Abstract
Rho GTPases have been shown recently to be important for cell polarity and motility of the trunk mesoderm during gastrulation in Xenopus embryos. This work demonstrated that Rho and Rac have both distinct and overlapping roles in regulating cell shape, and the dynamic properties, polarity and type of protrusive activity of these cells. Overexpression of activated or inhibitory versions of these GTPases also disrupts development of the head in Xenopus embryos. In this study, we have undertaken a detailed analysis of Rho and Rac function in migrating anterior mesendoderm cells. Scanning electron micrographs of these cells in situ revealed that their normal shingle arrangement is disrupted and both the cells and their lamellipodia are disoriented. Anterior mesendoderm explants plated on their natural blastocoel roof matrix, however, still migrated towards the animal pole, although the tendency to move in this direction is reduced compared to controls. Analysis of a number of parameters in time-lapse recordings of dissociated cells indicated that Rho and Rac also have both distinct and overlapping roles in the motility of the prospective head mesoderm; however, their effects differ to those previously seen in the trunk mesoderm. Both GTPases appear to modulate cell polarization, migration and protrusive activity. Rho alone, however, regulates the retraction of the lagging edge of the cell. We propose that within the gastrulating Xenopus embryo two types of mesoderm cells that undergo different motilities have distinct responses to Rho GTPases.
Keywords: cell motility, Rho GTPases, Rho, Rac, gastrulation, head mesoderm, Xenopus, anterior mesendoderm
INTRODUCTION
Cell movements are essential for the embryogenesis of all vertebrates and most invertebrates. They begin at gastrulation when changes in cell shape, adhesion, and motility result in a series of tissue rearrangements that establish the basic body plan of the embryo. In Xenopus, gastrulation involves several distinct types of cell movement, including epiboly of the ectoderm; rotation of the endoderm; involution of the mesoderm; migration of prospective head mesoderm cells and convergent extension of the trunk mesoderm (Keller and Winklbauer, 1992; Winklbauer and Schurfeld, 1999; Keller et al., 2003). The morphological details of these cell movements are well defined, however, their molecular basis is not fully understood. Our recent work demonstrated a role for Rho GTPases in the intercalation movements of the trunk mesoderm (Tahinci and Symes, 2003) and showed that the cellular responses to activated or inhibitory versions of Rac and Rho are distinct from those previously identified in cultured fibroblasts. To determine whether this is also true for migratory embryonic cells, we have examined the role of these GTPases in prospective head mesoderm cell motility. These cells, in contrast to trunk mesoderm cells, migrate as a sheet across a fibronectin rich extracellular matrix that lines the inner surface of the blastocoel roof and form leading edge protrusions such as lamellipodia and filopodia in direct contact with the extracellular matrix (Keller and Winklbauer, 1992; Ramos and DeSimone, 1996).
Rho GTPases have been shown to control a large variety of biological responses, including chemotaxis, cytokinesis, axon guidance, endocytosis, cell cycle progression and differentiation (Etienne-Manneville and Hall, 2002), and up to 20 members of the family have now been identified in a variety of organisms (Burridge and Wennerberg, 2004). Perhaps the best-characterized roles for this family, however, are that of Rho, Rac and Cdc42 in controlling specific rearrangements of the actin cytoskeleton (Raftopoulou and Hall, 2004), an essential process in cell motility. Initially characterized in cultured fibroblasts, but now shown to act in a variety of cell types, Rho regulates the assembly of contractile actin:myosin filaments, while Rac and Cdc42 control actin polymerization forming filopodia and lamellipodia, respectively (Ridley and Hall, 1992b; Ridley et al., 1992; Kozma et al., 1995; Nobes and Hall, 1995). In addition, all three GTPases promote the assembly of integrin based matrix adhesion complexes.
Most work characterizing Rho GTPase function has been carried out using cultured mammalian cells (for review see (Burridge and Wennerberg, 2004)), however, more recently, their roles in embryonic development have been investigated. In Drosophila and Caenorhabditis elegans, Rho and Rac participate in several developmental processes that involve rearrangement of the actin cytoskeleton such as in establishing cell polarity and cell motility (Settleman, 2001). Moreover, in both species, genetic analyses demonstrate that these functions are regulated by the planar cell polarity (PCP) signaling pathway that controls rearrangement of the actin cytoskeleton and gene regulation (Strutt et al., 1997; Fanto et al., 2000; Weber et al., 2000; Winter et al., 2001). This pathway has now been shown to play similar roles in vertebrate development. For example, convergent extension of the Xenopus trunk mesoderm requires the PCP pathway in which Rho and Rac are activated downstream of Wnt11/Fz7 and Dishevelled (Habas et al., 2001; Wallingford et al., 2002; Habas et al., 2003). Rho activation proceeds through a novel intermediate, Daam1, and possibly an unknown guanine nucleotide exchange factor, whereas Rac activation is independent of Daam1 (Habas et al., 2001; Habas et al., 2003; Kim and Han, 2005). Both GTPases result in the activation of the JUN N-terminal kinase (JNK) (Habas et al., 2001; Habas et al., 2003; Kim and Han, 2005). This splitting of the PCP pathway suggests that Rho and Rac act in parallel but ultimately converging pathways to regulate cell polarity in this process (Tahinci and Symes, 2003). In addition to Xenopus, Rho GTPases have been shown to be involved in the polarity and motility of different cell types throughout the development of a variety of vertebrates including mouse, chick, and zebrafish (Liu and Jessell, 1998; Sugihara et al., 1998; Marlow et al., 2002). Moreover, as they are activated in response to a wide range of growth factors and cell adhesion molecules it is likely that mechanisms other than the PCP pathway are also involved (Burridge and Wennerberg, 2004).
Different cell types respond to Rho GTPase activation in different ways, for example, activation of Rho in fibroblasts stimulates the formation of focal adhesions and stress fibers that flatten the cells. In contrast, activation of Rho in neuronal cells induces neurite retraction and cell rounding (Kozma et al., 1997). Moreover, when cells round up during mitosis, Rho activity is increased (Ren et al., 1999; Maddox and Burridge, 2003). Despite these opposing responses to Rho activation, the underlying biochemical mechanism may be the same. The formation of a contractile actin-myosin network on activation of Rho, may allow fibroblasts to maintain their flattened appearance through focal adhesions, whereas neuronal cells do not form focal adhesions and mitotic cells loose stress fibers and focal adhesions and, therefore, round up. Rac also has distinct effects in different cell types. For example, Rac stimulates cell-cell adhesion in keratinocytes (Braga et al., 1997), whereas it leads to cell scattering in Madin-Darby canine kidney epithelial cells stimulated with hepatocyte growth factor (Ridley et al., 1995).
Previous work demonstrated that Rho and Rac play essential roles in Xenopus convergent extension movements (Habas et al., 2001; Habas et al., 2003; Tahinci and Symes, 2003). Overexpression of activated (ca) or inhibitory (dn) versions of these GTPases, however, also disrupts head development in these embryos, although mesoderm induction occurs normally (Wunnenberg-Stapleton et al., 1999; Tahinci and Symes, 2003).
To determine how Rho and Rac affects the prospective head mesoderm during gastrulation anterior mesendoderm cells were examined in situ by scanning electron microscopy, as dispersed cell cultures by time-lapse confocal microscopy, and as explants plated on their natural, blastocoel roof matrix. We found that like the trunk mesoderm, Rho and Rac induce distinct cell behaviors in head mesoderm cells, but there are also differences between the two cell types. Both Rho and Rac are required to establish cell orientation and the polarity of their cytoplasmic protrusions. We further find that inhibition of either Rac or Rho dramatically reduces the ability of dissociated cells to migrate. These cells have a slower rate of movement, decreased numbers of transient lamellipodia, and the lifespan of transient lamellipodia is increased. In addition, in the case of Rho, inhibition of its signaling increases the number of stable lamellipodia while constitutive activation decreases it. Cell migration is also less continuous in cells overexpressing caRho, which undergo a sudden release of the rear of the cell that causes the cell body to collapse and the cell to move forward rapidly. Disruption of Rho or Rac, however, reduces but does not abolish the normal directed movement of prospective head mesoderm explants. Taken together these data indicate that within the gastrulating Xenopus embryo two types of mesoderm cells that undergo different motilities have distinct responses to Rho GTPases.
RESULTS
Disruption of RhoA or Rac1 inhibits normal head formation in Xenopus embryos
Our previous work showed that disruption of RhoA (Rho) or Rac1 (Rac) by overexpression of dominant negative (dn) or constitutively active (ca) forms caused Xenopus embryos to develop with gastrulation defects, including spina bifida, shortened anterior-posterior axis and reduced or missing anterior structures (Tahinci and Symes, 2003); Figure 1). This work also demonstrated that RhoA and Rac1 are not required for mesoderm induction or differentiation in Xenopus and that changes in the motility, polarity, and protrusions of trunk mesoderm cells depend on Rho GTPase signaling. The disruption of anterior mesoderm structures in these embryos further suggested that the motility of the head mesoderm is abnormal. In this study, we test that notion by examining the disruption of RhoA and Rac1 in anterior mesendoderm cells in intact embryos, and as explants and dissociated cells in culture.
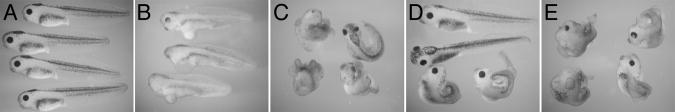
Figure 1. Rho and Rac are important for head development.
Embryos were microinjected at the 2-cell stage into the dorsoanterior marginal zone with 2ng of mRNA encoding (A) GAP43-GFP, (B) dnRho, (C) caRho, (D) dnRac, or (E) caRac. Note the disruption of anterior and axial structures in Rho GTPase-injected embryos compared to GFP controls.
Rho and Rac control normal polarity of prospective head mesoderm cells
During gastrulation, the prospective head mesoderm cells migrate as a sheet across the inner surface of the blastocoel roof (BCR), which is lined with a network of fibronectin fibrils (Nakatsuji, 1975; Keller and Schoenwolf, 1977; Nakatsuji et al., 1985). These cells are polarized with lamelliform protrusions extending towards the animal pole, in the direction of movement, and their trailing edges underlapping one another in a distinct pattern that resembles shingles on a roof ((Winklbauer and Nagel, 1991); see Figure 2A,B). To view anterior mesendoderm cell morphology under in vivo conditions, the BCR was removed from fixed stage 11 embryos. The substrate-apposed surface of the migrating mesoderm was then viewed by scanning electron microscopy (SEM; Figure 2).
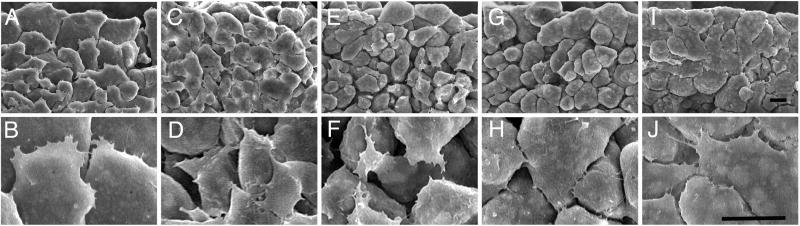
Figure 2. SEM analysis of anterior mesendoderm cells in situ.
Uninjected embryos (Con) or embryos that had been injected with mRNAs encoding different Rho GTPases into the future anterior mesoderm at the 2-cell stage were fixed at stage 11. The blastocoel roof was removed and the embryos were then processed for SEM. (A,B) Uninjected control embryos. (C,D,) dnRho, (E,F) caRho, (G,H) dnRac, or (I,J) caRac. Scale bars in I and J are 20 μm and refer to A,C,E,G,I and B,D,F,H,J, respectively.
In uninjected embryos, cells are loosely packed, yet spread against the substratum (Figure 2A). Lamelliform protrusions of cells (Figure 2B) point animally, generating the characteristic shingle arrangement of this cell population that is indicative of directional migration (Winklbauer and Nagel, 1991). The mesoderm in embryos expressing dnRho appears very similar to that of control embryos, with loosely packed cells spread against the substratum, however, the shingle arrangement of the cells and the polarity of their lamellipodia is disrupted (Figure 2C,D). In contrast, the cells expressing caRho are rounded and are more loosely packed (Figure 2E) than control cells (Figure 2A). In addition, these caRho expressing cells have randomly oriented lamelliform protrusions (Figure 2F). Expression of dnRac also leads to the rounding up of very loosely packed cells (Figure 2G), and the cytoplasmic processes are rare and filiform instead of lamelliform (Figure 2H). Like expression of caRho, caRac causes the random orientation of lamelliform protrusions relative to the animal pole (Figure 2I,J), however the cells are flattened against the substratum and appear more tightly packed (Figure 2I,J) than control cells (Figure 2A,B). Thus, all GTPase constructs disrupt the shingle arrangement of mesoderm cells. In addition, Rac seems to be required for lamellipodia formation and spreading on the BCR substratum, whereas inhibition of Rho function has little effect on spreading or protrusion formation in the intact embryo.
To examine individual cells in more detail, the prospective head mesoderm was dissociated and the cells cultured on a fibronectin substratum. In addition, to visualize the actin cytoskeleton, the cells were stained with rhodamine phalloidin (see Experimental Procedures). When individually cultured in this way, control anterior mesendoderm cells are typically elongate or triangular, with 1–3 lamellipodia at the ends (Winklbauer et al., 1991); Figure 3A). Expression of dnRho resulted in less pronounced lamellipodia (Figure 3B) than controls (Figure 3A). Similarly, dnRac diminishes lamellipodia size (Figure 3D). In addition, these cells have decreased actin staining of the cell body at the substrate level (Figure 3D). In contrast, expression of either caRho (Figure 3C) or caRac (Figure 3E) produces well-developed lamellipodia and caRac promotes the formation of additional small lateral processes (Figure 3E).
Figure 3. Rhodamine phalloidin staining of dissociated anterior mesendoderm cells.
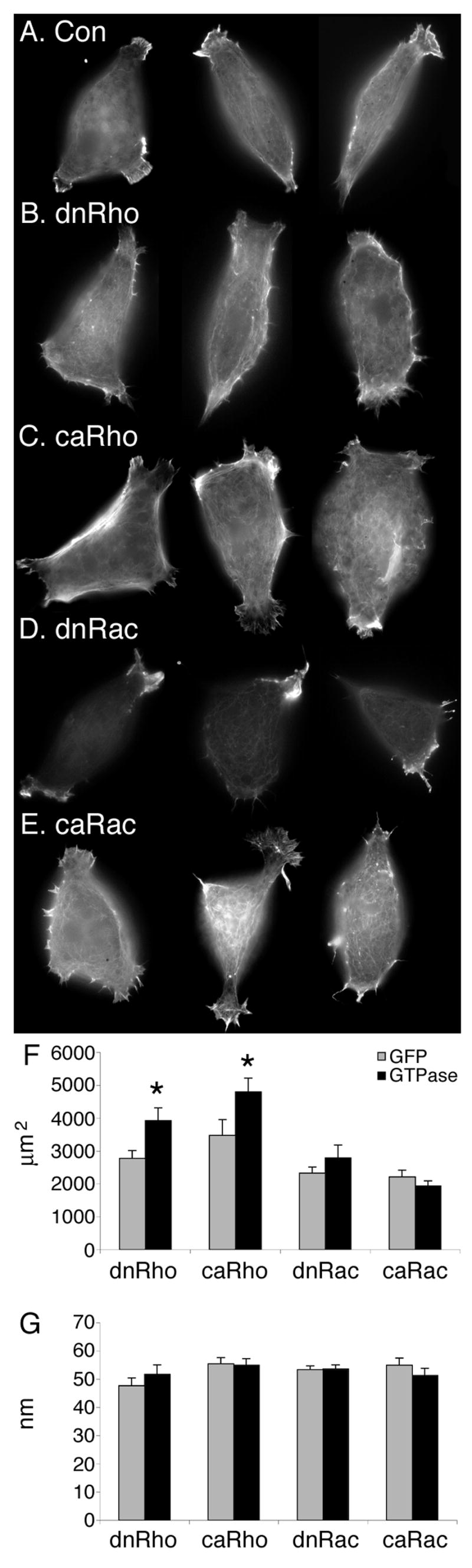
mRNAs encoding GAP43-GFP (Con) and different Rho GTPases were microinjected into the future anterior mesoderm at the 2-cell stage. Prospective head mesoderm cells were then dissected from these embryos at the beginning of gastrulation (stage 10) the cells were then fixed at stage 10.25 and the actin cytoskeleton was visualized using rhodamine phalloidin. (A–D) Con, (E) dnRho, (F) caRho (G) dnRac, (H) caRac. The cell area at the substrate surface (F) and the cell depth (G) was calculated, control GFP-injected (grey bars) and RhoGTPase-injected (black bars). Error bars represent standard error, * marks differences that are statistically significant (P < 0.05).
Interestingly, disruption of Rho affects cell spreading in vitro (Figure 3B,C and F,G). The cell area in contact with the substrate is increased in both caRho (4801±418 μm2) and dnRho (3923±391 μm2) expressing cells compared to control (3474±488 μm2 and 2778±232 μm2, respectively) and Rac expressing (dnRac 2793±391 μm2 and caRac 1936±156 μm2) cells (Figure 3F). The overall height of the cells, however, is not increased (Figure 3G). This increase in the size of Rho expressing cells is likely due to a slight delay in cell division. Nevertheless, it is translated into increased cell area in contact with the matrix instead of increased cell height, suggesting that the Rho-disrupted cells have a greater tendency to spread on fibronectin.
These data indicate that Rac1 is needed for lamellipodia formation both in vivo and on a fibronectin substratum in vitro. In contrast, RhoA appears to affect lamellipodia formation and the spreading of cells on fibronectin in vitro, but less so on the natural BCR substratum. To investigate the role of these GTPases in mesoderm cell motility further, time-lapse analysis of dissociated anterior mesoderm cells was performed.
Rho GTPase signaling is required for head mesoderm cell migration during gastrulation
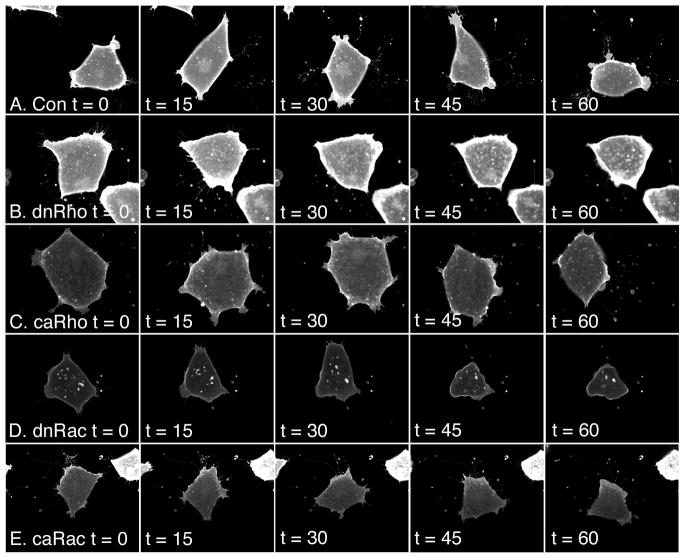
Time-lapse confocal movies were made of dissociated prospective head mesoderm cells moving on a fibronectin-coated surface. The movies were generated by the capture of one image every minute for one hour beginning at stage 10.5 (the early-mid gastrula stage). Five time points (0, 15, 30, 45, and 60 minutes) of a representative movie for each treatment are shown in Figure 4. In all cases, head mesoderm cells did move, however, motility was greatly reduced in cells in which RhoA or Rac1 was inhibited. To confirm this observation, the rate of cell movement was calculated for each cell throughout the movie (see Experimental Procedures; Figure 5A). Overexpression of constitutively active RhoA or Rac1 did not significantly affect the rate of cell motility (caRho 2.41±0.04 μm/min and caRac 1.85±0.31 μm/min, respectively compared to the control rates of 2.00±0.31 μm/min and 2.54±0.53 for those experiments). In contrast, and in parallel to the diminished lamellipodia size, inhibition of either RhoA or Rac1 decreased the rate of cell motility (dnRho 0.96±0.37 μm/min and dnRac 0.88±0.06 μm/min, respectively compared to the control cell rates of 2.57±0.54 μm/min and 2.25±0.64 μm/min for those experiments). These data suggest that the translocation of head mesoderm cells on fibronectin requires normal Rho GTPase signaling because inhibition of either RhoA or Rac1 signaling is sufficient to reduce the rate of motility. Interestingly, this rate is not affected by overexpression of either constitutively active Rho GTPase, but the pattern of movement is altered.
Figure 4. Time-lapse movies of migrating prospective head mesoderm cells.
mRNAs encoding GAP43-GFP (Con) and different Rho GTPases were microinjected into the future anterior mesoderm at the 2-cell stage. Prospective head mesoderm cells were then dissected from these embryos at the beginning of gastrulation (stage 10). The cells were dissociated, plated on a fibronectin matrix and monitored using time-lapse confocal microscopy during early-mid gastrula stage (beginning at stage 10.5). Five time points from a representative movie for each set of injections are shown. (A) Con, (B) dnRho, (c) caRho, (D) dnRac, (E) caRac. t equals time in minutes. Note, the loss of cell adhesion over time when Rac is inhibited (D).
Figure 5. Analysis of time-lapse confocal movies.
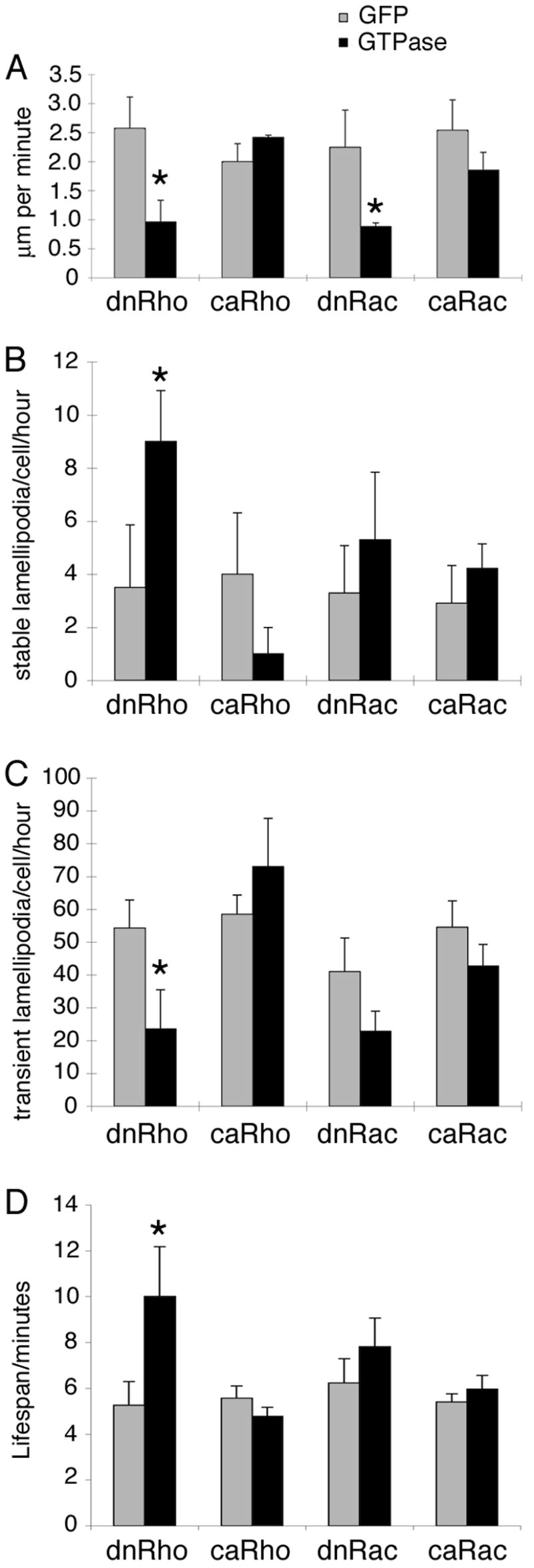
The (A) rate of motility, number of (B) stable and (C) transient lamellipodia, and the (D) lifespan of transient lamellipodia were assessed from the time-lapse movies (see Experimental Procedures). Data for each Rho GTPase (black bars) is shown next to that for sibling controls (grey bars). Error bars represent standard error, * marks differences that are statistically significant (P < 0.05).
RhoA controls the retraction of the lagging edge of the cell
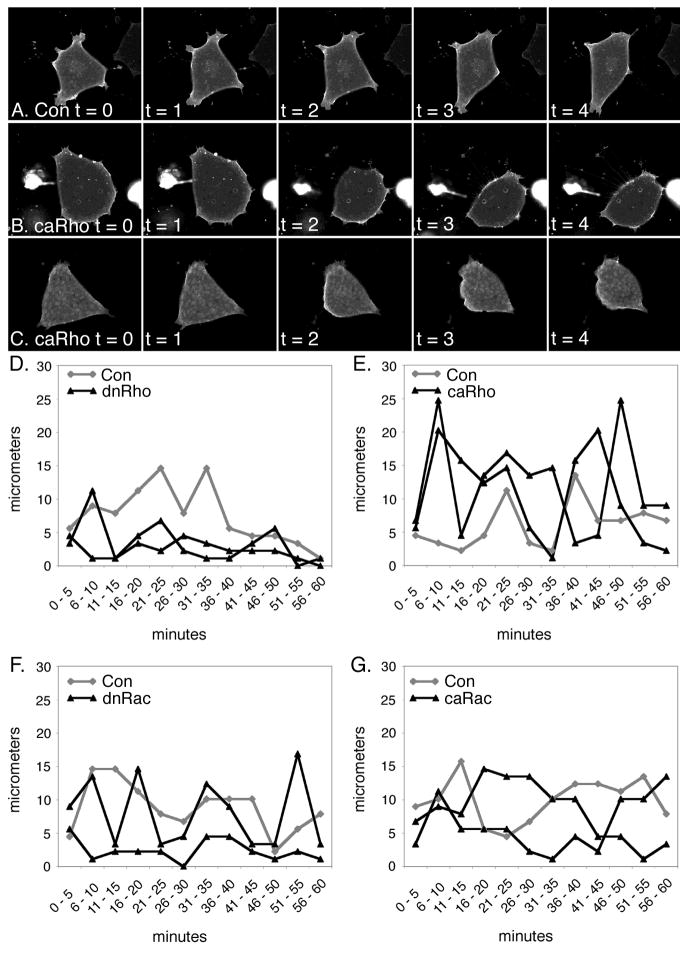
During the analysis of the time-lapse movies, we observed that head mesoderm cells overexpressing caRho, had an erratic motion compared to control cells and to those overexpressing other Rho GTPase forms. While the pattern of motility of control cells or cells overexpressing dn Rho, dnRac or caRac was similar and continuous (Figure 6A,D,F,G), the cells overexpressing caRho exhibited a rapid retraction of the rear of the cell and subsequent collapse of the cell body (Figure 6B,C,E). Thus, these cells move very little for several consecutive frames followed by a sudden increase in speed (Figure 6E). These data suggest that RhoA is important in the control of lagging edge retraction and further provide a mechanistic explanation for why constitutive activation of RhoA results in a disruption of anterior mesoderm structures in the embryo.
Figure 6. Rho controls lagging edge retraction.
The analysis of the time-lapse movies revealed that while control head mesoderm cells moved in a fluid motion those overexpressing caRho move erratically. Five time (t) points (one frame per minute) from representative movies for (A) Con, (B,C) caRho are shown. (D–E) The distance moved in consecutive 5 minute periods for control (grey) and Rho GTPases (black) are shown on graphs. Each graph has one example of a control cell and two examples of a Rho GTPase. (D) dnRho, (E) caRho, (F) dnRac, (G) caRac. Note the sudden spikes in the distance traveled for caRho. These spikes correspond to the retraction of the lagging edge and subsequent collapse of the cell body and forward movement.
Rho affects the stability of lamellipodia of head mesoderm cells
Our previous work in trunk mesoderm cells suggested that both Rho and Rac are also important for the formation and stability of leading edge protrusions such as lamellipodia during Xenopus gastrulation. To determine the role of Rho GTPases in leading edge protrusions in head mesoderm cells, lamellipodia were analyzed in the first 15 minutes of each of the time-lapse confocal movies. Two classes of lamellipodia were counted, stable lamellipodia that are present throughout the entire 15 min time period (Figure 5B), and transient lamellipodia that develop and retract within that time (Figure 5C). In addition, as a reflection of their stability, the lifespan of the transient lamellipodia was calculated (Figure 5D).
We found that neither mutants of Rac, dnRac nor caRac, affected the number of stable lamellipodia per cell per hour (5.3±2.5 and 4.2±0.9, respectively, compared to control cells 3.3±1.8 and 2.9±1.4, respectively). RhoA, however, has a significant effect on the number of stable lamellipodia (Figure 5B). Overexpression of dnRho increased the number of stable lamellipodia per cell per hour (dnRho 9.0±1.9 compared to control 3.5±2.4) while constitutive activation of Rho decreased them (caRho 1.0±1.0 compared to control 4.0±2.3). In contrast, transient lamellipodia are affected by both GTPases (Figure 5C,D). Neither constitutively active mutant had a significant effect on the number (caRho 73.0±14.5 compared to control 58.5±5.9; caRac 42.7±6.6; control 54.6±8.0; Figure 5C) or lifespan of transient lamellipodia (caRho 4.8±0.4 compared to control 5.6±0.5; caRac 6.0±0.6; control 5.4±0.4; Figure 5D). Inhibition of Rho or Rac, however, both reduces the number of transient lamellipodia (dnRho 23.5±12.0 compared to control 54.3±8.6; dnRac 22.8±6.2 compared to control 41.0±10.3) and increases their lifespan (dnRho 10.0±2.2 min compared to control 5.3±1.0 min; dnRac 7.8±1.3 min compared to control 6.2±1.1 min) suggesting that both GTPases are required for lamellipodia turnover in head mesoderm cells on fibronectin.
Anterior mesendoderm explants migrate in the normal direction
Anterior mesendoderm cells normally migrate as a sheet towards the animal pole, however, in GTPase-compromised embryos these cells appear disoriented in situ (see Figure 2). Dissociated cultures plated on a fibronectin matrix indicate that these cells are motile (see Figure 4A), however, such isolated cells cannot be used to study directional migration (Winklbauer et al., 1991). To determine whether RhoA and Rac1 are important for the directed migration of prospective head mesoderm cells, the movement of anterior mesendoderm explants was assessed in vitro on BCR-conditioned substratum (Nagel et al., 2004). In this assay, 90% of control explants (n=38) move towards the animal pole (Figure 7). Animal pole preference is highly significantly reduced in caRac (n=41), dnRac (n=52), caRho (n=39) and dnRho (n=48) expressing explants (Figure 7). However, dnRho explants, like controls, are highly significantly (α=0.001) different from random migration, where explants would move in either direction in 50% of the cases. In addition, at a moderate significance level of α=0.05, explants in all groups have a preference for the animal pole. All experimental explants migrate at about the same velocity as controls (about 40 μm/hour), and significantly slower than isolated cells.
Figure 7. Directed migration of head mesoderm explants is not controlled by Rho GTPases.
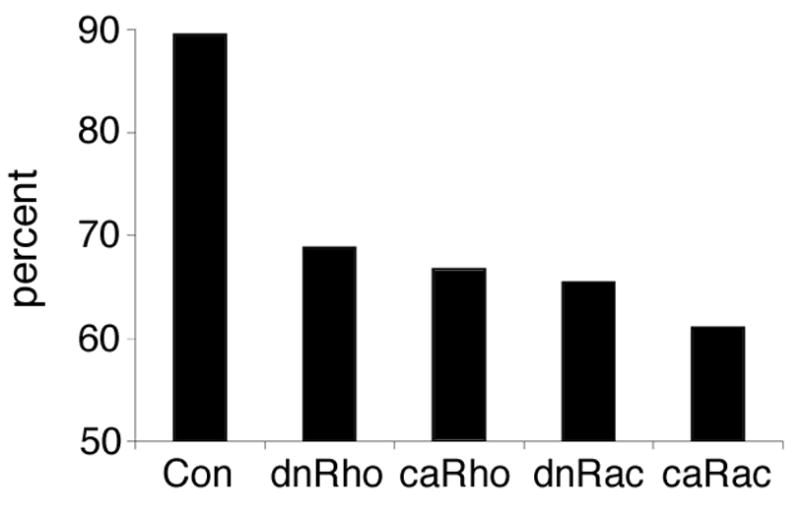
Anterior mesendoderm explants, derived from uninjected (Con) or RhoGTPase injected embryos, were plated on their natural blastocoel roof matrix and the direction of their movement assessed. Note that all explants tend to migrate in their normal direction although this tendency is reduced in explants expressing RhoGTPases.
Taken together, these data provide an explanation for why disruption of Rho or Rac signaling affects the normal development of anterior structures. Cell motility requires coordinated extension and retraction of cytoplasmic protrusions such as lamellipodia, as well as correct cell orientation and polarization. We find that both Rho and Rac are required for the normal shingle organization of anterior mesendoderm cells, and for efficient directional migration. In addition, Rac is required for the formation of lamelipodia in the embryo. In vitro, both GTPases seem to regulate the stability of lamellipodia of head mesoderm cells. When dnRho or dnRac is overexpressed, head mesoderm cells produce fewer transient lamellipodia with a longer average lifespan. DnRho also increases the number of stable lamellipodia. It may be the cumulative effect of these disruptions that significantly reduces the tendency of anterior mesendoderm explants to move in the normal direction. We speculate that this results in an overall decrease in directed head mesoderm translocation during gastrulation and consequently, defective head structures.
DISCUSSION
Rho and Rac are well-known modulators of the actin cytoskeleton that have been shown to be important for cell polarity and motility in a variety of cell types in culture, as well as during embryonic development. While the biochemical effects of specific GTPases are likely to be similar in most cells, the response of specific cell types to their activity varies. Our previous work examined the behavior of individual cells of the Xenopus trunk mesoderm during gastrulation and showed that distinct functions of Rho and Rac are required for the polarized mediolateral cell intercalation that drives convergent extension. The repertoire of cellular responses to perturbations of Rho and Rac activity, however, was different to that previously reported in cultured cell lines. This work also revealed that disruption of Rho or Rac inhibits head development in these embryos (Tahinci and Symes, 2003) and is consistent with previous work in Xenopus suggesting that RhoA is important for head formation (Wunnenberg-Stapleton et al., 1999). These anterior defects appeared to be due to morphogenetic problems and not to be consequence of earlier failures in mesoderm induction, since goosecoid, a gene specific to prospective head mesoderm, was still appropriately expressed (Tahinci and Symes, 2003). Thus, in this study, prospective head mesoderm cells were analyzed to identify the roles of Rho and Rac in their migration.
Mesoderm migration is mostly studied by using dispersed cells seeded onto an artificial fibronectin substratum, since the opaqueness of the Xenopus gastrula prevents in situ observations. However, in the Xenopus gastrula, mesoderm cells do not move individually, but as a coherent cell mass. The substratum for this movement, the inner surface of the BCR, is rather complex. Fibronectin in the BCR extracellular matrix is essential for migration (Ramos and DeSimone, 1996; Winklbauer and Keller, 1996), justifying the use of fibronectin substrata in vitro. In addition, a matrix-binding form of PDGF-A is involved in the control of mesoderm spreading and translocation (Symes and Mercola, 1996; Nagel et al., 2004). Moreover, mutual repulsion between migratory mesoderm cells and the cells of the BCR substratum is essential for mesoderm translocation (Wacker et al., 2000; Winklbauer et al., 2001). Since all these factors can possibly modulate the context for Rac and Rho function in head mesoderm cells, we compared the effects of these GTPases in single cells on fibronectin in vitro, in cells moving as aggregates on BCR matrix in vitro, and in the embryo.
All Rac and Rho mutant forms, whether constitutively active or dominant negative, disrupted the shingle arrangement of migratory mesoderm cells in the embryo, which is considered indicative of normal oriented migration of these cells towards the animal pole. Consistent with this, they also diminish the directionality of mesoderm explant migration on conditioned substratum, which is not a general response to overexpression of factors in the head mesoderm (for example see (Nagel et al., 2004)). However, in each case, explants are still significantly biased towards the animal pole, suggesting that compromised Rac or Rho signaling does not interfere with recognition of the orienting cues itself, but perhaps with their efficient translation into directional explant movement. Alternatively, it is possible that experimentally modulating the activity of each GTPase separately is not sufficient to completely abolish directionality. In other cell types, for example, the neutrophil-like HL-60 cells, cell polarity depends on the activity of Rac and Rho in two divergent pathways (Xu et al., 2003). Activation of Rac regulates the “front” of the cell through Gi-mediated production of 3′-phosphoinositol lipids and F-actin whereas activated Rho controls the “rear” and “sides” via a Rho-dependant kinase and myosin II (Xu et al., 2003). Whether the effects that are common to all mutant forms are caused by the same primary defect, for example in the cellular response to an orienting, PDGF-dependent signal, or due to different deficiencies remains to be elucidated.
In addition to this common effect on the efficiency of directional migration, specific roles for Rac and Rho are apparent. As often found for cultured cell lines (Ridley and Hall, 1992a), Rac seems to stimulate lamellipodia formation in head mesoderm cells. Dominant negative Rac diminishes lamellipodia both in vitro and in the embryo, and activated Rac has the opposite effect. The observed decreased or increased spreading of mesoderm cells on the BCR, respectively, could be an indirect consequence of this effect, but we cannot exclude an additional, direct role of Rac in cell attachment to the BCR. Also, the reduced velocity of mesoderm cell migration in vitro is probably a consequence of impaired lamellipodia formation.
Interestingly, Rho seems to have different effects in vitro and in the embryo. Inhibiting Rho function in the embryo has little effect on cell morphology, except cell orientation, and on conditioned substrate, directionality of migration is mildly diminished, but migration velocity is normal. This suggests that Rho function is not essential for migration when mesoderm cells move as an aggregate, as in the embryo or in explants on conditioned substratum. In fact, that caRho decreases spreading of cells on the BCR in vivo implies that Rho activity must be kept at a low level in the migrating mesoderm. However, in isolated cells on fibronectin, Rho appears to be active since its inhibition, as well as its increased activation, modulate cell shape, lamellipodial dynamics and the mode of translocation dramatically. For example, we found that activation of Rho affects migration by controlling the retraction of the lagging edge of the cell in dispersed cultures. A similar observation has also been made in other cell types including antigen-presenting dendritic cells (DC cells), monocyte and hemocytes (Worthylake et al., 2001; Swetman et al., 2002; Stramer et al., 2005). It is not clear however, whether some of these effects are also present in the embryo, and responsible for impaired cell orientation, but went unnoticed since cell behavior could not be observed directly.
In contrast to head mesoderm cells, the motility of trunk mesoderm cells engaged in convergent extension has only been studied in explants, since isolated cells do not engage in lamellipodia or filopodia formation (Symes et al., 1994). This makes it difficult to compare the effects of Rho and Rac mutant forms in both tissues, given our presently available data. Obviously, a cell’s environment can impact its response dramatically. For example, when human mammary epithelial cells are spatially organized in three-dimensional basement membrane cultures, growth and adhesion pathways are coupled and bidirectional, but when cultured as a two-dimensional monolayer, the same signaling pathways become uncoupled (Wang et al., 1998). In addition, within tissues mechanical forces may affect a cells’ response to Rho. It has been shown that extrinsically applied force can promote the assembly of stress fibers and focal adhesions in cells in which myosin activity is inhibited (Riveline et al., 2001). Nevertheless, a reduction in the rate of migration on inhibition of Rho or Rac is also seen in trunk mesoderm cells in intact explants (Tahinci and Symes, 2003) as well as in other cell types and organisms including border cell migration in the Drosophila ovary (Murphy and Montell, 1996), the migration of mammalian epithelial cells (Valles et al., 2004), and smooth muscle cell migration (Melnychuk et al., 2004). Currently, we cannot distinguish whether the observed differences in the trunk and head mesoderm with respect to lamellipodial dynamics are due to the cell environment, the kind of motility (intercalating versus migratory), or cell type. Taken together however, these data suggest that, like intercalating trunk mesoderm cells, Rho and Rac have distinct functions in head mesoderm cell motility. The challenge for future studies is the identification of the mechanisms underlying the differences between these two cell types.
EXPERIMENTAL PROCEDURES
Embryos
Xenopus embryos were fertilized in vitro, dejellied in 2% cysteine, pH 7.8, and cultured in 10% Marc’s Modified Ringer (0.1× MMR, (Peng, 1991)) at temperatures between 14°C and 23°C as previously described (Ataliotis et al., 1995). Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967).
mRNA Synthesis and Microinjection
Synthetic mRNA transcripts encoding the dominant negative (RhoN19, RacN17) or constitutively active forms (RhoV14, RacV12) of RhoA and Rac1 were transcribed from the SP6 promoter of pCS2-RhoN19, pCS2-RacN17, pCS2-RhoV14 and pCS2-RacV12 respectively, using the mMessage mMachine kit (Ambion, Austin, TX) as previously described (Tahinci and Symes, 2003). Similarly, a membrane-targeted form of the Green Fluorescent Protein (GAP43-GFP) was transcribed from pCS2+/GAP43-GFP (a generous gift from Dr. E. M. De Robertis, University of California, Los Angeles; (Kim et al., 1998)).
Embryos were injected into both dorsoanterior blastomeres at the 2–4 cell stage, targeting to the prospective head mesoderm (according to Xenopus fate maps; (Lane and Sheets, 2002)). A total of 2ng mRNA was injected per embryo. 300–1000pg mRNA encoding dominant negative (dn) or constitutively active (ca) RhoA or Rac1 was coinjected with mRNA encoding GAP43-GFP. GAP43-GFP mRNA was used as an inert control for injections.
Microdissection
For dissociated cells, embryo dissections were performed at the beginning of gastrulation (stage 10). Anterior mesoderm was excised from early gastrulae with mounted eyebrow hairs in 1× Modified Barth’s Solution (1× MBS, (Peng, 1991)). Dissections were carried out under GFP fluorescence to ensure only GFP-labeled cells were taken. These explants were then dissociated into single cells in Ca2+- and Mg2+-free MBS containing 0.1mM EDTA (Winklbauer et al., 1991). The cells were then plated in 1×MBS on glass coverslips for confocal microscopy or Greiner tissue culture dishes (Greiner Bio-One Inc., Longwood, FL) for fluorescence microscopy. Both the acetone rinsed coverslips and tissue culture dishes were coated with 50μl of 200 μg/ml bovine plasma fibronectin (FN; Sigma-Aldrich) for 45min, and then blocked with 100μl of 50 mg/ml bovine serum albumin. The dispersed cells were then monitored by time-lapse confocal microscopy (Zeiss Inverted Axiovert 100 M BP Laser Scanning Microscope 510; see below) or were fixed after 1 hour in 4% formaldehyde/0.1% Triton-X-100, and stained with 5μM TRITC-conjugated phalloidin (Sigma-Aldrich) for 30min.
To assay directional migration in vitro, substrate was conditioned according to (Nagel and Winklbauer, 1999). Stage 10 blastocoel roof (BCR) explants were held against the bottom of tissue culture dishes (Greiner Bio-One Inc., Longwood, FL) for 2 hours. After removal of the BCR, the substratum was saturated with 50 mg/ml of BSA. The movement of anterior mesendoderm explants containing about 200 cells each on conditioned substratum was observed by recording explant positions initially, and 1 hour later. Changes of positions were scored as being towards the animal pole or away from it and analyzed statistically using the one-sided Welch-test, with significance levels high α<0.005 and moderate α=0.05.
Time-lapse movies
Time-lapse movies were made of dispersed anterior mesoderm cells by the capture of an image once every minute for one hour. The total distance traveled by each cell was determined by calculating the sum of the distance its center moved between continuous frames of a movie. This figure was then used to calculate the rate of cell migration. In addition, using the first 15 minutes of each movie, every cell was evaluated for changes in the number and stability of its lamelliform protrusions, elaborate sheet-like extensions of the plasma membrane. The activity of each lamellipodia was classified according to whether it was present in every frame of the sequence (stable), or it appeared and then disappeared (transient). The lifespan of the transient protrusions was measured by calculating the time elapsed between appearance and retraction. Cell area was calculated in NIH image 1.62 by outlining the cell. A minimum of 5 cells in 3 independent experiments was analyzed for each condition. All measurements and data analyses were performed by using NIH image 1.62. Overall cell height was calculated by addition of the total number of optical sections through a cell in a Z-series. Statistical analysis was carried out using the Student t-test (P<0.05).
Scanning Electron Microscopy
Embryos were fixed in 2.5% formaldehyde and 2.5% glutaraldehyde in MBS, and BCRs were removed with needles. Specimens were postfixed in 2% OsO4, dehydrated in a graded ethanol series, critical point dried, and sputter coated with gold-palladium.
Acknowledgments
We would like to thank Eddie De Robertis for GAP43-GFP and Alan Hall, Marc Symons, Kim Tolias and Lewis Cantley for the Rho and Rac plasmids. We would also like to thank Vickery Trinkaus-Randall for her help and generosity in using the confocal microscope and Melanie Van Stry and John Lynch for help with data analysis and critical reading of the manuscript. This work was support by NIH/NCI RO1 CA87375 to KS, Massachusetts Department of Public Health Breast Cancer Research Grant (DPH-34080066071) to ET and by Canadian Institutes of Health Research grant (MOP-53075) and a Canada Foundation for Innovation grant to RW.
Grant information: Karen Symes: NIH/NCI CA 87375 Emilios Tahinci: Massachusetts Department of Public Health Breast Cancer Research Grant (DPH-34080066071) Rudi Winklbauer: CIHR (MOP-53075) and CFI
References
- Ataliotis P, Symes K, Chou MM, Ho L, Mercola M. PDGF signalling is required for gastrulation of Xenopus laevis. Development. 1995;121:3099–3110. doi: 10.1242/dev.121.9.3099. [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–988. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Gen Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Keller R, Winklbauer R. The cellular basis of amphibian gastrulation. Curr Top Dev Biol. 1992;27:39–89. doi: 10.1016/s0070-2153(08)60532-3. [DOI] [PubMed] [Google Scholar]
- Keller RE, Schoenwolf GC. An SEM study of cellular morphology, contact and arrangement as related to gastrulation in Xenopus laevis. Wilhelm Roux’s Arch Dev Biol. 1977;182:165–186. doi: 10.1007/BF00848055. [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125:4681–4690. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The ras-related proteins Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MC, Sheets MD. Rethinking axial patterning in amphibians. Dev Dyn. 2002;225:434–447. doi: 10.1002/dvdy.10182. [DOI] [PubMed] [Google Scholar]
- Liu JP, Jessell TM. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development. 1998;125:5055–5067. doi: 10.1242/dev.125.24.5055. [DOI] [PubMed] [Google Scholar]
- Maddox AS, Burridge K. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J Cell Biol. 2003;160:255–265. doi: 10.1083/jcb.200207130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish rho kinase 2 acts downstream of wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Melnychuk RM, Streblow DN, Smith PP, Hirsch AJ, Pancheva D, Nelson JA. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Galpha12. J Virol. 2004;78:8382–8391. doi: 10.1128/JVI.78.15.8382-8391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AM, Montell DJ. Cell type-specific roles for Cdc42, Rac and RhoL in Drosophila oogenesis. J Cell Biol. 1996;133:617–630. doi: 10.1083/jcb.133.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- Nagel M, Winklbauer R. Establishment of substratum polarity in the blastocoel roof of the Xenopus embryo. Development. 1999;126:1975–1984. doi: 10.1242/dev.126.9.1975. [DOI] [PubMed] [Google Scholar]
- Nakatsuji N. Studies on the gastrulation of amphibian embryos: cell movement during gastrulation in Xenopus laevis embryos. Wilhelm Roux’s Arch Dev Biol. 1975;178:1–14. doi: 10.1007/BF00848358. [DOI] [PubMed] [Google Scholar]
- Nakatsuji N, Smolira MA, Wylie CC. Fibronectin visualized by scanning electron microscopy immunocytochemistry on the substratum for cell migration in Xenopus laevis. Dev Biol. 1985;107:264–268. doi: 10.1016/0012-1606(85)90395-1. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publishing Company; 1967. [Google Scholar]
- Nobes CD, Hall A. Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem Soc Trans. 1995;23:456–459. doi: 10.1042/bst0230456. [DOI] [PubMed] [Google Scholar]
- Peng HB. In: Solutions and ProtocolsXenopus laevis: Practical uses in cell and molecular biology. Kay BK, Peng HB, editors. San Diego: Academic Press; 1991. pp. 657–662. [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Ramos JW, DeSimone DW. Xenopus embryonic cell adhesion to fibronectin: position-specific activation of RGD/synergy site-dependent migratory behavior at gastrulation. J Cell Biol. 1996;134:227–240. doi: 10.1083/jcb.134.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the smal GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Distinct patterns of actin organization regulated by the small GTP-binding proteins Rac and Rho. Cold Spring Harb Symp Quant Biol. 1992a;57:661–671. doi: 10.1101/sqb.1992.057.01.072. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992b;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman J. Rac ’n Rho: the music that shapes a developing embryo. Dev Cell. 2001;1:321–331. doi: 10.1016/s1534-5807(01)00053-3. [DOI] [PubMed] [Google Scholar]
- Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, Martin P. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, Sakagami H, Kondo H, Nozawa S, Aiba A, Katsuki M. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17:3427–3433. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- Swetman CA, Leverrier Y, Garg R, Gan CH, Ridley AJ, Katz DR, Chain BM. Extension, retraction and contraction in the formation of a dendritic cell dendrite: distinct roles for Rho GTPases. Eur J Immunol. 2002;32:2074–2083. doi: 10.1002/1521-4141(200207)32:7<2074::AID-IMMU2074>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Symes K, Mercola M. Embryonic mesoderm cells spread in response to PDGF and signalling by PI3 kinase. Proc Natl Acad Sci USA. 1996;93:9641–9644. doi: 10.1073/pnas.93.18.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes K, Yordán C, Mercola M. Morphological differences in Xenopus embryonic mesodermal cells are specified as an early response to distinct threshold concentrations of activin. Development. 1994;120:2339–2346. doi: 10.1242/dev.120.8.2339. [DOI] [PubMed] [Google Scholar]
- Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Valles AM, Beuvin M, Boyer B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J Biol Chem. 2004;279:44490–44496. doi: 10.1074/jbc.M405144200. [DOI] [PubMed] [Google Scholar]
- Wacker S, Grimm K, Joos T, Winklbauer R. Development and control of tissue separation at gastrulation in Xenopus. Dev Biol. 2000;224:428–439. doi: 10.1006/dbio.2000.9794. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent Extension. The Molecular Control of Polarized Cell Movement during Embryonic Development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber U, Paricio N, Mlodzik M. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development. 2000;127:3619–3629. doi: 10.1242/dev.127.16.3619. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Keller RE. Fibronectin, mesoderm migration, and gastrulation in Xenopus. Dev Biol. 1996;177:413–426. doi: 10.1006/dbio.1996.0174. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Medina A, Swain RK, Steinbeisser H. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature. 2001;413:856–860. doi: 10.1038/35101621. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Nagel M. Directional mesoderm cell migration in the Xenopus gastrula. Dev Biol. 1991;148:573–589. doi: 10.1016/0012-1606(91)90275-8. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Schurfeld M. Vegetal rotation, a new gastrulation movement involved in the internalization of the mesoderm and endoderm in Xenopus. Development. 1999;126:3703–3713. doi: 10.1242/dev.126.16.3703. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Selchow A, Nagel M, Stoltz C, Angres B. Mesoderm cell migration in the Xenopus gastrula. In: Keller R, Clark WH, Griffin F, editors. Gastrulation: Movements, Patterns, and Molecules. New York: Plenum Press; 1991. pp. 147–168. [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunnenberg-Stapleton K, Blitz IL, Hashimoto C, Cho KW. Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development. 1999;126:5339–5351. doi: 10.1242/dev.126.23.5339. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]