidence that such a tissue resident population may exist is highlighted by the finding that donor lymphocytes remain present in transplanted human lungs up to 34 weeks post procedure (Fung et al., 1985). The presence of activation markers on lung T cells also implies that they have been stimulated and activation markers may represent a footprint of a cell which has encountered its target antigen, and is therefore at a heightened level of immune surveillance.
We therefore hypothesised that T cells expressing activation markers are resident within the lung whereas those which do not express activation markers, are more likely to be trafficking rapidly to the mediastinal lymph nodes having failed to encounter either cognate antigen or other activating signals. In support of this, we found that lung T cells expressing activation markers expressed more IFNγ and that these cells co-expressed the chemokine receptor CXCR6 which could be involved in lung T cell retention.
Materials and methods
Preparation of human lung filtrate
Fresh human lung specimens were obtained from patients undergoing lung resection for predominantly non-small cell carcinoma of the lung at Glenfield Hospital, Leicester, UK. All patients gave written consent for the study and the study was approved by the Leicestershire, Northamptonshire & Rutland Research Ethics Committee. The specimen was taken from macroscopically normal lung at some distance from the tumour and minced with fine scissors. The resulting suspension was then filtered through 100 μm gauze to remove large particles. The number of cells obtained depended on the amount of lung received, but was in the region of 107 cells per gram of lung tissue and a minimum of 5 g of lung tissue was used. The number of lymphocytic cells in each sample of lung filtrate varied from subject to subject but was generally in the region of 5%. The filtrate obtained was left overnight at 37 °C in large petri dishes to remove contaminating alveolar macrophages (AM) by adherence. The following day after washing with phosphate buffered saline (PBS; Invitrogen Life Technologies, Paisley, UK)/0.5% bovine serum albumin (BSA; Sigma) (PBS/BSA), red cell lysis was carried out by osmotic shock. Lung T cells from 24 patients were used for the experiments in this paper. The individual numbers of subjects used for each experiment are detailed in the figure legends.
Labelling cells for activation markers, intracellular cytokine and chemokine receptors for three colour flow cytometry
The antibodies used for flow cytometry are shown in Table 1. We used a combination of direct and indirect labelling approaches depending on the study design for each experiment and the antibodies that were available either commercially or as gifts within the limitations of three colour flow cytometry. Non-specific binding was eliminated in the lung filtrate cells by incubation with 10% fetal calf serum (Sigma) for 15 min. For the studies of the expression of the activation receptors described in Fig. 1, cells were labelled with a monoclonal mouse anti-human activation marker antibody (either CD69, CD103 or CD49a), which was then visualised by fluorescein (FITC) labelled secondary rabbit anti-mouse antibody (DAKO Cytomation). After blocking with 10% mouse serum (Sigma), cells were stained for CD3 PerCP and CD8 PE. For activation marker co-expression experiments, in the same way, the first marker was labelled indirectly with FITC followed by a second R-phycoerythrin (PE) labelled mouse anti-human activation marker antibody (either CD69 PE, CD103 PE or CD49a PE) and CD3 PerCP.
Table 1.
| Isotype | Final concentration (mg/ml)a | Supplier | Clone | |
|---|---|---|---|---|
| CCR | ||||
| 1 | IgG2b | 20 | R&D | 53504.111 |
| 2 | IgG2b | 20 | R&D | 48607.121 |
| 3 | IgG2a | 6.35 | Millenium | 7B11 |
| 4 | IgG1 | 9 | Millenium | 1G1 |
| 5 | IgG2a | 10 | BD Pharmingen | 2D7/CCR5 |
| 6 | IgG2b | 5 | R&D | 53103.111 |
| 7 | IgG2b | 8.9 | Millenium | 7H12- (12-2) lot |
| 9 | IgG2a | 11 | Millenium | GPR-96-1 |
| 10 | IgG2a | 10 | Millenium | IB 508070 Pool#1
F13-20 |
| CXCR1 | IgG2a | 10 | R&D | 42705.111 |
| CXCR2 | IgG2a | 10 | R&D | 48311.211 |
| CXCR3 | IgG1 | 10 | R&D | 49801.111 |
| CXCR4 | IgG2b | 10 | R&D | 44716.111 |
| CXCR6 | IgG2b | 10 | R&D | 56811 |
| Cytokine | ||||
| IFN PE | IgG1 | 4 | BD Pharmingen | B27 |
| Surface marker | ||||
| CD3PerCP | IgG1 | 1.25 | BD | SK-7 |
| CD3 ECD | IgG1 | 1:32 dilution | Immunotech | UCHT1 |
| CD8 PE | IgG1 | 5 | DAKO | DK25 |
| CD8 APC Cy7 | IgG2a | 1:32 dilution | Caltag | 3B5 |
| Activation marker | ||||
| CD69 | IgG1 | 22.5 | DAKO | FN50 |
| CD69PE | IgG1 | 10 | DAKO | FN50 |
| CD69 APC | IgG1 | 1:4 dilution | BD Pharmingen | FN50 |
| CD49 | IgG1 | 1:50 dilution | BD | SR84 |
| CD49aPE | IgG1 | 1:8 dilution | BD Pharmingen | SR84 |
| CD103 | IgG1 | 9.5 | DAKO | Ber-ACT8 |
| CD103PE | IgG1 | 10 | DAKO | Ber-ACT8 |
| CD103 FITC | IgG2a | 1:16 dilution | Immunotech | 2G5 |
| Controls | ||||
| MoIgG PerCP | IgG1 | b | BD | X40 |
| MoIgGPE | IgG1 | b | DAKO | DAK-GO1 |
| MoIgGFITC | IgG1 | b | DAKO | DAK-GO1 |
| IgG1 | IgG1 | b | DAKO | DAK-GO1 |
| IgG2a | IgG2a | b | DAKO | DAK-GO5 |
| IgG2b | IgG2b | b | DAKO | DAK-GO9 |
BD Pharmingen, Oxford, UK; Caltag Laboratories, California, USA; DAKO Cytomation, Cambs, UK; Immunotech, Marseille, France; R&D Abingdon, UK.
Where concentration not available, dilution factor of reagent used is given.
Concentration of isotype control used equivalent to the primary antibody used.
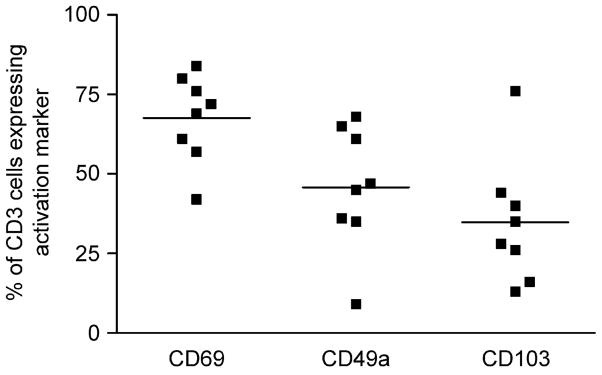
Fig. 1.
Flow cytometry data showing the percentage of CD3+ cells expressing each of the receptors CD69, CD49a and CD103 in lung filtrate samples. Cells were indirectly labelled with the activation markers and directly labelled with CD3PerCP and CD8PE as indicated in the methods section (n = 8). Subject numbers 1–8 were used for these experiments.
For intracellular cytokine measurements, as above, indirect labelling of either CD69 or CD103 was performed prior to stimulation for 4 h at 37 °C in medium containing either phorbol 12-myristate 13-acetate (Sigma) 5 ng/ml, calcium ionophore (Sigma) 250 ng/ml and brefeldin (Sigma) 10 μg/ml, or Staphylococcal enterotoxin B (SEB) and brefeldin (both at 10 μg/ ml). Following CD3 PerCP surface marker staining, cells were fixed and permeabilised using 4% paraformaldehyde (Sigma)/0.1% Saponin (Sigma) for 15 min at 4 °C. After intracellular blocking with 10% mouse serum, cells were incubated with PE labelled mouse anti-human IFNγ.
Finally, the expression of CD69 and CD103 in conjunction with the chemokine receptors CCR1, 2, 6, CXCR1, 2, 3, 4, 6, CCR3, 4, 7, 9, 10 (kind gift of Millenium Pharmaceuticals, MA, USA) and CCR5 was performed. All chemokine receptor antibodies were mouse monoclonals and appropriate isotype controls (DAKO) were used throughout. Lung filtrate cells were FITC stained indirectly for the chemokine receptor of interest then labelled with mouse anti-human CD3 PerCP and either CD69 PE or CD103 PE.
Samples were acquired using a BD FACScan and results were analysed using CellQuest Pro software, version 4.0.2 (BD Biosciences). Lymphocytes were initially gated on the basis of their forward/side scatter properties and CD3 expression.
Labelling of cells for activation markers, intracellular cytokine and chemokine receptors for five colour flow cytometry
In order to study a more diverse population of activation marker expressing cells together with their ability to secrete cytokine or express chemokine receptors (particularly CXCR6), we devised a five colour flow experiment enabling simultaneous analysis of two activation markers, CD3, CD4, CD8 and either IFNγ or CXCR6.
Briefly, non-specific binding on cells was abolished by incubation with 10% fetal calf serum as before. Unlabelled primary CXCR6 antibody or mouse control was incubated with the cells for 45 min at 4 °C. After washing, cells were incubated for a further 45 min on ice with a biotinylated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, PA, USA) at a dilution of 1:50. After a further blocking step using 10% normal mouse serum, the primary antibody was visualised using streptavidin conjugated PECy7 (Cedar-lane Laboratories, Ont., Canada) at a dilution of 1:100. At the same time, a cocktail of other direct labelled antibodies was added to the cells, CD103 FITC, CD49a PE, CD3 ECD and CD8 APC Cy7 and incubated for a further 45 min at 4 °C. Cells were then washed in SM-2 buffer (PBS, 0.1% sodium azide and 10% fetal calf serum) before fixation in 1% PFA prior to shipping to the USA for analysis.
Experiments measuring IFNγ secretion were carried out in a similar fashion. Unlabelled CD49a was initially used followed by the same biotinylated and streptavidin conjugated antibodies as above. The cocktail of direct labelled antibodies was as above except that no PE labelled antibody was added. After incubation, cells were washed in medium prior to 4 h stimulation with PMA, calcium ionophore and brefeldin as set out in methods above. Intracellular staining for IFNγ was as described for the three colour flow cytometry experiments. Cells were fixed and shipped to the USA for further analysis. In all experiments, isotype control antibodies were used throughout.
Samples were acquired using a MoFlo cytometer (DAKO Cytomation) and analysed using Summit 3.1 software (DAKO Cytomation).
Statistical analysis
All statistical analysis was done using Graphpad Prism (Graphpad Software Inc., San Diego, CA). Student’s t test was used to compare differences between groups. p values of <0.05 were considered significant. All values are expressed as means±S.D.
Results
Expression of activation markers on human lung T cells
Expression of CD69, CD103 and CD49a on T cells from human lung filtrate were assessed in experiments on eight different lung donors. The expression of these markers on human lung T cells varied widely. An average of 68±13.8% of CD3 cells expressed CD69 whereas for CD49a and CD103 the levels of expression were lower at 46±19.5% and 35±19.8%, respectively. There was no statistically significant bias towards expression of either CD69 or CD103 on CD8 positive or negative cells (Fig. 1).
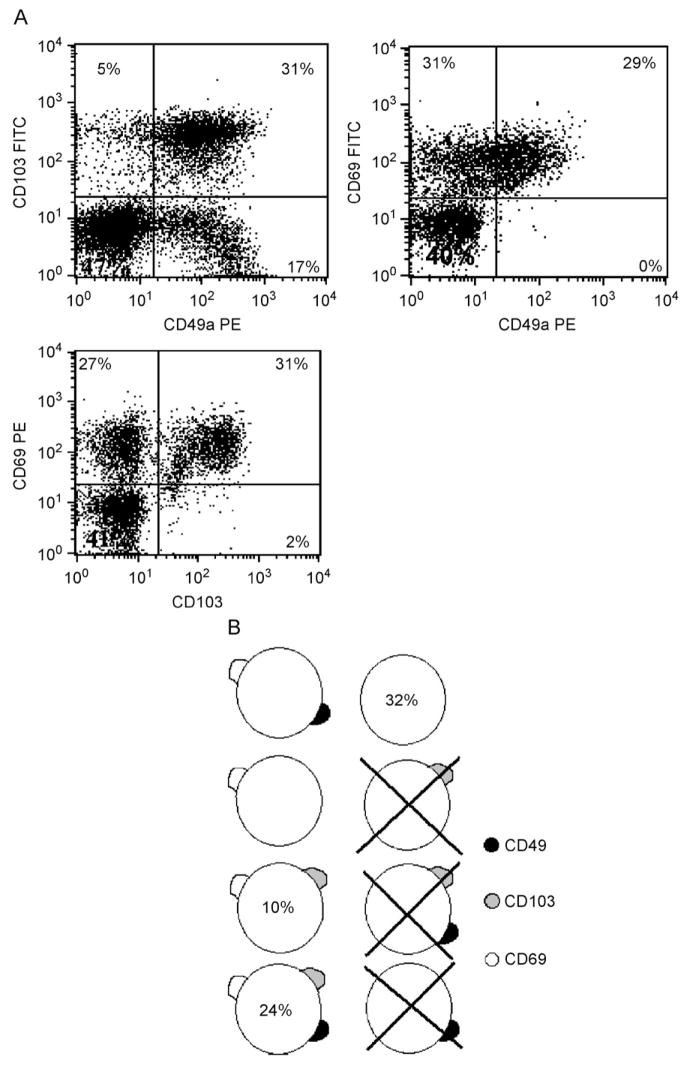
Analysis of the co-expression of the activation markers revealed a number of subsets expressing different combinations. Of particular note, CD69– cells were unlikely to express either CD49a or CD103. Thus, of cells which lack CD69 expression, 96.6±3.7% and 88.8±16.4% also fail to express CD49a and CD103, respectively (n = 8, Fig. 2A). Therefore, lack of CD69 expression appears to serve as a good marker for cells which are negative for all three of the activation markers (‘unactivated’). In contrast of CD3 cells which express CD103, an average of 68±12.7% and 89.3±10.3% also express CD49a and CD69, respectively (n = 8, Fig. 2A), thus making CD103 a marker for cells co-expressing all three activation markers (‘activated’). Three activation markers give a possible eight different combinations of activation marker expression. Analysis of our results as outlined above allows for a reasonable estimate as to the sizes of some of these populations (Fig. 2B). In this way, we have shown that some combinations such as CD3+CD69−CD49a−CD103+ cells essentially do not exist on human lung T cells. The limitations of a three colour flow cytometer mean that for populations which lie between the two extremes listed above it is difficult to make accurate estimations with regard to population size.
Fig. 2.
(A) Example of three colour flow cytometry dot plots from two separate experiments showing the typical co-expression patterns of the three activation markers studied. All plots are gated on live lymphocytes by forward/side scatter and then on CD3+ cells. (B) Schematic diagram showing the potential combinations of activation marker expression on individual T cells according to the results of three colour flow cytometry experiments. Phenotypes which appear not to exist are crossed out, for other phenotypes where possible, an estimate as to the percent of cells falling into this phenotypic group are denoted by the figure inside the cell.
Relationship between activation marker expression and cytokine secretion
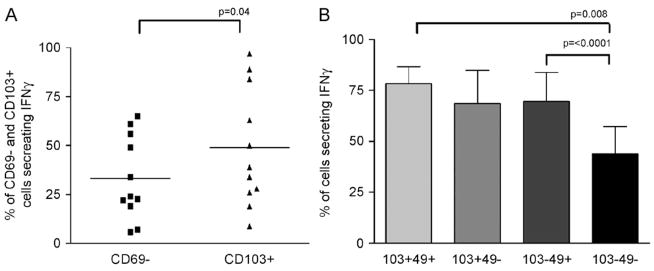
We compared the activated (CD103+) and the unactivated (CD69−) populations in terms of their ability to secrete cytokine when stimulated by either PMA/ionomycin or SEB. Following PMA/ionomycin stimulation there was a significant difference in the ability of these cell populations to secrete IFNγ when stimulated in vitro, with 33.2±21.2% of CD69– cells secreting this cytokine as compared to 48.9±30.2% of CD103+ cells (p = 0.044) (Fig. 3A). When cells were stimulated by SEB, there was dramatically less IFNγ production detectable, with an average of 11.4±4.8% of CD3+ cells positive for IFNγ compared to 44.9±27.6% of cells with PMA/ionomycin stimulation. There was a trend towards increased secretion of IFNγ by CD103+ cells in that 13.1±3.1% of these cells secrete IFNγ compared to 9.8±5.2 of CD69– cells; however, these differences were not statistically significant (data not shown) (subjects 9–13 were used for the experiments with SEB).
Fig. 3.
(A) Flow cytometry data showing the percent of CD103+ cells (‘activated’) and CD69– cells (‘unactivated’) secreting IFNγ after stimulation for 4 h with PMA/ionomycin. Cells were indirectly labelled with either CD69 and CD103 followed after stimulation by CD3 PerCP. They were then permeabilised before staining for IFNγ as indicated in the methods. Mean values represented by horizontal bars (n = 11) (subjects 1–4 and 6–12 were used for these experiments). Significantly more ‘activated’ than ‘unactivated’ cells produced IFNγ (p<0.4). (B) Five colour flow cytometry data showing the percentages of T cells lying within intermediate populations secreting IFNγ after stimulation for 4 h with PMA/ionomycin, n = 4 (subjects 9–12 were used for these experiments). Staining was undertaken as described in the methods. IFNγ production correlated with the number of activation receptors expressed; p value by paired t test.
Further analysis of intermediate populations using five colour flow cytometry revealed a significant difference between CD103+49+ cells and CD69−49– cells in terms of their ability to secrete IFNγ with 78.3±8.4% of double positive cells secreting this cytokine compared to 43.9±13.3% of double negative cells, p = 0.008 (Fig. 3B). There was no difference in cytokine production between CD8+ and CD8– populations (data not shown).
Chemokine receptor expression on polarised T cell populations
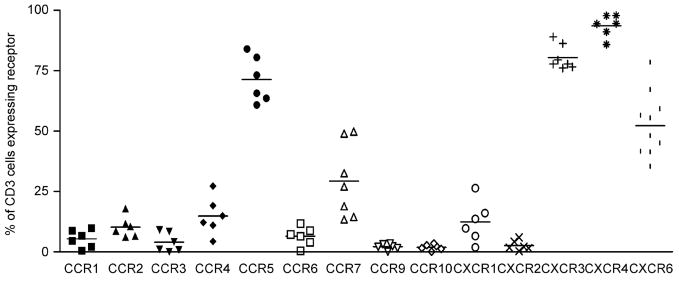
Analysis of chemokine receptor expression on human lung T cells demonstrated that the average chemokine receptor expression for all lung T cells varies according to the receptor in question (Fig. 4), but generally can be divided into those receptors which are highly expressed in the lung (>80%) such as CXCR3 and CXCR4, those with low expression (<10%) such as CCR1, 3, 6, 9, 10 and CXCR2 and the remainder with moderate expression between these levels.
Fig. 4.
Flow cytometry data showing the expression of each CR studied on T cells from lung filtrate. Mean values are represented by a bar. Cells were stained indirectly with the chemokine receptor antibody followed by staining with directly conjugated CD3 PerCP and either CD69 PE or CD103 PE. The percent expression was subtracted from the isotype control (n = 6). Subjects numbers 15–24 were used for these experiments. Subjects 15–20 were used for the majority of the data and subjects 21–24 were used for the additional data on CXCR6.
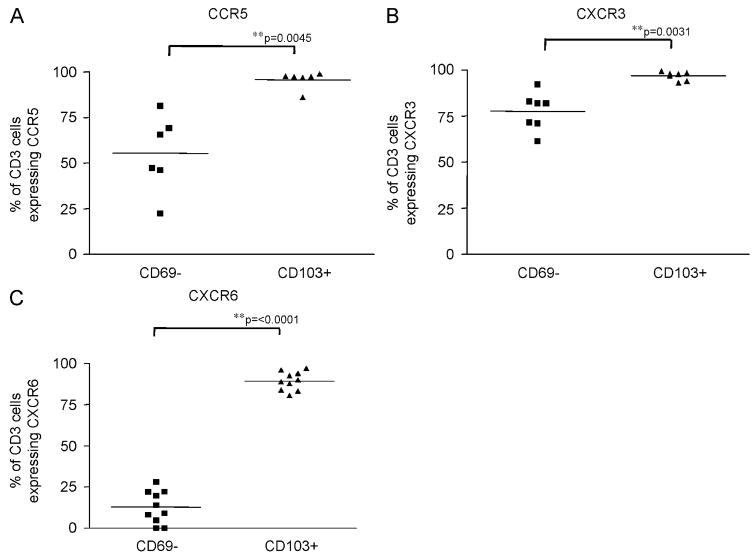
We hypothesised that activated T cells would preferentially express chemokine receptors that are involved in retaining the cells within the lung mucosa. We therefore compared expression of a panel of CR (Table 1) on CD103+ and CD69– cells. Three of the fourteen receptors measured showed significant differences in expression between the two polarised populations (Fig. 5A–C). CCR5 was expressed by 95.6±4.8% of CD103+ cells compared to 55.3±21% of CD69– cells (p = 0.0045, Fig. 5A) whereas CXCR3 was expressed by 96.8±2.3% of CD103+ cells compared to 77.6±10.2% of CD69– cells (p = 0.0031, Fig. 5B). The most marked difference was for CXCR6, with 89.5±5.5% of CD103 cells expressing CXCR6 compared to 12.8±9.8% of CD69– cells (p = <0.0001, Fig. 5C).
Fig. 5.
Flow cytometry data showing the expression of CCR5 (A) (subjects 15, 16, 17, 19, 20, 21), CXCR3 (B) (subjects 15–21) and CXCR6 (C) (subjects 15–24) in CD69– cells (‘unactivated’) and CD103+ cells (‘activated’). Cells were stained as in Fig. 4. Mean values are represented by a bar.
CCR7 was one of only two receptors whose expression was less on CD103+ T cells compared to CD69– cells, the other being CXCR2. Values for CCR7 expression were 22.5±10.7% and 48.5±30% in CD103+ cells and CD69– cells, respectively. However, this difference did not reach statistical significance (p = 0.1).
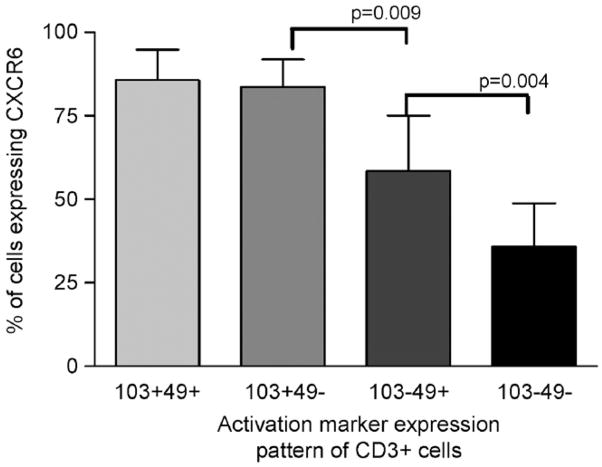
Further analysis of intermediate populations using five colour flow cytometry revealed there was a graded response of CXCR6 expression dependent upon activation marker expression with the most likely cells to express CXCR6 being CD103+49a+, followed by CD103+49a−, then CD103–49a+ and finally the least likely to express this chemokine receptor being CD103−49a– cells (Fig. 6). We found that there was no significant difference between CD3+CD8+ and CD3+CD8– cells in the pattern of CXCR6 expression amongst activation marker subgroups.
Fig. 6.
Five colour flow cytometry data showing the percentage of T cells expressing different combinations of activation markers and their expression of CXCR6 in five separate experiments. Cells were stained with unlabelled primary CXCR6 antibody followed by biotinylated goat anti-mouse secondary antibody and a further staining step using streptavidin conjugated PECy7 together with the directly labelled antibodies (CD103 FITC, CD49a PE, CD3 EDC, and CD8 APCCy7). Significant differences are represented on the graph (subjects 9–12 and 14 were used for these experiments).
Discussion
Lung T cells, like tissue T cells, in general have increased expression of activation markers. However, the mechanisms and implications of this expression have not been explored in detail. We have shown for the first time that these receptors co-express in a random fashion with two clearly polarised populations, one expressing none of the receptors and one expressing all three. We have shown that these two populations differ markedly in terms of their expression of chemokine receptors, especially CXCR6, and their ability to secrete cytokines. We hypothesise that the ‘activated’ cells represent a resident lung population whereas the ‘unactivated’ cells are just passing through.
We hypothesised that if these cells were truly activated then they would have greater potential for cytokine release. We have found that CD103 positive cells which generally co-express both CD69 and CD49a, contained a significantly greater number of cytokine producing cells than the cells expressing none of these receptors, exemplified by CD69 negative cells. It was a limitation of our technology that we had to use CD103 as a surrogate for triple expression of the activation receptors when 30% of these cells did not express CD49a. However, the difference in cytokine expression was accentuated when analysis of intermediate populations was made possible using five colour flow cytometry. Unfortunately, we were unable to stain for CD69 in the five colour experiments but there was a marked difference between the cells that expressed CD103 and CD49a (which will also be CD69 positive) and the cells which were negative for these receptors most of which will be triple negative. The above results were obtained with a maximal non-physiological stimulus (PMA/ calcium ionophore) and were also seen with a more physiological polyclonal stimulus (SEB). It seems therefore that cells expressing activation markers do appear to be more ‘activated’ in the sense of being able to produce more cytokines. In these experiments we did not study expression of the Th2 cytokines IL-4 or IL-13 or the regulatory associated cytokine IL-10. The number of T cells expressing Th2 cytokines whether from BAL or lung resection T cells is in the region of 2% (data not shown) which would make co-expression with activation markers difficult to interpret. We were unable to detect IL-10 secretion by lung T cells using this technique.
One of the major implications of expression of slow onset activation markers such as CD103 and CD49a is that these cells must be retained within the tissue, otherwise expression of these markers would be seen in the peripheral blood. It would seem plausible that cells not expressing these markers contain the population of cells that are not retained but are trafficking to the regional lymph nodes on their way back to the circulation. One can speculate that the retained cells have encountered cognate antigen and are being held in the tissue by signals provided by APC. If this were the case we would expect cells expressing activation markers to express receptors responsive to retention signals from APCs. We report here for the first time the novel and potentially important observation that cells expressing all three activation markers were virtually all positive for CXCR6, whereas the majority of cells which were activation marker negative did not express this receptor. These observations were confirmed using five colour flow cytometry on each different set of cells using a different set of antibodies. We observed increased expression of CXCR6 with increasing expression of activation markers although expression of CXCR6 appears to be most closely related to CD103 expression.
CXCR6 was initially recognised as a fusion co-factor for the HIV and SIV-1 viruses (Deng et al., 1997; Matloubian et al., 2000). It is predominantly expressed by a small subset of peripheral blood T cells and a subset of dendritic cells (Berahovich et al., 2006). Expression is increased on T cells from a number of tissues including the lung, gut, synovium and liver (Agostini et al., 2005; Heydtmann et al., 2005; Morgan et al., 2005; Nanki et al., 2005; Stenstad et al., 2006). The sole ligand for CXCR6 is CXCL16, which like CX3CL1 is a transmembrane chemokine which is present in both a full length form expressed on the cell surface and a smaller secreted moiety (Ludwig and Weber, 2007). CXCL16 is also a scavenger receptor which is thought to be involved in atherosclerosis (Sheikine et al., 2006). We have recently found that this chemokine is highly expressed by AM and in BAL from normal subjects. Although it is difficult to determine the precise concentration of CXCL16 at the site of action the concentrations present in BAL fluid and secreted by alveolar macrophages and bronchial epithelial cells is in the nanogram range (paper in preparation). This is therefore one of the highest constitutively produced chemokines in the lung and in a range that is likely to mediate recruitment of CXCR6 T cells. We have therefore speculated that CXCR6 and CXCL16 are involved in constitutive migration of T cells into the lung (Morgan et al., 2005). CXCL16 is a transmembrane chemokine and is expressed both on the surface of AM as well as in soluble form in supernatants from AM. CXCL16 has also been shown to be expressed by dendritic cells. (Tabata et al., 2005). We therefore hypothesise that CXCR6 positive cells recruited into the lung, become attracted to AM and to other APCs expressing CXCL16. This suggestion has also been made by Latta et al. (2007). If those cells are also expressing cognate antigen then this results in up-regulation of CD69, CD49a and particularly CD103 which are involved in anchoring the T cells within the tissue possibly through adherence to the APCs themselves or to collagen (VLA-1) or E-cadherin in the case of CD103. In most cases, this would result in a tolerogenic signal (as T cells in the lung are not constitutively producing cytokine) and this would be consistent with the down-regulatory role of AM on T cell activation. We cannot discount the possibility however that CXCR6 is a marker of cognate interaction with APCs and does not relate to the migratory function of these cells.
T cells from lung filtrate are derived mostly from current or ex-smokers with lung cancer, and we cannot completely discount the possibility that our findings are influenced by this. However, expression of both the activation markers CD49a and CD103, as well as the majority of CRs studied are similar in BAL T cells from normal volunteers which makes this possibility less likely although we have not performed the same subset analysis on BAL cells due to paucity of cell numbers (data not shown). Additionally, Ancochea et al. (1993) have reported similar expression values for CD69 in BAL from healthy controls compared to our results from lung filtrate T cells. The absence of naïve (CD45RA+ or CD45RO−) T cells from these lung preparations strongly suggests that contamination from peripheral blood during the preparation of the sample is insignificant. Lastly the CD69– population appear phenotypically more similar to the cells expressing activation markers than peripheral blood T cells in terms of their expression of both IFNγ and other chemokine receptors.
In summary, therefore, we have shown that lung T cells with an ‘activated’ phenotype are much more likely to express CXCR6 compared with cells with a ‘non-activated phenotype’. We propose that CXCR6, together with its ligand CXCL16, play an important role in the retention of antigen stimulated T cells within the lung mucosa.
Acknowledgments
This study was funded by Asthma, UK and the Wellcome Trust.
Abbreviation
- AM
alveolar macrophages
- BAL
bronchoalveolar lavage
- SEB
staphlococcal enterotoxin B
References
- Agostini C, Cabrelle A, Calabrese F, Bortoli M, Scquizzato E, Carraro S, Miorin M, Beghè B, Trentin L, Zambello R, Facco M, Semenzato G. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T-cell alveolitis in sarcoidosis. Am J Respir Crit Care Med. 2005;172:1290–1298. doi: 10.1164/rccm.200501-142OC. [DOI] [PubMed] [Google Scholar]
- Ancochea J, Gonzalez A, Sanchez MJ, Aspa J, Lopez-Botet M. Expression of lymphocyte activation surface antigens in bronchoalveolar lavage and peripheral blood cells from young healthy subjects. Chest. 1993;104:32–37. doi: 10.1378/chest.104.1.32. [DOI] [PubMed] [Google Scholar]
- Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ. Evidence for NK cell subsets based on chemokine receptor expression. J Immunol. 2006;177:7833–7840. doi: 10.4049/jimmunol.177.11.7833. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, Andrew DP, Wu L, Butcher EC, Wardlaw AJ. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–2848. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- Corrigan CJ, Hartnell A, Kay AB. T lymphocyte activation in acute severe asthma. Lancet. 1988;21:1129–1132. doi: 10.1016/s0140-6736(88)91951-4. [DOI] [PubMed] [Google Scholar]
- Deng HK, Unutmaz D, Kewalramani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Fung JJ, Zeevi A, Kaufman C, Paradis IL, Dauber JH, Hardesty RL, Griffith B, Duquesnoy RJ. Interactions between bronchoalveolar lymphocytes and macrophages in heart-lung transplant recipients. Hum Immunol. 1985;14:287–294. doi: 10.1016/0198-8859(85)90236-8. [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestine immune compartment: Epithelial expression of tissue-specific chemokines as an organising principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta M, Mohan K, Issekutz TB. CXCR6 is expressed on T cells in both T helper type 1 (Th1) inflammation and allergen-induced Th2 lung inflammation but it is only a weak mediator of chemotaxis. Immunology. 2007;121:555–564. doi: 10.1111/j.1365-2567.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Weber C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb Haemost. 2007;97:694–703. [PubMed] [Google Scholar]
- Matloubian M, David E, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Guillen C, Symon FA, Huynh TT, Berry MA, Entwisle JJ, Briskin M, Pavord ID, Wardlaw AJ. Expression of CXCR6 and its ligand CXCL16 in the lung in health and disease. Clin Exp Allergy. 2005;35:1572–1580. doi: 10.1111/j.1365-2222.2005.02383.x. [DOI] [PubMed] [Google Scholar]
- Nanki T, Shimaoka T, Hayashida K, Taniguchi K, Yonehara S, Miyasaka N. Pathogenic role of the CXCL16-CXCR6 pathway in rheumatoid arthritis. Arthritis Rheum. 2005;52:3004–3014. doi: 10.1002/art.21301. [DOI] [PubMed] [Google Scholar]
- Pabst R, Binns RM. Lymphocytes migrate from the bronchoalveolar space to regional bronchial lymph nodes. Am J Respir Crit Care Med. 1995;151:495–499. doi: 10.1164/ajrccm.151.2.7842212. [DOI] [PubMed] [Google Scholar]
- Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR) 4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltini C, Hemler ME, Crystal RG. T lymphocytes compartmentalized on the epithelial surface of the lower respiratory tract express the very late activation antigen complex VLA-1. Clin Immunol Immunopathol. 1988;46:221–233. doi: 10.1016/0090-1229(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Sheikine Y, Bang CS, Nilsson L, Samnegard A, Hamsten A, Jonasson L, Eriksson P, Sirsjo A. Decreased plasma CXCL16/SR-PSOX concentration is associated with coronary artery disease. Atherosclerosis. 2006;188:462–466. doi: 10.1016/j.atherosclerosis.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, Agace WW. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood. 2006;107:3447–3454. doi: 10.1182/blood-2005-07-2860. [DOI] [PubMed] [Google Scholar]
- Tabata S, Kadowaki N, Kitawaki T, Shimaoka T, Yonehara S, Yoshie O, Uchiyama T. Distribution and kinetics of SR-PSOX/CXCL16 and CXCR6 expression on human dendritic cell subsets and CD4+ T cells. J Leukoc Biol. 2005;77:777–786. doi: 10.1189/jlb.1204733. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. Advances in Immunology: T-cell function and migration-two sides of the same coin. N Eng J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ. Textbook of Respiratory, Cell and Molecular Biology. Martin Dunitz; London: 2002. Immunological basis of lung disease; pp. 47–71. [Google Scholar]