Abstract
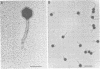
We characterized UC-1, a previously undescribed Escherichia coli phage. UC-1 was observed to have an icosahedral head and a long, flexible, noncontractile tail: its genome consisted of linear double-stranded DNA having a molecular weight of 34 X 10(6). The product of the tonA gene served as at least part of the receptor for UC-1. E. coli tonA strains neither plated nor adsorbed UC-1 well, tonA mutants were selected on the basis of UC-1 resistance, and ferrichrome, a siderophore which utilizes TonA as its receptor, blocked infection. Restriction analyses, DNA-DNA hybridization experiments, and guanine-plus-cytosine determinations demonstrated that UC-1 DNA was unrelated to that of other phages (T1, T5, and phi 80) which employ TonA as a receptor. Also, mutants specifically resistant to UC-1 were isolated. UC-1 may be useful as a probe for investigating TonA, which functions as a receptor for more ligands than any other membrane protein. Study of the resistant mutants may improve our understanding of how phage DNA penetrates the cell envelope.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Braun V., Hantke K., Stauder W. Identification of the sid outer membrane receptor protein in Salmonella typhimurium SL1027. Mol Gen Genet. 1977 Oct 20;155(2):227–229. doi: 10.1007/BF00393164. [DOI] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Dawid I. B. DNA-DNA hybridization on membrane filters: a convenient method using formamide. Biochim Biophys Acta. 1977 Jul 15;477(2):191–194. doi: 10.1016/0005-2787(77)90235-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GAUSE G. F. Recent studies on albomycin, a new antibiotic. Br Med J. 1955 Nov 12;2(4949):1177–1179. doi: 10.1136/bmj.2.4949.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A. C., Stocker B. A. Genetics of sensitivity of Salmonella species to colicin M and bacteriophages T5, T1, and ES18. J Bacteriol. 1977 Jun;130(3):1214–1223. doi: 10.1128/jb.130.3.1214-1223.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Henning U., Jann K. Two-component nature of bacteriophage T4 receptor activity in Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):664–666. doi: 10.1128/jb.137.1.664-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe M. M. Transduction by bacteriophage MU-1. Virology. 1973 Sep;55(1):103–117. doi: 10.1016/s0042-6822(73)81012-8. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Heller K., Coulton J. W., Braun V. Genetic control of hydroxamate-mediated iron uptake in Escherichia coli. J Bacteriol. 1980 Jul;143(1):256–264. doi: 10.1128/jb.143.1.256-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba P. E., McIntosh M. A., Neilands J. B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982 Mar;149(3):880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard K. R., Kleinschmidt A. K., Lake J. A. Caulobacter crescentus bacteriophage phiCbK: structure and in vitro self-assembly of the tail. J Mol Biol. 1973 Dec 15;81(3):349–365. doi: 10.1016/0022-2836(73)90146-0. [DOI] [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Neilands J. B. Iron transport in Salmonella typhimurium LT-2: prevention, by ferrichrome, of adsorption of bacteriophages ES18 and ES18.h1 to a common cell envelope receptor. J Bacteriol. 1976 Aug;127(2):1036–1037. doi: 10.1128/jb.127.2.1036-1037.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Wayne R., Neilands J. B. In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun. 1975 May 19;64(2):687–693. doi: 10.1016/0006-291x(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Reeves P. Outer membrane of Escherichia coli K-12: TSX mutants (resistant to bacteriophage T6 and colicin K) lack an outer membrane protein. Biochem Biophys Res Commun. 1976 Jul 26;71(2):466–471. doi: 10.1016/0006-291x(76)90810-x. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Furukawa H., Mizushima S. Role of lipopolysaccharide and outer membrane protein of Escherichia coli K-12 in the receptor activity for bacteriophage T4. J Bacteriol. 1978 Nov;136(2):693–699. doi: 10.1128/jb.136.2.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Old R., Murray K., Boizes G. Recognition sequence of restriction endonuclease III from Hemophilus influenzae. J Mol Biol. 1975 Feb 25;92(2):331–339. doi: 10.1016/0022-2836(75)90232-6. [DOI] [PubMed] [Google Scholar]
- Pickett C. L., Earhart C. F. Iron uptake in pseudorevertants of Escherichia coli K-12 mutants with multiple defects in the enterochelin system. Arch Microbiol. 1981 Feb;128(4):360–364. doi: 10.1007/BF00405913. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ N. M. SUPPRESSION OF A LAC O-O MUTATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:996–1001. doi: 10.1128/jb.88.4.996-1001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Horn V., Yanofsky C. On the production of deletions in the chromosome of Escherichia coli. J Mol Biol. 1970 Oct 14;53(1):49–67. doi: 10.1016/0022-2836(70)90045-8. [DOI] [PubMed] [Google Scholar]
- Sugden B., De Troy B., Roberts R. J., Sambrook J. Agarose slab-gel electrophoresis equipment. Anal Biochem. 1975 Sep;68(1):36–46. doi: 10.1016/0003-2697(75)90676-4. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Henson J. M., Lee C. S. Isolation and characterization of plaque-forming lambdadnaZ+ transducing bacteriophages. J Bacteriol. 1977 Apr;130(1):354–365. doi: 10.1128/jb.130.1.354-365.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmaar S. O., Cohen J. A. A quantitative assay for DNA-DNA hybrids using membrane filters. Biochem Biophys Res Commun. 1966 Aug 23;24(4):554–558. doi: 10.1016/0006-291x(66)90356-1. [DOI] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamagishi H., Eguchi G., Matsuo H., Ozeki H. Visualization of thermal inactivation in phages lambda and phi80. Virology. 1973 May;53(1):277–282. doi: 10.1016/0042-6822(73)90486-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- van Alphen W., Lugtenberg B., Berendsen W. Heptose-deficient mutants of Escherichia coli K12 deficient in up to three major outer membrane proteins. Mol Gen Genet. 1976 Sep 23;147(3):263–269. doi: 10.1007/BF00582877. [DOI] [PubMed] [Google Scholar]