Abstract
The recent availability of internal tobacco industry documents provides significant insight into industry knowledge and manipulation of tobacco smoke delivery. One critical area of research is the role of smoke chemistry in determining the absorption and effects of smoke constituents, especially harm producing or pharmacologically active compounds. Independent scientific research has suggested that the nicotine dosing characteristics, hence the addiction potential of cigarettes, may be determined in part by the amount of free‐base nicotine in cigarette smoke and its effects on the location, route, and speed of absorption in the body and on the sensory perception effects of the inhaled smoke. Tobacco industry documents describe the use of a number of methods internally for measuring free‐base nicotine delivery. These include the common use of cigarette “smoke pH” as a means to estimate the fraction of free‐base nicotine in the particulate matter (PM) in cigarette smoke, as well as efforts to measure free‐base nicotine directly. Although these methods do not provide accurate absolute measures of free‐base nicotine in smoke, consistencies observed in the findings across the various manufacturers indicate: (1) real relative differences in the acid/base chemistry of the smoke from different brands of cigarettes; (2) a connection between differences in free‐base levels and brand‐dependent differences in sensory perception and smoke “impact”; and (3) levels of free‐base nicotine that are greater than have typically been publicly discussed by the industry. Furthermore, the results of these methods are generally consistent with those of a recent study from the Centers for Disease Control and Prevention which directly measured the free‐base fraction of nicotine across a range of cigarette types. Consideration of the likely fundamental importance of free‐base nicotine levels in cigarette smoke, together with the efforts discussed in the tobacco industry documents to measure such levels, indicates that the public health community would benefit from additional research to assess directly the delivery of free‐base nicotine in cigarette smoke across brands. This may be especially useful for those products (“light”, “ultralight”, “reduced carcinogen”, etc) that have been promoted, either explicitly or implicitly, as “harm reducing”.
Keywords: nicotine, nicotine/analysis, nicotine/pharmacology, tobacco use disorder, tobacco industry
Internal tobacco industry documents, made publicly available as a requirement of the 1998 Master Settlement Agreement,1 provide an important resource for understanding tobacco industry knowledge and intent regarding the delivery of nicotine and other smoke constituents to smokers. In this article, we examine internal documents to assess industry‐measured differences among brands in the forms of nicotine delivered to the smoker. Our research extends observations by earlier reviewers. For example, Hurt and Robertson concluded that the form of nicotine deposited in the lungs has implications for addiction, based on their review of industry documents,2 and Pankow's review of internal RJ Reynolds Tobacco Company (RJRTC) research on the importance of volatilisation in the respiratory tract deposition of nicotine extended those conclusions.3 Their findings indicate that the chemistry of cigarette smoke may be manipulated through intended or unintended changes to product design, with direct implications for the deposition, metabolism, and effects of specific smoke constituents.
Although it is now accepted that the establishment of nicotine dependence by cigarette smoking requires that cigarettes provide adequate doses of nicotine, there has been relatively little study outside of the tobacco industry of what constitutes minimally acceptable and addictive doses of nicotine.2,4,5 Scientific assessment has conventionally focused on the per cigarette nicotine dose, either through measurement of the total mainstream smoke nicotine produced under standard machine smoking conditions, or as inferred from studies of the metabolised nicotine present in the body after smoking.4,6,7 The addiction potential of a drug can, however, depend on a number of factors beyond dose—for example, the speed of delivery and receptor activation, as well as subjective sensory effects associated with drug administration.8,9,10,11,12 It is thus plausible that the addiction potential of commercial cigarettes may be significantly altered by changes in cigarette design that affect the form of nicotine delivered, the associated sensory effects, the location and route of absorption, and/or the speed with which the cigarette produces pharmacological effects.13,14 The forms of nicotine delivered by cigarettes have consequently received increasing attention in recent discussions of the implications of cigarette smoking on public health.15,16 These discussions include considerations of statements found in the tobacco industry documents.
Acid/base chemistry of nicotine
In tobacco smoke, nicotine can be found in both the smoke particulate matter (PM) and in the gas phase of the smoke.3 The conventional view has been that most of the nicotine in the mainstream smoke is in the PM phase, with 1% or less initially in the gas phase.17,18 In smoke PM, nicotine can exist in three pH‐dependent forms: diprotonated, monoprotonated, and unprotonated (“free‐base”). The diprotonated form has usually been assumed to be of negligible importance in tobacco smoke.3,19,20 While application of the concept of pH to tobacco smoke PM is considerably more complex than its use in aqueous systems,3,21 nevertheless the fraction of free‐base nicotine in PM always increases as the alkalinity of the PM phase smoke increases. In the gas phase, nicotine is found only as free‐base nicotine.
Drawing on principles of aerosol chemistry and physics, Pankow has described how tobacco smoke PM serves as a carrier that allows nicotine to be transported into the lower respiratory tract.3 In the complete absence of smoke particles, the only nicotine present would be gaseous free‐base nicotine. This nicotine is rapidly removed on inhalation by deposition in the mouth and throat,22 and may produce sensory effects (for example, “impact”) or other physiological responses in the smoker (table 1).22 The majority of deeply inhaled smoke nicotine is retained in the lower respiratory tract.23 While it has been argued that most of this nicotine deposits as protonated PM‐phase nicotine,24 Pankow theorised that PM‐phase nicotine that reaches the lower respiratory tract can deposit there in particle form, or can volatilise (“off‐gas”) as free‐base nicotine with subsequent rapid deposition from the gas phase onto lung tissue.3 The work of Watson et al,25 in which tobacco smoke particulate was captured separately from the gas‐phase and then free nicotine was allowed to off‐gas from the particulates as a way of measuring free nicotine fractions in the particulate, provides further evidence of the volatilisation process. Overall, it appears both plausible, and consistent with assumptions made in tobacco industry documents, that the more free‐base nicotine delivered to the lungs, the greater the initial dose strength and the greater the addiction potential.2,3
Table 1 Possible effects of increases in amount of free‐base nicotine delivered.
| Increased oral absorption | → | Increased activation of oral sensory receptors | → | Heightened sensory “cue” for nicotine delivery |
| Increased efficiency of respiratory deposition | → | Greater retention of total smoke nicotine | → | Greater availability of nicotine for deposition/absorption |
| Increased volatility from inhaled particles | → | More rapid absorption of nicotine in lung tissue | → | Reduced time course of delivery to brain |
Measuring free‐base nicotine
In practice, discussions of the role of free‐base nicotine in cigarette smoke have been complicated by differences of opinion as to how to best measure free‐base nicotine delivery as it is controlled by the acid/base conditions in the smoke. Despite the considerable conceptual differences that exist between measured “smoke pH” values and aqueous pH,20,26,27,28 measurements of cigarette “smoke pH” have been widely attempted as a means to calculate free‐base nicotine deliveries for smoke PM. Such “smoke pH” measurements have generally yielded acidic values that are ∼6.5 or less, resulting in calculated percent free‐base values that range from ∼0.001 to a maximum of ∼0.03.29 At such levels, the amount of free‐base nicotine in the PM that is available for evaporative deposition in the lungs has been presumed to be small.
In response to the fact that typical “smoke pH” measurements are now widely recognised as being unreliable in absolute terms,3,24,30,31 Pankow et al have determined that an assessment of differences among brands can best be obtained by the direct measurement of percent free‐base values in tobacco smoke PM samples.3,21,29 This approach has been separately adopted in recent studies by Pankow et al25 and Watson et al.29
Goals
This paper examines industry measures of free‐base nicotine delivery in order to assess the likelihood of the existence of differences in delivery among brands. These free‐base nicotine values are also examined in the context of industry‐obtained subjective measures of smoke acceptability, including perceived “sensory effects” and smoker “satisfaction”. Finally, the results are considered in terms of the absolute reliabilities of the industry measures of free‐base nicotine as based on “smoke pH” and other methods.
METHODS
More than 7 000 000 tobacco industry documents have been disclosed by the major tobacco companies during litigation processes, and then made publicly available as a result of the 1998 Master Settlement Agreement.1 The documents used in this paper were accessed via the internet through the interface and archival database maintained at Tobacco Documents Online (www.tobaccodocuments.org). Document identification was performed using an index‐based word search of titles, authors, recipients, and other document characteristics (such as date, document type, original file location), as well as keywords and abstracts. Whenever available, full‐text optical character recognition was also used. The searches were conducted employing combinations of terms paired across a set of five term groups as listed below (for example, “nicotine and pH”, “free‐base and nicotine”):
nicotine
free, free‐base, bound, protonated, unprotonated, volatile, extracted, extractable
pH, smoke pH, whole smoke, particulate, vapour phase
method, measure, measurement, absorption, deposition, evaporative, transfer, uptake, denuder, bubbler, nuclear magnetic resonance, NMR, oil/water, o/w
sensory, impact, satisfaction, subjective, perception, pharmacology, physiology.
The relation of terms to each other in the text (that is, paired, or appearing within a specified number of words) was used to refine searches, and word stemming (for example, “‐s”, “‐ed”, “‐ing”) was included where appropriate.
Relevancy was determined based on whether documents described: (1) industry attempts to estimate or directly measure free‐base nicotine levels in cigarette smoke; (2) correlation of free‐base nicotine with sensory perception or subjective measures; or (3) differences in sensory or pharmacological response among cigarette brands. Relevant documents were abstracted and indexed. The resulting document set was surveyed for recurring authors, keywords, codes, or project names that would suggest further avenues for retrieval. An indexed set of 471 documents can be accessed (as of 15 June 2005) at: http://tobaccodocuments.org/product_design/list.php?field_id = 9&resource_id = 23577. Of these, 70 documents are referenced in this paper.
A number of unique difficulties associated with the use of internal industry documents as a source of scientific information must be considered. Industry research was not generally subjected to careful peer review, and details regarding the experimental methods used and the resulting quality of the data are often unavailable, making it difficult to assess the reliability of the science. In addition, the available documents do not always represent the totality of the internal research that was conducted on a particular topic—as indicated by the existence of many partial reports and memos. Finally, within each given company, the documents are authored by numerous different researchers from a range of departments over tens of years, and so findings are sometimes inconsistent and occasionally even contradictory. Comparisons of the documents reveal real company‐to‐company differences in approach to the issues of studying nicotine in tobacco smoke, a finding that must be taken into account.
RESULTS
Industry use of “smoke pH” for estimating free‐base nicotine in tobacco smoke PM
Industry documents indicate a long‐term and industry‐wide reliance on measurements of “smoke pH”, “tar”, and total nicotine delivery as a common means to understand the relative properties of the smoke produced by different cigarette brands.32,33 The “smoke pH” methods that have been utilised internally by tobacco manufacturers are summarised in table 234,35,36,37,38,39; industry uses of “smoke pH” values are summarised in table 3.40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56
Table 2 Survey of internal industry methods for determining “smoke pH”34,35,36,37,38,39.
| Method | Description | Industry use |
|---|---|---|
| Sensabaugh and Cundiff (1967) | Puff‐by‐puff measure of whole smoke (vapour and PM) using solvent‐coated glass electrode in smoke aerosol | RJ Reynolds Tobacco Company (RJRTC): Sensabaugh technique used internally for “several years” before 1971 |
| Brown & Williamson Tobacco Company (BWTC): Used experimentally… “Work reported by Honeycutt, Frank, and Johnson showed a range of over one pH unit… for similar cigarettes of the same brand style.” (1984–1989) | ||
| Backhurst (1966) | Aqueous (cold‐trapped) whole smoke measure using pH electrode | BWTC: Experimental use only |
| Artho and Grob (1966) | Measure of smoke pH of PM collected on Cambridge pad and dissolved in water | BWTC: Adopted as standard method, used until 1987, and in altered form through 1997 |
| Lorillard Tobacco Company (LTC): Used internally by Ihrig, others 1969–1973 | ||
| Linder and Frank (1984) | Puff‐by‐puff measure of smoke pH using PM collected on Cambridge pad | BWTC: “this work was reported in a little‐known file note” in 1984 |
| “Philip Morris” method (1970) | Whole smoke measure, no puff profile | Philip Morris (PMTC): Standard method, at least as early as 1970 |
| Lakritz (1975) | Measure of both whole smoke and vapour phase using platinum‐sliver, silver chloride electrode | LTC: Internal method |
| Harris and Hayes (1977) | Whole smoke measure using pH electrode, modifying Sensabaugh method (with no puff profile) | BWTC: Used experimentally in 1990 |
| ISFET whole smoke method (1997) | Whole smoke measure, modifying Harris‐Hayes method with use of solid‐state probes (not glass electrode) | BWTC: Adopted in 1997 |
Table 3 Summary of internal industry uses of “smoke pH” measures.
| • Across all major manufacturers, as a comparative (relative) measure of free‐base nicotine levels40,44,45,46 |
| • As a means, in conjunction with total nicotine levels, for estimating free‐base nicotine levels47,48 |
| • For comparisons of brands across manufacturers,49 for analysis of consumer preferences,50 and to provide “additional information for development of new brands”51 |
| • To predict changes in product perception based on form of nicotine delivered, as in a 1977 RJR panel study: “Panelists seem to respond to relatively small changes in pH near the region where free nicotine would exist.”52 |
| • As a correlating parameter with smoker perceptual responses including: (a) perceived “impact”,*53,54 and electrophysiological and other responses55; (b) pharmacological satisfaction44; and (c) perception of smoke characteristics such as irritation, tobacco taste, and menthol52,56 |
*“Impact” is considered to be an important sensory cue that is linked to the pharmacological effects of smoking.
As noted above, the main perceived utility of “smoke pH” values to the industry has involved the assumption that they can be used to compute free‐base nicotine deliveries. Indeed, in industry documents, “smoke pH” is frequently discussed as a surrogate for free‐base nicotine levels. For example, in one series of RJRTC documents, “smoke pH” is used as a parameter to assess the implications of different deliveries of free‐base nicotine for brand market share.40,41,42,43 For the period 1964 to 1973, the RJRTC researchers note:
“Our preliminary correlations strongly suggest… that the vigorous, sustained growth in sales of Marlboro (and other Philip Morris brands) and Kool correlates closely with the increased smoke pH, hence increased “free” nicotine and nicotine impact of those brands… Our emphasis should be directed toward free nicotine while pH would provide us with a measure of or tool to effect free nicotine.”41
Other industry approaches for estimating free‐base nicotine deliveries in tobacco smoke PM
Although measured “smoke pH” values have been used extensively for estimating free‐base nicotine in tobacco smoke PM, other techniques were also developed and used by the industry. These techniques are described below and summarised in table 4.
Table 4 Survey of internal industry methods for measurement of free‐base nicotine45,49,56,58,62,76,77,78,79,80.
| Method | Description | Industry comments | Industry use |
|---|---|---|---|
| Shmuk (1953) | Selective solvent extraction, with quantification by chromatography or spectroscopy | LTC (1976): “this should not be a difficult analysis.” | LTC: “Quick check” GC method in place; however, the proposed standard assay “was never acted upon” |
| Extractable nicotine (BWTC ‐1965) | Chloroform extraction of free base nicotine from aqueous solution of smoke PM | BWTC (1965): Good agreement with strength perception, and smoke pH measurements | BWTC: Standard use of extractable nicotine measurements maintained at least until 1984 |
| BWTC (1974, 1977): Unusually high results/adjustments required for low‐tar, low weight cigarettes | |||
| Nuclear magnetic resonance (PMTC ‐1975) | Measure of changes in the chemical shift of the n‐methyl resonance of nicotine. | PMTC (1975): “The results obtained by this method indicate that the condensates are not acidic and the majority of nicotine in condensate exists as the free base” | PMTC: Published research using NMR technology in 1977; further internal use undetermined |
| Headspace nicotine (BWTC ‐1984) | Headspace GC determined for PM collected via Cambridge pad | BWTC: Experiments in 1984 | |
| Gas phase nicotine‐ denuder tubes (PMTC ‐1991) | Use of open‐tube denuders to separate vapour phase from PM nicotine | PMTC (1991): “technique seems to be sufficiently definitive for any significant changes in relative deliveries of nicotine in the vapor phase”; not effective for defining small differences | PMTC: Experiments throughout 1991 |
| (RJRTC ‐1990) | RJRTC (1990): “low rates of nicotine removal relative to expected vapor deposition rates” | RJRTC: used in 1990, replaced by bubbler adsorption 1991 | |
| RJRTC (1991): “uncertainty that the inner walls of the syringe remained sufficiently wetted throughout the smoke aging to act as perfect sinks for nicotine vapor.” | |||
| Bubbler adsorption—volatile nicotine (RJRTC ‐1991) | Smoke passed through bubbler containing water as adsorption medium | RJRTC (1991): Mimics vapour removal with minimal particle deposition characteristic of the human airway | RJRTC: “converted to standard method” in 1991, with major improvement in results; technique “being applied to many studies.” |
| Spinning denuder‐volatile nicotine (RJRTC ‐1991) | Denuder‐based method, using spinning to ensure uniform solvent coating | RJRTC (1991): “Incorporates physical properties of upper airway”… “quantitative measures… can be related to human experience” | RJRTC: Proposed in 1991; results unknown |
BWTC, Brown & Williamson Tobacco Company; LTC, Lorillard Tobacco Company; PMTC, Philip Morris Tobacco Company; RJRTC, RJ Reynolds Tobacco Company.
“Extractable nicotine”
From the 1960s through the 1980s, Brown & Williamson Tobacco Company (BWTC) utilised an internally‐developed extraction method in which smoke PM collected on a glass fibre filter (“Cambridge filter pad”) was equilibrated by placing the “pad” in initially‐pure water. The water was then extracted with chloroform,57 and the nicotine extracted into the chloroform was measured by gas chromatography. The result was expressed as a percentage of the total nicotine delivery.57 This methodology was apparently developed based on the assumption that it is primarily the free‐base nicotine originally in the PM that ends up in the chloroform.
Although it was observed internally that “it has not been established completely that the nicotine extracted with chloroform is the free base”,58 and although the measured “extractable nicotine” values were consistently higher than free‐base nicotine levels calculated from “smoke pH” measures,58,59 the quantity “extractable nicotine” was nevertheless used as a proxy measure for free‐base nicotine delivery.59 Moreover, after accounting for differences in the amount of smoke PM collected, the BWTC researchers concluded that: (1) there was good correlation between “extractable nicotine” deliveries and “smoke pH”,60 and that (2) “extractable nicotine” levels were useful when comparing brands.59,60,61
For example, in one BWTC study,59 domestic market brands were classified as being either “lower” or “higher” in the delivery of “extractable nicotine”. The “lower” products were associated with reduced or flat market sales, and “higher” products were associated with increasing sales. The authors concluded: “The data seem to indicate a demand in the domestic market for those brands in the higher extractable nicotine range.”59
BWTC researchers also concluded that perceived sensory “impact” was well correlated with “extractable nicotine”,61,62 and that “impact” correlated more closely with “extractable nicotine” than with total nicotine.61 Those studies thus hypothesised that free‐base nicotine delivery (as approximated by “extractable nicotine”) is an important factor in determining the perceived strength of cigarette smoke.61,62,63 An empirical equation to predict “impact” was developed based on “extractable nicotine”, “non‐extractable nicotine”, and the draw resistance of the cigarette.64
“Oil/water partitioning”
Another variation of the “extractable nicotine” method developed by BWTC scientists was referred to as the “oil/water partitioning” (“O/WP”) method,65 in which cigarette smoke was bubbled through an aqueous buffer solution (rather than initially‐pure water), followed by extraction (“partitioning”) of the buffer solution with chloroform (the “oil”). It was argued that the nicotine level obtained by this “O/WP” method could be used to predict “impact”, and that such predictions correlated closely with the predictions of “impact” based on “extractable nicotine”.65
“Volatile nicotine”
In the early 1990s, RJRTC researchers sought to develop a method that could be used to determine the ability of nicotine to evaporate from inhaled smoke, and then deposit from the gas phase onto respiratory tract surfaces.66 One method involved bubbling smoke through initially‐pure water, followed by measurements of both the nicotine in the water, and collected on a “Cambridge filter pad” placed downstream of the bubbler.67 It was assumed that volatilised nicotine would be absorbed into the water, and that most of the nicotine remaining in the smoke particles would pass through the bubbler unretained. The resulting “volatile nicotine” values were found to correlate with “impact”, sensory strength, and throat harshness.68,69 Also, “volatile nicotine” was found to be more consistently correlated with “impact” and harshness than either the tar/nicotine ratio, or total nicotine.70,71 Overall, it was concluded that this method gave useful results,68 though it was noted that:
“Smoke pH should be expected to have some relationship to ‘volatile' nicotine, but measurements [e.g., “smoke pH”] made by standard methodology yielded inconsistent correlations.”72
Some internal discussion distinguished between “volatile nicotine” results and a true measurement of free‐base nicotine, and one scientist concluded:
“[T]here is no[t] any analytical procedure available to distinguish the volatile nicotine from the non‐volatile nicotine in smoke or in tobacco.”73
Nevertheless, results obtained by the “volatile nicotine” method were considered to be of sufficient value that a 1992 RJRTC memo suggested that standard ISO (International Standards Organization) measurements of total nicotine be replaced company‐wide by “volatile nicotine” measurements.70
Direct methods
It would be optimal to probe the smoke PM phase by a technique that gives a signal that is directly related to free‐base nicotine. Philip Morris Tobacco Company (PMTC) researchers in the mid‐1970s attempted to utilise nuclear magnetic resonance spectroscopy in this type of effort, the results of which suggested that the majority of nicotine in PM exists in free‐base form.74 We note that the interpretation of their results may have been confounded by their addition of acetone as a dilution solvent to the smoke‐PM phase. Similarly, liquid chromatography‐based research conducted in 1977 by RJRTC failed to achieve determination of levels of free‐base nicotine in extracts and was eventually abandoned.75
“Smoke pH” and free‐base nicotine deliveries measured by the tobacco industry
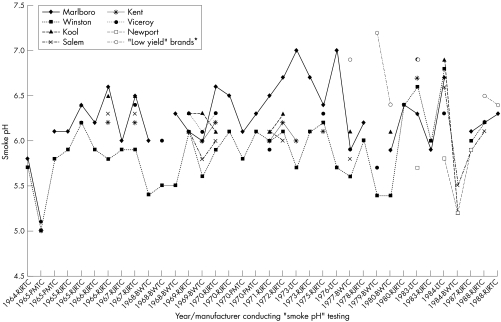
As noted above, the most common internal industry method for calculating free‐base nicotine deliveries utilised measured “smoke pH” values. Unfortunately, document‐to‐document comparisons of “smoke pH” values are severely compromised by differences in “smoke pH” methodology and complicated by incomplete information on the methods used. Figure 1 provides a sample of measured “smoke pH” values found in tobacco industry documents from the period 1960 to 1990 for selected commercial brands of cigarettes.41,43,44,53,59,81,82,83,84,85,86,87,88,89,90,91,92 Table 5 summarises the free‐base nicotine deliveries obtained by industry measurements for selected brands.44,49,53,59,64,78,82,84,85,94,95,96
Figure 1 Sample of industry document measured “smoke pH” values for selected commercial brands, 1964–1988.41,43,44,53,59,81,82,83,84,85,86,87,88,89,90,91,92 *Cambridge, Now, Merit. BWTC, Brown & Williamson Tobacco Company; PMTC, Philip Morris Tobacco Company; RJRTC, RJ Reynolds Tobacco Company.
Table 5 Sample of industry document measured free‐base nicotine values (%) for selected brands, 1968 to 199244,49,53,59,64,78,82,84,85,94,95,96.
| Method | Company/year | Marlboro | Kool | Winston | Viceroy | Other brands |
|---|---|---|---|---|---|---|
| Extractable nicotine | BWTC/1968 | 18% | 25% | |||
| Extractable nicotine | BWTC/1969 | 23% | 24% | 16% | 24% | Salem 19% |
| Extractable nicotine | BWTC/1976 | 36% | 28% | 30% | ||
| Extractable nicotine | BWTC/1977 | 29% | 30% | 20% | 28% | Salem 27% |
| Merit 50% | ||||||
| Now 50% | ||||||
| Extractable nicotine | BWTC/1980 | 29% | 25% | Merit 50% | ||
| (method unknown) | BWTC/1984 | 25% | ||||
| Extractable nicotine | BWTC/date unknown | 29% | Cambridge 59% | |||
| Barclay 64% | ||||||
| Headspace nicotine | BWTC/1984 | 11% | 9% | 10% | Merit 13% | |
| (method unknown) | LTC/1978 | Kent III 29%/20% | ||||
| Gas‐phase nicotine | PMTC/1991 | 0%/4% | ||||
| Volatile nicotine | RJRTC/1991 | 4% | 5% | Marlboro Lights 6% | ||
| Winston Lights 5% | ||||||
| Volatile nicotine | RJRTC/1992 | 5% | 5% | Camel‐ 4% |
BWTC, Brown & Williamson Tobacco Company; LTC, Lorillard Tobacco Company; PMTC, Philip Morris Tobacco Company; RJRTC, RJ Reynolds Tobacco Company.
The “smoke pH” values in fig 1 vary from 5.0 to 7.2. The document‐based evidence suggests that at least in some cases, the measured brand‐to‐brand differences were reflective of real product differences.52,53,93 Also of interest to tobacco manufacturers internally were the observable variations over time of “smoke pH” within brands.34,41,42 The majority of the values in fig 1 fall in the range 5.5 and 6.5. When applied directly (but therefore inappropriately) to estimation of free‐base nicotine, these values give a corresponding range of calculated free‐base nicotine percentages between ∼0.3% and ∼3%. In contrast, an actual “effective pH” range for contemporary domestic cigarettes (calculated from measures of the fraction of free‐base nicotine in the smoke PM phase) was reported by Pankow et al to be ∼1 to ∼36%.29 A close evaluation of the historical variations in fig 1 is not possible because of differences among the methods used.
Internal industry observations based on testing methods other than “smoke pH” often report free‐base nicotine percentages that are much higher than would be inferred based on calculations using “smoke pH”. For example, a 1980 BWTC study observed “smoke pH” values between 5.3 and 6.4 for commercial brands,43 while “free nicotine” percentages (presumably measured by the “extractable nicotine” method) were between 23% and 50%—much higher than “smoke pH” calculated yields. Similarly, a 1984 BWTC study reported “smoke pH” values for Kool cigarettes of 4.8 to 5.8, and “free nicotine” percentages of 15–35%.53 In both of the above studies, the “smoke pH” values were correlated with free‐base values.
Although each tobacco industry method has limitations and none of them provide an absolute determination of free‐base nicotine deliveries, each method appears to have allowed some degree of relative comparison of free‐base nicotine deliveries among brands, and there appears to be general consistency in the findings across methods and studies.
Relative brand differences
Overall, the “smoke pH” values indicate considerable brand‐to‐brand variations. Despite the many stated limitations of the data, consistent relationships are observed between brands for internal “smoke pH” measurements (fig 1). For example, during the period 1965–1980, Marlboro exhibited consistently‐higher “smoke pH” values relative to Winston, regardless of testing method or manufacturer; after 1980, both brands gave similar “smoke pH” values. Similarly, “low yield” brands such as Merit and Barclay show higher values relative to “regular” brands. Consistent patterns of relative brand differences such as these explain the long‐term use of “smoke pH” measurements by the tobacco industry, and contrasts with public claims by the industry that little or no variation in “smoke pH” exists among brands.97,98
Other measures of free‐base nicotine, including “extractable nicotine” and “volatile nicotine” measures, also indicate significant brand‐to‐brand differences. A consistent finding from these measures is the strong correlation demonstrated between the sensory perception of throat “impact” and estimated free‐base nicotine levels. Measures specifically targeting free‐base nicotine also provided stronger correlation with “impact” than more traditional measures, including total (Federal Trade Commission, FTC) nicotine, tar/nicotine ratio, or even measures of “smoke pH”.47 The successful correlation of “smoke pH” measurements with differences among brands in consumer perception of sensory cues such as “impact”, strength, and harshness, supports the view that these measures have had some utility in relative comparison of brands. Indeed, in each of these cases the details of what is being measured appear to be of secondary importance to the value of a consistent and reproducible measurement that reflects smokers' perceptions of the cigarette.
In addition to percent free‐base nicotine, the industry documents discuss the relevance of brand comparisons based on measurements of the delivery of total free‐base nicotine. For example, in the 1980 B&W study cited above,44 two cigarette brands with different total nicotine deliveries were compared. These were Marlboro (1.15 mg) and Merit (0.64 mg). While Marlboro was reported to average 27.8% “free nicotine”, Merit was reported to average 50%. The total measured “free nicotine” deliveries were thus discussed as being essentially the same at ∼0.3 mg. The authors note:
“In theory, a person smoking these cigarettes would not find an appreciable difference in the physiological satisfaction from either based on the amount of free nicotine delivered.”44
This suggests that studies of the form of nicotine delivered may need to consider not only the percent free‐base nicotine in the smoke, but also the total free‐base nicotine delivered.
“Low yield” cigarettes
Some industry documents argue that the role of free‐base nicotine may be of greatest significance in so‐called “low yield” products. For example, industry documents make the point that the assumed relationship between “smoke pH” and free‐base nicotine could be used to develop products with reduced tar and nicotine deliveries while maintaining adequate “impact” from the free‐base nicotine delivery. A typical passage is:
“This suspected relationship between free nicotine concentration and smoke impact implies that we could create a[n] ultra‐low tar cigarette that produces much more impact than its delivery would suggest. Therefore, it is recommended that this relationship be evaluated.”47
A Lorillard Tobacco Company (LTC) study in which free‐base nicotine was added directly to cigarette smoke was considered to be confirmation of this hypothesis: “Only a small addition of free nicotine was needed to provide the impact of a higher nicotine cigarette.”99 Internal results obtained for “low yield” brands are consistent with the view that such brands may compensate for reduced total nicotine delivery by maintaining a certain level of free‐base nicotine delivery (fig 1, table 5).
Further research needs suggested by the tobacco industry documents
The industry documents considered here indicate that further independent laboratory research is needed to assess more accurately the differences that exist in nicotine delivery among different cigarette brands. Recent studies of commercial cigarettes indicate the types of measurements of free‐base nicotine in PM phase smoke that can now be carried out.25,29
Studies of how free‐base nicotine is delivered in the lower respiratory tract, with subsequent effect on the speed of nicotine delivery, are clearly needed. For example, some industry documents make reference to “chest impact”, suggesting deposition of free‐nicotine deeper in the respiratory tract than the pharynx. Thus, a 1978 LTC marketing document recommended:
“…development of new flavors to help mask the lower chest impact of low T/N cigarettes, without necessarily revealing to the consumer the existence of the new flavor.”100
However, direct measures of the site and rate of nicotine uptake are not available either in the open literature, or in the tobacco industry documents considered in this study. In an example for the latter case, while a 1990 PMTC study of cigarettes with similar total nicotine deliveries did note that “[higher levels of] the base produced enhanced electrophysiological and subjective responses”,54 it did not characterise the associated sensory effects (for example, “impact”), or the effects of site and rate of absorption, and only proposed additional study of the effects of chemical variables on “smoke pH”. As summarised in a 1994 internal PMTC memo,
“[A]n influence of smoke pH on nicotine kinetics in the lower respiratory tract cannot be excluded: pH‐enhanced gas phase diffusion of nicotine to the mucosa might increase its uptake rate.”101
Another promising study area involves investigation of the differences among cigarettes in free‐base nicotine delivery rates to the brain. For example, PMTC researchers argued internally for the importance of studying the electrophysiological effects of differences in free‐base nicotine delivery based on the pattern‐reversal evoked potential.102 A 1988 RJRTC study compared smokers' electroencephalogram (EEG) patterns before and after they smoked Winston or Marlboro cigarettes,103 and based on prior internal findings, hypothesised that the time course of nicotine delivery to the brain following each individual puff may differ between the two brands. To assess this possibility, company researchers proposed a separate study designed to measure EEG changes reflecting the time‐course of the arrival of nicotine in the brain, but the study appears to have been suspended after further research.104
What this paper adds
Despite the widespread historical use of a variety of methods to measure differences in free‐base nicotine delivery across cigarettes, more recent assertions by tobacco manufacturers deny the existence of meaningful brand differences.
This study demonstrates that internal industry measures consistently reflect significant, measurable differences across brands, which mirror subjective smoker perceptions. These findings confirm the need to consider the role of smoke chemistry in determining exposure, including how free‐base nicotine deliveries interact with other substances to influence cigarette addiction potential.
DISCUSSION
The public release of internal tobacco industry documents has led to increasing sophistication by independent scientists in the characterisation of the design and function of tobacco products. One critical area of research is the role of smoke chemistry in determining the delivery, absorption, and effects of smoke constituents, especially harm producing or pharmacologically active compounds. The adverse health effects of smoking are a function of the toxicity of smoke constituents, as well as the amount and duration of exposure to those toxins, in combination with individual differences in metabolism of toxic compounds and susceptibility. Conventional measurements of smoke delivery, such as ISO measures, have generally focused on “tar” or nicotine in smoke PM as collected from the Cambridge filter using standard machine smoking protocols. Recent findings suggest that these measures fail to account for or describe important design‐based differences in smoke chemistry, which may alter exposure or toxicological impact.
Tobacco industry documents indicate that the major US tobacco manufacturers have routinely sought to measure the amounts of free‐base nicotine delivered by their own as well as competing brands using a range of internal methods. Many of the documents make reference to specific, brand‐dependent free‐base nicotine deliveries in units such as mg per cigarette. While those historic determinations are now understood to have been unreliable in absolute terms, they may nevertheless retain utility as relative measures of smoke alkalinity and free‐base nicotine delivery. For example, the differences observed among brands by these methods were found to correlate with differences in sensory perception and “impact” in a manner that appears to be best explained in terms of different relative free‐base nicotine deliveries.
Because of the enormous historic interest in “smoke pH” values that is made evident in the tobacco industry documents, public health researchers and governmental entities (for example, Texas, Massachusetts) have recently focused attention on “smoke pH” as a proxy to determine free‐base nicotine delivery. For example, since 1997, Massachusetts has required “smoke pH” testing as a component of its nicotine disclosure regulations.105 This requirement has been the subject of industry criticisms, including the claim that the resulting measurements of “smoke pH” show only minor differences across brands, and that any differences in these “smoke pH” values are likely due to differences in methodologies across companies.97,98
The public statements made by tobacco manufacturers are not consistent with decades of industry use of “smoke pH” and other methods to differentiate commercial brands according to smoke alkalinity. They are also not consistent with the recent determinations by Pankow et al and Watson et al.25,29 Criticisms by Pankow and others, that “smoke pH” methods are not capable of providing absolute measures of free‐base nicotine delivery,3,29,31 suggest that traditional “smoke pH” methods should be supplanted by more accurate approaches for measuring free‐base nicotine delivery. This point is confirmed by observations made internally by tobacco manufacturers. The method of Pankow et al for measuring effective pH values of collected smoke PM samples holds promise in this regard.29 The Watson et al research from the Centers for Disease Control and Prevention laboratory is particularly important because it used sophisticated techniques to measure directly the free base fraction of nicotine in cigarette smoke.25 These findings largely substantiated those of Pankow. Watson et al also found that the free base fraction increased in direct relation to increasing ventilation, consistent with the hypothesis that decreasing concentrations of smoke aerosol may result in an increasing fraction of nicotine “off gassing” in the unionized free‐base form.
The FTC has requested comments on its tobacco smoke testing methods and has asked the Secretary of Health and Human Services for guidance on how to improve the current testing programme. The World Health Organization Framework Convention on Tobacco Control has also proposed additional in‐depth testing of tobacco products to provide more meaningful assessments as to the actual deliveries and exposure of nicotine and other substances.106,107,108,109,110 We conclude that government and public health agencies must seek to better understand the role of smoke chemistry in determining exposure, including how free‐base nicotine deliveries interact with other substances to influence cigarette addiction potential. One possible regulatory strategy would include the required disclosure by manufacturers of free‐base nicotine deliveries for marketed brands. In addition, regulatory strategies targeting tobacco product dependence could consider imposing limits on free base nicotine delivery.
For the scientific community, areas requiring study include: (1) patterns of free‐base nicotine deliveries among regular and low yield cigarettes; (2) correlation of free‐base nicotine deliveries with market share; (3) differences in measurable effects of percent free‐base nicotine versus total free‐base nicotine delivery; and (4) effects of free‐base nicotine delivery on addiction potential. The latter should include studies that measure: (a) “impact” response and sensory effects; (b) EEG and other studies that directly measure physiological effects; (c) the mechanisms and locations of free‐base nicotine deposition in the respiratory tract; (d) uptake rates within the lung; and (e) delivery rates to the brain.
Abbreviations
BWTC - Brown & Williamson Tobacco Company
EEG - electroencephalogram
FTC - Federal Trade Commission
ISO - International Standards Organization
O/WP - oil/water partitioning
PM - particulate matter
PMTC - Philip Morris Tobacco Company
RJRTC - RJ Reynolds Tobacco Company
Footnotes
Funding for this research was provided through the National Cancer Institute, grant RO1 CA87477‐05. Dr Henningfield's efforts were supported by the Innovators Awards Program of the Robert Wood Johnson Foundation at the Johns Hopkins University School of Medicine.
Competing interest: Drs Henningfield and Connolly have served as expert witnesses in litigation against the tobacco industry
References
- 1.Master Settlement Agreement (1998) Full text available (as of 1 April 2004) at: http://www.naag.org/upload/1032468605_cigmsa.pdf
- 2.Hurt R D, Robertson C R. Prying open the door to the tobacco industry's secrets about nicotine: the Minnesota Tobacco Trial. JAMA 19982801173–1181. [DOI] [PubMed] [Google Scholar]
- 3.Pankow J F. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol 2001141465–1481. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz N L, Henningfield J E. Establishing a nicotine threshold for addiction: The implications for tobacco regulation. N Engl J Med 1994331123–125. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration 21 CFR Part 801, et al. Regulations restricting the sale and distribution of cigarettes and smokeless tobacco to protect children and adolescents; final rule. Federal Register 19966144396–45318. [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services The health consequences of smoking: nicotine addiction. A report of the Surgeon General, 1988. Rockville, Maryland: Public Health Service, Centers for Disease Control, Office on Smoking and Health, 1988, (DHHS Publication No (CDC) 88‐8406. )
- 7.Jarvis M J, Boreham R, Primatesta P.et al Nicotine yield from machine‐smoked cigarettes and nicotine intakes in smokers: evidence from a representative population survey. J Natl Cancer Inst 200193134–138. [DOI] [PubMed] [Google Scholar]
- 8.Henningfield J E, Radzius A, Cone E J. Estimation of available nicotine content of six smokeless tobacco products. Tob Control 1995457–61. [Google Scholar]
- 9.Balfour D J. The neurobiology of tobacco dependence: a commentary. Respir 2002697–11. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien C P. Drug addiction and drug abuse. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, eds. Goodman & Gilman's the pharmacological basis of therapeutics. New York: McGraw‐Hill, 1996557–577.
- 11.Royal College of Physicians Nicotine addiction in Britain: a report of the Tobacco Advisory Group of the Royal College of Physicians. London, UK: Royal College of Physicians of London, 2000
- 12.Panlilio L V, Yasar S, Nemeth‐Coslett R.et al Human cocaine‐seeking behavior and its control by drug‐associated stimuli in the laboratory. Neuropsychopharmacology 200530433–443. [DOI] [PubMed] [Google Scholar]
- 13.Henningfield J E, Fant R V. Tobacco use as drug addiction: the scientific foundation. Nicotine Tob Res 19991(suppl 2)S31–S35. [DOI] [PubMed] [Google Scholar]
- 14.Henningfield J E, Benowitz N L, Connolly G N.et al Reducing tobacco addiction through tobacco product regulation. Tob Control 200413132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler D A. Statement on Nicotine‐Containing Cigarettes: Hearings before the House Subcommittee on Health and the Environment (testimony of Kessler DA). Statement on Nicotine‐Containing Cigarettes. 1994
- 16.Robertson C.State of Minnesota, et. al., vs. Philip Morris, et. al. C1‐94‐8565. February 4, 1998. 12, 2198‐2369. (Testimony of Channing Robertson.)
- 17.Armitrage A K, Turner D M. Absorption of nicotine in cigarette and cigar smoke through the oral mucosa. Nature 19702261231–1232. [DOI] [PubMed] [Google Scholar]
- 18.Lauterbach J H. A critical assessment of recent work on the application of gas/particle partitioning theories to cigarette smoke. Beitrage zur Tabakforschung Int. (Contributions to Tobacco Research) 20001965–83. [Google Scholar]
- 19.Morie G P. Fraction of protonated and unprotonated nicotine in tobacco smoke at various pH values. Tob Sci 197216167 [Google Scholar]
- 20.Brunnemann K D, Hoffmann D. Chemical studies on tobacco smoke. XXV.The pH of tobacco smoke. Fd Cosmet Toxicol 197412115–124. [DOI] [PubMed] [Google Scholar]
- 21.Pankow J F, Mader B T, Isabelle L M.et al Conversion of nicotine in tobacco smoke to its volatile and available free‐base form through the action of gaseous ammonia [Erratum: Environ Sci Technol 1999;33:1320]. Environ Sci Technol 1997312428–2433. [Google Scholar]
- 22.Bergström M, Nordberg A, Lunell E.et al Regional deposition of inhaled 11C‐nicotine vapor in the human airway as visualized by positron emission topography. Clin Pharmacol Ther 199557309–317. [DOI] [PubMed] [Google Scholar]
- 23.Armitrage A K, Hall G H, Morrison C F. Pharmacological basis for the tobacco smoking habit. Nat 1965217331–334. [DOI] [PubMed] [Google Scholar]
- 24.Russell M A H, Feyerabend C. Cigarette smoking: a dependence on high nicotine boli. Drug Metab Rev 1975529–57. [DOI] [PubMed] [Google Scholar]
- 25.Watson C H, Trommel J S, Ashley D L. Solid‐phase microextraction‐based approach to determine free‐base nicotine in trapped mainstream cigarette smoke total particulate matter. J Agric Food Chem 2004527240–7245. [DOI] [PubMed] [Google Scholar]
- 26.Sensabaugh A J, Cundiff R H. A new technique for determining the pH of whole tobacco smoke. Tob Sci 19671125–30. [Google Scholar]
- 27.Rickert W S.Partial characterization of 10 “common” brands of American cigarettes. Project Report Prepared for: Massachusetts, Ontario, Canada: Department of Public Health 1997
- 28.Henningfield J E, Fant R V, Radzius A.et al Nicotine concentration, smoke pH, and whole tobacco aqueous pH of some cigar brands and types popular in the United States. Nicotine Tob Res 19991163–168. [DOI] [PubMed] [Google Scholar]
- 29.Pankow J F, Tavakoli A D, Luo W.et al Percent free‐base nicotine in the tobacco smoke particulate matter of selected commercial and reference cigarettes, Chem Res Toxicol2003161014–1018. [DOI] [PubMed] [Google Scholar]
- 30.Hurt R D.State of Minnesota, et. al., vs. Philip Morris, et. al. C1‐94‐8565. January 28, 1998. 12, 1345‐1346. (Testimony of Richard D. Hurt. )
- 31.Henningfield J E, Pankow J F, Garrett B E. Ammonia and other chemical base tobacco additives and cigarette nicotine delivery: issues and research needs. Nicotine Tob Res 20046199–205. [DOI] [PubMed] [Google Scholar]
- 32.Lewis L S, Moser H E. Chemical Research, Henley W.M., and Piehl, D.H. Comparison of chemical and physical analyses of selected 1978 competitive brands. (TDO website.) July 3, 1979. http://tobaccodocuments.org/product_design/500608639‐8673.html
- 33.Honeycutt R H, Lin O C. Chemical characterization of US cigarette brands Rd‐B014‐84. (TDO website). February 9, 1984. http://tobaccodocuments.org/product_design/78645.html
- 34.Lauterbach J H, B&W PGS‐B‐001‐97. A retrospective review of B&W's smoke Ph method and results. (TDO website). February 19, 1997. http://tobaccodocuments.org/product_design/13134072.html
- 35.Will F., III Ph of smoke your memo to me 700313. (TDO website). March 17, 1970 (est.). http://tobaccodocuments.org/product_design/2050870983.html
- 36.Anon pH of Smoke. (TDO website). 1977 (est). http://tobaccodocuments.org/product_design/1000128444.html
- 37.Chen, and Leighton Ph of smoke, a review. (TDO website). July 12, 1976. http://tobaccodocuments.org/product_design/2925.html
- 38.Price‐B B & W. Smoke Ph. (TDO website). February 27, 1997. http://tobaccodocuments.org/product_design/13134077.html
- 39.Dobbins J T, Jr, Harrell T G, Markunas P C.et al A study of the puff by puff measurement of cigarette smoke Ph. (TDO website). November 8, 1971. http://tobaccodocuments.org/product_design/500605825‐5831.html
- 40.Piehl D. H. Quarterly section research report. (TDO website). June 13, 1973. http://tobaccodocuments.org/product_design/500997469‐7475.html
- 41.Teague C E. Implications and activities arising from correlation of smoke Ph with nicotine impact, other smoke qualities, and cigarette sales. (TDO website). 1973. http://tobaccodocuments.org/product_design/344.html
- 42.Dickerson J P. Historical trends in tar, nicotine and smoke Ph of Winston and Marlboro. (TDO website) December 27, 1977. http://tobaccodocuments.org/rjr/509308839‐8849.html
- 43.Phillip Morris Key issues. (TDO website). 1980. http://tobaccodocuments.org/product_design/511367302‐7326.html
- 44.Gregory C F. Observation of free nicotine changes in tobacco smoke/#528. (TDO website). Januray 4, 1980. http://tobaccodocuments.org/product_design/3355.html
- 45.Ireland M S. Subject: Research proposal ‐ development of assay for free nicotine. (TDO website). August 16, 1976. http://tobaccodocuments.org/product_design/00044522‐4523.html
- 46.Lephardt J O. Subject: Puff by puff Ph measurement. (TDO Website). November 11, 1991. http://tobaccodocuments.org/product_design/2050871038.html
- 47.Schori T R. Free nicotine: its implications on smoke impact. (TDO website). October 22, 1979. http://tobaccodocuments.org/product_design/166104.html
- 48.Blevins R A., Jr [Free‐base nicotine explains some of the differences in performance between Winston, Marlboro, Salem and Kool]. (TDO website). July 12, 1973. http://tobaccodocuments.org/product_design/501327012‐7012.html
- 49.Crellin R. Project Ship ‐ July/August 1984 ‐ progress review. (TDO website). September 20, 1984. http://tobaccodocuments.org/product_design/11979114.html
- 50.Wilson J B. Adjustment in the smoke Ph of Winston cigarettes. (TDO website). February 23, 1970. http://tobaccodocuments.org/product_design/504414927‐4928.html
- 51.Kruszynski A J. The determination of the smoke Ph and its organoleptic evaluation. (TDO website). 1982. http://tobaccodocuments.org/product_design/2028560587‐0599.html
- 52.Neumann C L. Project 1250 nicotine/satisfaction. (TDO website). April 12, 1977. http://tobaccodocuments.org/product_design/512974285‐4286.html
- 53.Mosser L B & W. Effects of varying smoke Ph on Kool Ks/244. (TDO website). March 19, 1984. http://tobaccodocuments.org/product_design/12175389.html
- 54.Gullotta F P, Hayes C S, Martin B R. Subject: The electrophysiological and subjective consequences of tobacco filler pH modifications: A proposal. (TDO website). December 14, 1990. View on TDO, http://tobaccodocuments.org/product_design/2022262774‐2775.html
- 55.Anon The effects of cigarette smoke pH on nicotine delivery and subjective evaluations. (TDO website). June 1, 1994. http://tobaccodocuments.org/product_design/3155.html
- 56.Dryden M J. The potential role of nicotine in dry/stale complaints. (TDO website). January 27, 1992. http://tobaccodocuments.org/product_design/512354471‐4493.html
- 57.Frank M S. Standard method number SM‐92 procedure for the determination of extractable nicotine in cigarette smoke. (TDO website). July 25, 1979. http://tobaccodocuments.org/product_design/376373.html
- 58.Backhurst J D, Hughes I W. A relation between the ‘strength' of a cigarette and the ‘extractable nicotine' in the smoke. (TDO website). November 16, 1965. http://tobaccodocuments.org/product_design/NcSmBAT19651116.Rg.html
- 59.Leach J, Shockley L. TPM aqueous extract pH and extractable nicotine studies of major cigarette brands from B&W and some domestic competitive companies. (TDO website). June 13, 1969. http://tobaccodocuments.org/product_design/996330.html
- 60.Canon A B. Extractable nicotine values for low delivery cigarettes/408. (TDO website). July 26, 1977. http://tobaccodocuments.org/product_design/19054.html
- 61.Hirji T, Wood D J. Impact: Its relationship with extractable nicotine and with other cigarette variables (Report No. Rd. 1052‐R). (TDO Website). October 29, 1973. http://tobaccodocuments.org/product_design/70021.html
- 62.Brooks G O, Cousins A R, Crellin R A. Puff by puff impact ‐ extractable nicotine studies on Hallmark cigarettes from Australia report No. Rd. 1108‐R. (TDO website). May 21, 1974. http://tobaccodocuments.org/bw/70032.html
- 63.Maynor B W, Rosene C J. A comparison of the extractable nicotine content of smoke from Barclay and Cambridge cigarettes. (TDO website). January 20, 1981. http://tobaccodocuments.org/bw/329242.html
- 64.Hirji T, Wood D J. Equations relating impact with extractable nicotine and other cigarette variables report No. Rd.1337 restricted. (TDO website). March 22, 1976. http://tobaccodocuments.org/bw/400593.html
- 65.Hook R G. O/WP nicotine, extractable and non‐extractable nicotine. (TDO website). August 9, 1976. http://tobaccodocuments.org/bw/70086.html
- 66.Ingebrethsen B J, Lyman C, Coleman W.et al Smoke nicotine ‘volatility' measurement methods. (TDO website). August 28, 1991. http://tobaccodocuments.org/rjr/508276435‐6446.html
- 67.Ingebrethsen B J, Lyman C, Coleman W.et al Smoke nicotine ‘volatility' measurement methods. (TDO website). August 28, 1991. http://tobaccodocuments.org/rjr/508276435‐6446.html
- 68.Ingebrethsen B J, Lyman C S. 1991 (910000) 2nd quarter report on research. (TDO website). 1991. http://tobaccodocuments.org/rjr/509744255‐4258.html
- 69.RJ Reynolds Highlights. Biobehavioral research ‐ Project Xb. Support to Project Xb ‐ nicotine transport/nicotine volatility studies. (TDO Website). June 3, 1991. http://tobaccodocuments.org/rjr/508944574‐4577.html
- 70.RJ Reynolds The potential role of nicotine in dry/stale complaints. (TDO Website). January 27, 1992. http://tobaccodocuments.org/rjr/512795107‐5129.html
- 71.Reynolds J H. [Summary of ongoing and recently completed nicotine studies]. (TDO website). November 13, 1991. http://tobaccodocuments.org/rjr/507985491‐5495.html
- 72.Ingebrethsen B J. Performance profile 1991 year end review. (TDO website). February 27, 1992. http://tobaccodocuments.org/rjr/507986060‐6072.html
- 73.Shu K. Subject: Analysis of nicotine. (TDO website). June 22, 1992. http://tobaccodocuments.org/rjr/511619689‐9689.html
- 74.Bassfield R, Ferguson R, Whidby J. An NMR method for the determination of free nicotine base of cigarette smoke condensate. (TDO Website). 1975 (est.). http://tobaccodocuments.org/pm/1001823838‐3849.html
- 75.Clapp W L. Quarterly section research report. Instrumentation ‐ chromatography section. (TDO website). June 23, 1977. http://tobaccodocuments.org/rjr/502803940‐3946.html
- 76.B&W Smoke Ph vs. FTC nicotine. (TDO website). 1997. http://tobaccodocuments.org/product_design/13134074.html
- 77.Canon A B. Extractable nicotine values for low delivery cigarettes/408. (TDO website). July 26, 1977. Bates: 650100485, http://tobaccodocuments.org/product_design/19054.html
- 78.Watson D C. Gas phase nicotine ‐ Status (Reference: D.C. Watson to R. Ferguson, ‘Gas Phase Nicotine', September 3, 1991). (TDO website). October 30, 1991. http://tobaccodocuments.org/product_design/2023569357‐9362.html
- 79.Anon One a) volatile nicotine measures developed and shown to be more consistently correlated with sensory attribute of throat harshness than T/N ratio of FTC nicotine. (TDO Website). November 14, 1991. http://tobaccodocuments.org/product_design/515465307‐5310.html
- 80.Ingebrethsen B J. Volatility of nicotine in TPM. (TDO website). January 1990. http://tobaccodocuments.org/product_design/509778516‐8524.html
- 81.Ingebrethsen B J, Lyman C, Coleman W.et al Smoke nicotine ‘volatility' measurement methods. (TDO website). May 1, 1991. http://tobaccodocuments.org/rjr/508276436‐6445.html
- 82.Shockley I. pH of TPM studies. I. Effect of added stem and PCL on the pH of the TPM and extractable alkaloids of cigarette smoke/313. (TDO website). September 6, 1968. http://tobaccodocuments.org/product_design/979418.html
- 83.Shockley L L. pH studies of TPM ‐ V. Viceroy vs. Winston. (TDO website). November 15, 1968. http://tobaccodocuments.org/product_design/979423.html
- 84.Maynor H W. GLC extractable nicotine ‐ smoke pH. (TDO website). May 9, 1977. http://tobaccodocuments.org/product_design/1459685.html
- 85.Maynor H W. Determination of pH, free and bound nicotine in pads and filters. (TDO website). 1900. http://tobaccodocuments.org/product_design/979424.html
- 86.Moss B L. Subject: Smoke pH analysis on commercial brands. (TDO website). November 29, 1982. http://tobaccodocuments.org/product_design/01591377.html
- 87.Reynolds R J. Inside RJR: Exploring smoke pH and nicotine. (TDO website). 1994 (est.). http://tobaccodocuments.org/product_design/2063146325.html
- 88.Charles J L. Determination of pH of whole smoke on a puff‐by‐puff basis. (TDO website). June 10, 1970. http://tobaccodocuments.org/product_design/2501421262‐1263.html
- 89.Ikeda R M, Palmer A M, Resnik F E. Completion report the determination of the pH of whole smoke and TPM. (TDO Website). October 19, 1965. http://tobaccodocuments.org/product_design/2051205946‐5961.html
- 90.Reynolds R J. Smoke Ph Values. (TDO website). 1988. http://tobaccodocuments.org/product_design/507955308‐5308.html
- 91.Reynolds R J, Dufour W M. Subject: Winston KS R&D team meeting February 18, 1983 (830218). (TDO website). February 23, 1983. http://tobaccodocuments.org/product_design/504361245‐1247.html
- 92.Hawley R W, Grimes R I I I, Porter T J., Jret al PM blend comparison study. Rd&M90 046. (TDO website). February 27, 1990. http://tobaccodocuments.org/product_design/512528825‐8896.html
- 93.Creighton D E, Hirji T. The significance of pH in tobacco and tobacco smoke. (TDO website). January 1988. http://tobaccodocuments.org/product_design/3223.html
- 94.Ihrig A M. % free nicotine on Kent III and some experimental cigarettes. (TDO website). 1978 (est.). http://tobaccodocuments.org/product_design/00112616.html
- 95.Lyman C S. [Determine volatile nicotine for four types of cigarettes with 70mm additional filters]. (TDO website). November 6, 1992. http://tobaccodocuments.org/product_design/509742993‐2997.html
- 96.Beard K A. Volatile nicotine' determinations for Winston and Marlboro cigarettes. (TDO website). October 1, 1991. http://tobaccodocuments.org/product_design/509742504‐2509.html
- 97.Philip Morris et al Comments before the Massachusetts Department of Public Health on proposed amendments to regulations entitled cigarette and smokeless tobacco products: Reports of added constituents and nicotine ratings. October 2 1998
- 98.Philip Morris Inc et al Submission Before the Massachusetts Department of Public Health Regarding Proposed Refinements in Sampling and Testing Procedures Set Forth in 105 CMR 660.500 and Certain Other Matters. April 8 1997
- 99.Larson T M, Morgan J P. Application of free nicotine to cigarette tobacco and the delivery of that nicotine in the cigarette smoke. (TDO website). June 8, 1976. http://tobaccodocuments.org/product_design/00398312‐8322.html
- 100.Vila B. [Regarding Focus Group Findings on Alternate Flavors and Sensations]. (TDO Website). June 16, 1978. http://tobaccodocuments.org/product_design/04325955‐5957.html
- 101.Reininghaus W. Bioavailability of nicotine. (TDO website). November 15, 1994. http://tobaccodocuments.org/product_design/2050878704‐8707.html
- 102.Gullotta F P, Hayes C S. Electrophysiological Studies ‐ November Monthly Progress Report. (TDO website). January 1991 (est.). http://tobaccodocuments.org/product_design/2024850235.html
- 103.Pritchard W S, Robinson J H. 1990 (900000) Winston/Marlboro Eeg study. (TDO Website). June 11, 1990. http://tobaccodocuments.org/product_design/515811707‐1709.html
- 104.Reynolds R J. [John H. Robinson's 1981–1991 Performance Evaluations]. (TDO website). No date. http://tobaccodocuments.org/product_design/512051633‐1649.html
- 105.Massachusetts Department of Public Health 105 CMR 660. 000 Cigarette and Smokeless Tobacco Products: Reports of Added Constituents and Nicotine Ratings,
- 106.World Health Organization Framework Convention on Tobacco Control (WHO FCTC), 2005. Accessed 14 June 2005. http://www.who.int/tobacco/framework/en/
- 107.World Health Organization, Scientific Advisory Committee on Tobacco Product Regulation (SACTob) Recommendation on health claims derived from ISO/FTC method to measure cigarette yield. Geneva: WHO, 2002
- 108.World Health Organization, Scientific Advisory Committee on Tobacco Product Regulation (SACTob) Recommendation on nicotine and the regulation in tobacco and non‐tobacco products. Geneva: WHO, 2002
- 109.World Health Organization, Scientific Advisory Committee on Tobacco Product Regulation (SACTob) Recommendation on tobacco product ingredients and emissions. Geneva: WHO, 2003
- 110.World Health Organization, Tobacco Regulation Study Group Study Group on Tobacco Product Regulation (TobReg) Recommendation: guiding principles for the development of tobacco product research and testing capacity and proposed protocols for the initiation of tobacco product testing. Geneva: WHO, 2004