Abstract
A boy with developmental delay, particularly of speech, a distinct face, antineutrophil cytoplasmic antibodies, and recurrent infections was found to have an apparently balanced de novo t(1;6)(q32.3;q22.3) translocation. Fluorescent in situ hybridisation with BAC/PAC clones and long range polymerase chain reaction products assessed in the human genome sequence localised the chromosome 1 breakpoint to a 9.8 kb segment within a hypothetical gene, LOC388735, and the chromosome 6 breakpoint to a 12.8 kb segment in intron 4 of the T‐cell lymphoma breakpoint‐associated target 1 (TCBA1) gene. Disruption and/or formation of TCBA1 fusion genes in T cell lymphoma and leukaemia cell lines suggests a role for this gene in tumorigenesis. The isolated mouse Tcba1 gene shows 91% amino acid sequence similarity with human TCBA1. It is expressed in fetal and adult brain and with lower levels in liver and testis. The human gene has been reported to be expressed exclusively in brain and thymus. Reduced TCBA1 expression in brain and thymus may explain at least some of the symptoms in this patient. It is concluded that germline alterations of the TCBA1 gene are associated with developmental delay and typical physical features.
Keywords: chromosome breakpoint, de novo translocation, developmental delay, recurrent infections, TCBA1 gene
De novo balanced chromosome rearrangements that disrupt or otherwise inactivate genes in the breakpoint regions can cause mental retardation or congenital malformations or both.1,2,3 The cytogenetic and molecular characterisation of disease associated balanced chromosome rearrangements offers a successful instrument for identifying genes involved in inherited disease. Over the past decade, well over 100 genetic loci and genes of Mendelian disorders have been found by such positional cloning approaches.4,5 Here we describe our analysis of a de novo t(1;6)(q32.3;q22.3) translocation in a dysmorphic and developmentally delayed boy with an early childhood history of recurrent chest infections. The constitutional chromosome breakpoint on chromosome 6q22 is of particular interest, because it coincides with a common breakpoint cluster region in T cell malignancies. The TCBA1 gene is thought to be a target gene for the tumour specific chromosome rearrangements; however, its function remains to be elucidated.6 Our patient provides important clues as to the phenotypic effects of constitutional TCBA1 haploinsufficiency.
Methods
Patient
The patient, a boy, was clinically assessed by one of us (DTP) and recruited to the study with parental consent. He was the oldest child of healthy non‐consanguineous parents. There was no history of miscarriages. He was born at 38 weeks' gestation. His birth weight was on the 50th centile, and his occipito‐frontal head circumference (OFC) was between the 75th and 91st centiles. He was found to have an atrioventricular septal defect, which closed spontaneously in the first few months of life, and a slightly dysplastic pulmonary valve. Macrocephaly was also noted and thought to be familial, with a paternal OFC of 5 cm above the 99.6th centile, and maternal OFC on the 91st centile. He had bilateral orchidopexies for undescended testes, and a fundoplication for marked gastro‐oesophageal reflux. Other problems included recurrent chest infections, which had improved, asthma, and enuresis. His development was delayed, and formal assessment at 17 months showed a mental age of 12.3 months (GQ = 78). He had general learning difficulties, especially in the area of expressive speech.
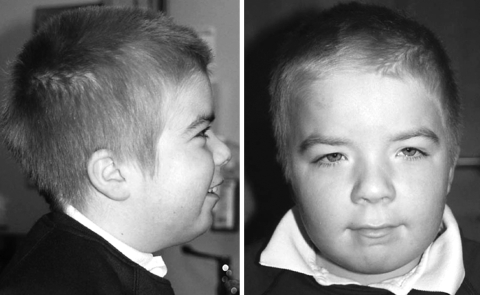
When assessed at the age of five years, his height was on the 25th centile, weight on the 75th centile, and OFC between the 75th and 91st centiles. He was very fair skinned and had very blond, almost white hair, although both his parents had darkish hair. He was hyperteloric, and had blue eyes and small palpebral fissures. He also had prominent corneal nerves. His nose was prominent and he had a thin, tented upper lip. He had fetal finger pads, overlapping third and fifth toes, very hyperextensible joints, and a slightly hirsute back. On follow up at the age of eight years his facial features had coarsened and his hair had become darker (fig 1). He had also been extensively investigated for frequent loose stools, for which so far no cause had been found. He continued to have difficulties with his motor skills, and communication, particularly in the area of expressive speech.
Figure 1 Profile and facial view of the t(1;6) translocation patient at the age of eight years. Written permission was obtained from the child's parents for these images to be reproduced.
Investigations have included cranial magnetic resonance imaging, which showed some enlargement of lateral and third ventricles with no evidence of aqueduct stenosis. A renal ultrasound, EEG, thyroid function test, sweat test, and lysosomal enzyme screen were normal. Immunological studies did not show any specific abnormalities of the lymphocyte subpopulations or of immunoglobulin and antibody production. However, he was found to have raised antineutrophil cytoplasmic antibodies of the cytoplasmic pattern (C‐ANCA), which are often associated with vasculitides.
Fluorescence in situ hybridisation mapping
BAC and PAC clones were selected from the Wellcome Trust Sanger Institute ensembl contigs (http://www.ensembl.org) and obtained from the resource centre primary database of the German Human Genome Project (http://www.rzpd.de). In order to amplify larger (approximately 10 kb) BAC/PAC subfragments, the Expand Long Template polymerase chain reaction (PCR) system (Roche Products, Basel, Switzerland) was used according to the recommendations of the manufacturer, with a series of primer pairs (table 1) chosen from the genomic sequence of breakpoint spanning clones. Genomic BAC/PAC DNAs and their long range PCR products were labelled with biotin‐16‐dUTP or digoxigenin‐11‐dUTP (Roche) by standard nick translation. Fluorescence in situ hybridisation (FISH) was carried out on metaphase spreads from EBV transformed lymphoblastoid cells using standard molecular cytogenetic techniques.3
Table 1 Long range polymerase chain reaction fragments of BAC/PACs for fluorescence in situ hybridisation mapping.
| Fragment | Forward primer (5′–3′) | Reverse primer (5′–3′) | Position in clone | |||
|---|---|---|---|---|---|---|
| 157N22A | gag aat gcc act gag cta ct | agc cct cac aga tga gat tc | 481–13590 bp | |||
| 157N22B | gga tag ctt tcc agc acc ta | aac tgt agt gag cgt gct tc | 14954–26020 bp | |||
| 157N22C | tgc tgt ttc ctc tgt ctc ag | gtt ctg ctg gaa ctg cag at | 51120–62350 bp | |||
| 157N22D | ggt ctg ggt tct atg tca ct | gta gaa tct tgg agc agc tc | 61421–73681 bp | |||
| 157N22E | agc tgt ggc tca gta gaa ac | atc aag cta gtc aga ggc tg | 26501–39350 bp | |||
| 338C15A | acc agc ttc atg tgg cta tc | act gag ctt gtc tgc cat ct | 7631–20200 bp | |||
| 338C15B | cct gtc aag tgc aca gaa tg | tct cag atg cca gaa tcc ag | 28441–42030 bp | |||
| 338C15D | tgg tga cac tgg agc att ct | cgt act gac tac gga tgt tc | 54011–63770 bp |
Isolation of the mouse Tcba1 gene
Total RNA was extracted from mouse tissues or whole embryos with Trizol reagent (Invitrogen, San Diego, California, USA) and reversely transcribed using Superscript III reverse transcriptase (Invitrogen). cDNA was synthesised from 3 μg of total RNA at 55°C with mouse Tcba1 reverse transcription primer (5′‐cgattcgcagagacttaga‐3′); 2 μl of this cDNA were then used as template in a PCR reaction with mouse Tcba1 forward (5′‐atgggttattgcagtggca‐3′) and reverse primers. PCR was carried out with an initial denaturation at 94°C for three minutes, 30 cycles of 94°C for 45 seconds, 56°C for 45 seconds, 72°C for 1.5 minutes, and a final 10 minute extension at 72°C. To obtain the full length cDNA sequence, rapid amplification of cDNA ends (RACE) was done with the SMART RACE cDNA amplification kit (Clontech, Palo Alto, California, USA), according to the manufacturer's instructions. Briefly, 5′‐RACE PCR was undertaken with a Tcba1‐specific primer (5′‐agcgatggtcttctggagcccagtctgg‐3′) and Universal Primer A mix from the kit. 3′‐RACE was done with another gene specific primer (5′‐gtcccccagtcacatcctttaggcgaacac‐3′) and Universal Primer A mix. PCR was carried out with five cycles of 94°C for 30 seconds, 72°C for three minutes, five cycles of 94°C for 30 seconds, 70°C for 30 seconds, 72°C for three minutes, and 25 cycles of 94°C for 30 seconds, 68°C for 30 seconds, 72°C for three minutes, with a final 10 minute extension at 72°C. The PCR products were cloned into pCR2.1‐TOPO vector (Invitrogen) and sequenced.
DNA sequence analysis
DNA sequence analysis was done using the genome BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/). Amino acid sequence alignment and transmembrane helix prediction were performed using the TmHMM and Clustal programs of Heidelberg Unix Sequence Analysis Resources (HUSAR) (http://genius.embnet.dkfz‐heidelberg.de).
Results and discussion
Chromosomal breakpoint mapping
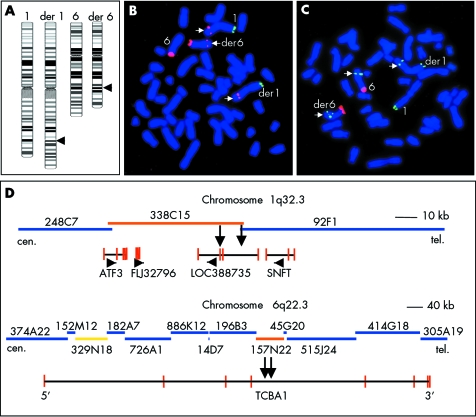
Chromosome banding analysis of this patient revealed an apparently balanced reciprocal translocation involving the exchange of material between the long arms of chromosomes 1 and 6 (fig 2A). The parental karyotypes were normal. In order to map the de novo translocation breakpoints at higher DNA resolution, we have assembled BAC/PAC contigs of the critical chromosome regions 1q32.3 and 6q22.3 according to the database (fig 2D). Individual BAC/PACs from the breakpoint regions were co‐hybridised with chromosome 1pter and 6pter identification probes to the patient's metaphase spreads. BAC RP11‐338C15 at 209.4 Mb on chromosome 1 and PAC RP1‐157N22 at 124.6 Mb on chromosome 6 produced FISH signals on the normal chromosome 1 and 6, respectively, and on both derivative chromosomes (data not shown), as expected for breakpoint spanning clones. To narrow down the breakpoint regions further, three long range PCR products—338C15A, 338C15B, 338C15D—were generated from the chromosome 1 breakpoint BAC and five PCR products—157N22A, B, C, D, and E—from the chromosome 6 breakpoint PAC (table 1). The 9.8 kb BAC fragment 338C15D produced a split hybridisation signal on the derivative chromosomes (fig 2B) and therefore must contain the 1q32.3 breakpoint. Similarly, the 6q22.3 breakpoint was localised to the 12.8 kb PAC fragment 157N22E, which hybridised to the normal chromosome 6 and both derivative chromosomes (fig 2C).
Figure 2 Molecular cytogenetic characterisation of the t(1;6)(q32.3;q22.3) translocation. (A) Ideograms of the patient's normal and derivative chromosomes 1 and 6. The breakpoints predicted by G band analysis are indicated by arrows. (B,C) High resolution fluorescence in situ hybridisation (FISH) mapping of BAC/PAC subfragments to patient's metaphase spreads. PACs CTB‐14E10 (labelled by FITC in green) and CTB‐62I11 (labelled by Cy3 in red) from the short arm ends of chromosome 1 and 6, respectively, were used for chromosome identification. The 1q32.3 breakpoint spanning polymerase chain reaction (PCR) fragment 335C15D (B; red signals indicated by arrows) produced signals on the normal chromosome 1, the der(1), and the der(6). The 6q22.3 breakpoint spanning PCR fragment 157N22E (C; green signals indicated by arrows) hybridised to the normal chromosome 6 and both derivative chromosomes. (D) BAC/PAC (blue, orange, and yellow bars) contigs of the breakpoint regions on chromosomes 1q32.3 and 6q22.3. The black lines below the contigs indicate the location of genes in the breakpoint regions, vertical red lines indicate exon sequences, arrowheads the direction of transcription. The breakpoint containing clones RP11‐338C15 and RP1‐157N22 are indicated by orange bars. The 1q32.3 and 6q22.3 breakpoint regions, respectively, are indicated by two vertical black arrows. The yellow clone RP3‐329N18 is reported7 to vary in copy number between individuals.
Disruption of the TCBA1 gene on chromosome 6q22.3
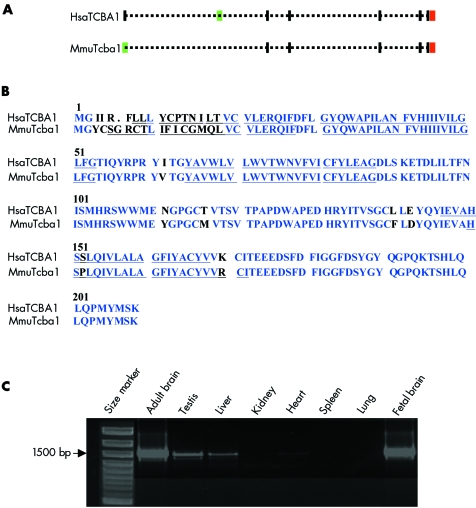
According to our high resolution FISH mapping results the translocation breakpoint on chromosome 6 lies within intron 4 of the TCBA1 gene (fig 2D), which encodes a transmembrane protein. The mouse Tcba1 cDNA (GenBank accession number AY940478) was obtained from mouse brain RNA by reverse transcriptase polymerase chain reaction (RT‐PCR) with primers directed against conserved regions of human TCBA1 and subsequent 5′‐ and 3′‐RACE PCRs. Human TCBA1 has eight exons with the initiation codon located in exon 2, whereas the mouse gene has only seven exons and the initiation codon in exon 1 (fig 3A). Mouse Tcba1 lacks the exon corresponding to human TCBA1 exon 2. In both genes the stop codon is located in the last exon. Both the human and the mouse proteins are endowed with four transmembrane helices and share 91% amino acid sequence similarity (fig 3B). This evolutionary conservation is considered a good indicator of the gene's functional significance.
Figure 3 (A) Genomic structure of human (Homo sapiens, HSA) TCBA1 and mouse (Mus musculus, MMU) Tcba1. Exons are indicated by vertical bars. The initiation codons (green bars) are located in exon 2 of human TCBA1 and exon 1 of mouse Tcba1, respectively; the stop codons (red bars) are located in the last exon. (B) Amino acid sequence alignments of human TCBA1 and mouse Tcba1. Conserved amino acids are marked in blue. The four predicted transmembrane helix domains are underlined. (C) Reverse transcriptase polymerase chain reaction carried out with mouse RNA preparations from adult brain, testis, liver, kidney, heart, spleen, lung, and fetal brain. Tcba1 is highly expressed in adult and fetal brain with lower expression in testis and liver.
The human TCBA1 gene is reported to be expressed exclusively in fetal and adult brain and thymus.6 Our RT‐PCR experiments in the mouse showed a high level of Tcba1 expression in fetal and adult mouse brain and lower expression levels in testis and liver (fig 3C). TCBA1 is involved in human T cell lineage specific chromosome aberrations at 6q21–q22.6 Similar to our patient, the T cell lymphoblastic lymphoma cell line, HT‐1, has a breakpoint in PAC RP1‐157N22 (within intron 4 of TCBA1), fusing TCBA1 to the SUMO‐I‐specific protease (SUSPI) gene. In the adult T cell leukaemia cell line, ATN‐1, a breakpoint is located in PAC RP1‐196B3 (within intron 3 of the TCBA1 gene). In this context, it is also interesting to note that PAC RP3‐329N18, which is located in intron 1 of TCBA1 (fig 2D, yellow bar), was reported to show copy number variation among normal individuals. In an array based comparative genomic hybridisation study, one of 39 unrelated healthy individuals showed a copy loss of the RP3‐329N18‐syntenic segment.7 However, by PAC FISH on metaphase spreads of 53 control individuals we always observed hybridisation signals of equal intensity on both chromosomes 6q22 (data not shown). Thus copy number gains or losses involving RP3‐329N18‐syntenic sequences appear to be relatively rare. Nevertheless, it is possible that loss of a large DNA segment from intron 1 affects TCBA1 regulation and thus contributes to interindividual differences in the susceptibility to haematopoetic malignancies. The clustering of constitutional and tumour breakpoints within TCBA1, as well as large scale copy number variations involving intronic sequences, suggests that this genomic region is highly dynamic and predisposes to chromosome rearrangements.
Alterations of TCBA1 have so far not been connected with C‐ANCA associated vasculitides such as Wegner's granulomastosis, microscopic polyangiitis, or Churg‐Strauss syndrome. However, the finding of raised C‐ANCA levels in our patient in association with his history of recurrent respiratory problems, and also his gastrointestinal symptoms, could be compatible with a vasculitis, and investigations are ongoing.
Disruption of a hypothetical gene on the chromosome 1q32.2
The breakpoint region on chromosome 1q32.3 contains two hypothetical and two known genes (fig 2D). The activating transcription factor 3 (ATF3) gene lies approximately 50 kb proximal and the small nuclear factor isolated from T‐cells (SNFT) gene approximately 30 kb distal to the breakpoint. The 1q32.3 breakpoint spanning fragment 338C15D is contained in the hypothetical gene LOC388735. However, so far the only evidence for the existence of this gene is a single EST derived from anaplastic oligodendroglioma. Neither RT‐PCR of RNAs from fetal and adult brain and multiple other tissues, nor PCR screening of a human brain cDNA library (RZPD No 588) with LOC38873 specific primers (forward 5′‐agtatgtgcatcaccagca‐3′; reverse 5′‐tgagactccaccattacag‐3′), nor northern blots have revealed any transcript(s), supporting this gene (data not shown).
Conclusions
Our patient with de novo translocation t(1;6)(q32.3;q22.3) provides evidence that constitutional inactivation (haploinsufficiency) of the TCBA1 gene causes developmental delay and a distinct phenotype. The expression patterns of the human and mouse genes in fetal and adult brain, thymus, liver, and testis are consistent with some features of our patient, in particular developmental delay and recurrent infections. Although we cannot exclude the formal possibility that a TCBA1 fusion gene is formed by the translocation, our data argue in favour of a loss of function mutation producing a truncated protein. Isolation and partial characterisation of the orthologous mouse Tcba1 gene is a first step towards functional studies in knock‐out mice.
Electronic database information
BLAST program: http://www.ncbi.nlm.nih.gov/BLAST/; Heidelberg Unix Sequence Analysis Resources: http://genius.embnet.dkfz‐heidelberg.de; Ensembl Genome Browser: http://www.ensembl.org; Resource Center Primary Database of the German Human Genome Project: http://www.rzpd.de
Acknowledgements
The work was supported by the German Research Foundation (HA 1374/5‐2) and the Boehringer Ingelheim Foundation.
Abbreviations
FISH - fluorescence in situ hybridisation
OFC - occipito‐frontal head circumference
RACE - rapid amplification of cDNA ends
Footnotes
Conflicts of interest: none declared
References
- 1.Tommerup N. Mendelian cytogenetics. Chromosome rearrangements associated with Mendelian disorders. J Med Genet 199330713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bugge M, Bruun‐Petersen G, Brondum‐Nielsen K, Friedrich U, Hansen J, Jensen G, Jensen P K, Kristoffersson U, Lundsteen C, Niebuhr E, Rasmussen K R, Rasmussen K, Tommerup N. Disease associated balanced chromosome rearrangements: a resource for large scale genotype–phenotype delineation in man. J Med Genet 200037858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wirth J, Nothwang H G, van der Maarel S, Menzel C, Borck G, Lopez‐Pajares I, Brondum‐Nielsen K, Tommerup N, Bugge M, Ropers H H, Haaf T. Systematic characterisation of disease associated balanced chromosome rearrangements by FISH: cytogenetically and genetically anchored YACs identify microdeletions and candidate regions for mental retardation genes. J Med Genet 199936271–278. [PMC free article] [PubMed] [Google Scholar]
- 4.Stankiewicz P, Lupski J R. Genome architecture, rearrangements and genomic disorders. Trends Genet 20021874–82. [DOI] [PubMed] [Google Scholar]
- 5.Abeysinghe S S, Stenson P D, Krawczak M, Cooper D N. Gross rearrangement breakpoint database (GRaBD). Hum Mutat 200423219–221. [DOI] [PubMed] [Google Scholar]
- 6.Tagawa H, Miura I, Suzuki R, Suzuki H, Hosokawa Y, Seto M. Molecular cytogenetic analysis of the breakpoint region at 6q21–22 in T‐cell lymphoma/leukemia cell lines. Genes Chromosomes Cancer 200234175–185. [DOI] [PubMed] [Google Scholar]
- 7.Iafrate A J, Feuk L, Rivera M N, Listewnik M L, Donahoe P K, Qi Y, Scherer S W, Lee C. Detection of large‐scale variation in the human genome. Nat Genet 200436949–951. [DOI] [PubMed] [Google Scholar]