Abstract
Objective
To study the incidence of conversion to tobacco dependence (TD) and the prevalence of the TD state in relation to several potential determinants in a sample of adolescent smokers.
Methods
Questionnaires were administered every 3–4 months to document TD symptoms, amount of cigarette consumption, and depression symptoms in a prospective cohort of 1293 grade 7 students in a convenience sample of 10 schools.
Results
Over 54 months of follow‐up, 113 of 344 novice smokers converted to TD. The referent series for the analysis of incidence comprised 823 person‐surveys. The prevalence series included 1673 person‐surveys, contributed by 429 smokers. Conversion to TD and TD status were associated with the intensity of recent (that is, past 3‐month) cigarette consumption (adjusted incidence rate ratio (aIRR) 1.63 (95% confidence interval (CI) 1.36 to 1.97) and adjusted prevalence odds ratio (aPOR) 1.35 (95% CI 1.23 to 2.48) per 100 cigarettes per month), slowest CYP2A6 activity (aIRR 4.19 (95% CI 1.38 to 12.76) and aPOR 2.30 (95% CI 1.29 to 4.09)), depression score (aIRR 1.61 (95% CI 1.17 to 2.21) and aPOR 1.47 (95% CI 1.22, 1.75) per 1‐unit change). Additional determinants included, for conversion to TD, time since onset of cigarette use (aIRR 0.76 (95% CI 0.58 to 1.00) per year) and, for the TD state, positive TD status six months ago (aPOR 3.53 (95% CI 2.41 to 5.19)).
Conclusions
TD risk in adolescents is associated with intensity of recent cigarette consumption, while the role of more distant cigarette consumption appears small; subjects with slow nicotine metabolism and those with more depression symptoms are at increased risk of becoming tobacco dependent. The risk of being tobacco dependent is considerably higher in subjects who had previously developed the TD state.
Keywords: tobacco dependence; tobacco use disorder; smoking, risk factors; adolescent
Tobacco dependence (TD) is a complex disorder1 resulting from the interplay of multiple factors beyond cigarette consumption.2,3,4 According to the nicotine sensitivity model,2,3 TD is strongly related to individual sensitivity to nicotine. However, current knowledge of risk factors for the onset and maintenance of TD as well as of indicators of increased susceptibility to the effects of tobacco (and, in particular, nicotine) is lacking, and the aetiologic contribution of possible risk factors has not been accurately quantified, or, in fact, clearly established.4,5 Even for the most obvious aetiologic factor—cigarette smoking—there is no consensus as to what the pattern of its association with TD is (temporally and otherwise),1,6 although several recent studies suggest that even short‐term exposure in novice smokers can result in the appearance of TD symptoms.7,8,9 Risk factor identification is further complicated by a lack of agreement about what TD actually is and how it should be measured, especially in youth.10,11,12 Previous research is characterised by the use of a variety of differing methodological approaches to the measurement of TD.10,13,14,15 The objective of this study was to investigate the occurrence of TD, assessed using a clinically relevant and psychometrically sound measure,13 in relation to a series of potential determinants. While it is important to identify the determinants of the initial conversion to TD, the developed TD state might not be inherently permanent such that a subject could move in and out of the TD state over time, depending on his/her characteristics, most notably, according to cigarette consumption.16 Thus, we investigated determinants of “being in the state of TD” over the course of the early smoking experience. Potential determinants investigated included cigarette consumption, age, sex, time since the onset of cigarette use, symptoms of depression, TD status history, and rapidity of nicotine metabolism as measured by activity status of CYP2A6, a genetically variable hepatic enzyme that is responsible for the majority of the metabolic inactivation of nicotine to cotinine.17 In addition, we studied potential modification of the effect of the determinants by CYP2A6 activity status.
METHODS
Study population
The McGill University Study on the Natural History of Nicotine Dependence (NDIT Study) is a six‐year (1999 to 2005) longitudinal investigation of 1293 students aged 12–13 years at baseline, recruited from all grade 7 classes in a convenience sample of 10 Montreal secondary schools. The schools were selected in consultation with local schools boards and school principals, to include a mix of French and English schools, urban, suburban, and rural schools, and schools located in high and low socioeconomic neighbourhoods. Over half (55.4%) of eligible students participated at baseline; the relatively low response was related to the need to take blood samples for genetic analysis and to a province‐wide labour dispute that resulted in some teachers refusing to collect consent forms. Self‐report questionnaires were administered every 3–4 months in the language of instruction of each school. More detailed descriptions of the methods have been published elsewhere.9,18 In this current analysis, we used data from the first 4.5 years of follow‐up; each respondent had data from up to 18 questionnaires available for analysis. Of the 1293 subjects originally recruited, 423 (32.7%) were lost to follow‐up in the course of the study because they either refused to continue (n = 84) or moved (n = 339).
Since TD occurs among subjects who smoke only (that is, it belongs in the domain19 of cigarette smoking2,5), data from surveys in which the average monthly number of cigarettes smoked in the previous three months was less than one or missing were excluded from analysis. The analysis of TD incidence was limited to respondents who initiated cigarette use during follow‐up; in these subjects, the follow‐up was terminated at the survey at which conversion to TD occurred or at the end of the follow‐up (either at the end of the study or at the moment of loss to follow‐up), whichever came first. Thus, the source base for the analysis of prevalence of positive TD status comprised a total of 2592 person‐surveys (contributed by 490 subjects) and the source base for the analysis of incidence of first conversion to TD comprised 1089 person‐surveys (contributed by 358 subjects).
Genotyping
Genotyping was performed for five variant alleles (CYP2A6 *1 (the wild‐type), *9, *12, *2, *4, and *1x2, where “*number” refers to the specific variant allele of the CYP2A6 gene) using established gene and allele‐specific two‐step PCR assays.17 The variants investigated were selected based on their impact on nicotine metabolism and on reported frequencies in different ethnic groups.17 The analyses identified two partially inactive variants (CYP2A6*9, *12), two fully inactive variants (CYP2A6*2, *4), and the increased activity gene duplication, CYP2A6*1x2.
Description of study variables
Tobacco dependence was measured in a dichotomised indicator based on the six criteria for tobacco dependence in the International Classification of Diseases, 10th revision (ICD‐10). These included: (1) a strong desire or sense of compulsion to take tobacco; (2) difficulties in controlling tobacco taking behaviour in terms of its onset, termination, or levels of use; (3) a physiological withdrawal state when tobacco use has ceased or been reduced, as evidenced by: the characteristic withdrawal syndrome for tobacco; or use of the same (or a closely related) substance with the intention of relieving or avoiding withdrawal symptoms; (4) evidence of tolerance, such that increased doses of tobacco are required in order to achieve effects originally produced by lower doses; progressive neglect of alternative pleasure or interests because of tobacco use, increased amount of time necessary to obtain or take the substance or to recover from its effects; (5) progressive neglect of alternative pleasure or interests because of tobacco use, increased amount of time necessary to obtain or take the substance or to recover from its effects; and (6) persisting with tobacco use despite clear evidence of overtly harmful consequences, such as depressive mood states consequent to periods of heavy substance use, or drug related impairment of cognitive functioning.20 Each of the six criteria included 2–4 questionnaire items for a total of 18 items. To create the indicator questionnaire items (each with four to five response choices) were designed to measure each criterion. Our approach to identify tobacco‐dependent adolescents was conservative; an item was considered positive only if the most extreme response choice was endorsed; a criterion was considered positive if the respondent endorsed any of its items (the withdrawal syndrome required that at least two of four items be endorsed).13 Respondents were categorised as tobacco dependent if they met three or more of the six ICD‐10 criteria.20 In previous work,13 this TD measure was shown to have very good psychometric properties.
Potential determinants of TD investigated included age, sex, time since the onset of cigarette use, cigarette consumption in the recent (0–3 months), intermediate (3–6 months), and distant (6–9 months) past, CYP2A6 activity status, symptoms of depression in the past three months, and, in the analysis of prevalence, TD status six months ago.
At each survey, participants provided data on cigarette consumption for each of the preceding three months, including number of days on which they had smoked each month and average number of cigarettes smoked per day (on days when they smoked) each month. These two measures were multiplied and averaged over each three‐month interval to produce an estimate of average monthly cigarette consumption for each survey.
CYP2A6 activity was grouped into two categories: normal/slower inactivators (two copies of *1 (the wild type active allele and/or the duplication variant) or one copy of *9 or *12) and slowest inactivators (1–2 copies of *2 or *4 or two copies of *9 and/or *12).17
Depression symptoms in the past three months were measured at each survey in a validated six‐item scale.21 A depression symptom score, created by summing responses to each item, ranged from 1 to 6, with higher scores indicating more depression symptoms.
Time since cigarette use onset was computed as the difference between the current date that a questionnaire was completed and the date on which the respondent reported a non‐zero amount of cigarettes consumed for the first time.
TD status history was denoted by TD status measured six months before a given survey. The six month time lag was selected to ensure that the effect of cigarette consumption in the intermediate past (that is, 3–6 months ago) is not adjusted for ND status at a time following the time period for cigarette consumption measurement, which could have led to “overadjustment” and dilution of the association between cigarette consumption in the intermediate past and current TD status.
Statistical analysis
Analysis of incidence of first conversion to TD
The incidence series comprised person‐surveys contributed by participants who converted to TD (that is, the TD case series), and person‐surveys contributed by the corresponding referent series.22 Univariate and multivariable regression models were fitted to estimate, respectively, the crude and adjusted associations between occurrence of first conversion to TD and each potential determinant. We used a generalised estimating equation (GEE) repeated‐measures generalisation of logistic regression,23 with an order 1 autoregressive correlation structure of residuals.24 Exponentiation of estimated regression coefficients from the logistic model provided estimates of the incidence rate ratio for each determinant. Empirical standard errors were used.
Analysis of prevalence of positive TD state
The prevalence series comprised person‐surveys contributed by participants in a positive or negative TD state.16,22 Univariate and multivariable regression models were fitted to estimate, respectively, the crude and adjusted associations between occurrence of a positive TD state and each potential determinant. Here too we relied on a GEE generalisation of the logistic regression. Exponentiation of estimated regression coefficients from the logistic model provided estimates of the prevalence odds ratio for each determinant. In addition to studying the “main effects” of each determinant, in the prevalence analysis we explored possible modification of the effect of cigarette consumption in the recent and intermediate past, depression symptoms, and TD status history by CYP2A6 activity status. In these analyses, appropriate product terms were added to the full model, one at a time.
Cigarette consumption in all the regression analyses was represented by two variables: average monthly cigarette consumption in the past three months (that is, recent past), and average monthly cigarette consumption 3–6 months before a given survey (that is, intermediate past). Cigarette consumption 6–9 months before a given survey (that is, the relatively distant past) was initially considered in preliminary analyses but was dropped because of an apparent absence of effect when conditioning on other potential determinants.
Because genotype was assessed in only 129 respondents (37.5% of 344 retained for analysis) in the incidence series and 159 respondents (37.1% of 429 retained for analysis) in the prevalence series, to increase the precision while estimating the associations with other variables, the CYP2A6 activity status was represented in multivariable analysis by two binary variables: an indicator of the availability of the activity status information, and an indicator of the actual status; respondents with no CYP2A6 data were assigned the value of zero for this second variable (19). Similarly, in the prevalence analysis, because time since cigarette use onset could not be reliably determined in 91 subjects (21.2% of 429 retained for analysis) who had initiated cigarette use before study entry, this determinant was represented by an indicator of the availability of these data and by a continuous variable representing the actual time since cigarette use onset. Respondents with no information on time since onset were assigned a value of zero for this variable.19 All analyses were performed using SAS version 8.2 version for Windows.
RESULTS
Analysis of incidence of first conversion to TD
Among 1089 person‐surveys available in the source database for the incidence analysis, those with missing values for cigarette consumption 3–6 months ago (n = 122, 11.2%) and of current depression symptoms score (n = 11, 1.0%) were excluded. In the data series retained for analysis, 113 of 344 novice smokers converted to TD. The size of the referent series22 retained for the incidence analysis was thus 823 person‐surveys.
Distributions of selected characteristics of the referent series for the incidence analyses are provided in table 1.
Table 1 Distribution of respondent characteristics in the incidence and prevalence series.
| Characteristic | Referent series for the incidence analysis (n = 823 person‐surveys) | Prevalence series (n = 1673 person‐surveys) | ||||
|---|---|---|---|---|---|---|
| Mean (median) | SD | % | Mean (median) | SD | % | |
| Age (years) | 14.6 | 1.5 | 14.8 | 1.4 | ||
| Depression symptoms score | 2.2 | 0.7 | 2.3 | 0.8 | ||
| Average cigarette consumption in the past 3 months (cig/month) | 54.6 (9.0) | 119.7 | 159.5 (67.0) | 219.6 | ||
| Average cigarette consumption in the past 3–6 months (cig/month) | 32.2 (1.5) | 92.9 | 133.7 (32.5) | 211.0 | ||
| Time since the onset of cigarette use (years) | 1.1 (1.0) | 1.1 | 1.6 (1.5) | 1.2 | ||
| Male sex | 34 | 29 | ||||
| Slowest CYP2A6 activity status | 3 | 5 | ||||
| Positive TD status 6 months ago | 24 | |||||
McGill University Study on the Natural History of Nicotine Dependence (NDIT Study), Montreal, Quebec, 1999–2004.
SD, standard deviation; TD, tobacco dependence.
The results of the logistic regression model with the occurrence of the event of first TD conversion as the dependent variable are presented in table 2. Recent cigarette consumption significantly increased the risk of the event of first TD conversion, while the effect of cigarette consumption in the intermediate past was non‐significant. Slowest CYP2A6 activity status and higher depression level were associated with significant increases in the risk of conversion. The incidence rate decreased with increasing time since cigarette use onset. The results for the associations with age and sex were non‐significant.
Table 2 Crude and adjusted incidence rate ratios for tobacco dependence (TD).
| Characteristic | Crude | Adjusted | ||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p Value | IRR | 95% CI | p Value | |
| Average monthly cigarette consumption in the past three months (per 100 cigarettes) | 1.54 | 1.32 to 1.79 | <0.0001 | 1.63 | 1.36 to 1.97 | <0.0001 |
| Average monthly cigarette consumption in the past 3–6 months (per 100 cigarettes) | 1.15 | 1.00 to 1.32 | 0.0461 | 0.94 | 0.79 to 1.12 | 0.5134 |
| Slowest CYP2A6 activity status | 4.11 | 1.48 to 11.39 | 0.0065 | 4.19 | 1.38 to 12.76 | 0.0117 |
| Depression symptoms score | 1.45 | 1.11 to 1.91 | 0.0064 | 1.61 | 1.17 to 2.21 | 0.0031 |
| Age (per year) | 0.87 | 0.76 to 0.99 | 0.0385 | 0.91 | 0.77 to 1.08 | 0.2870 |
| Time since the onset of cigarette use (per year) | 0.71 | 0.57 to 0.89 | 0.0032 | 0.76 | 0.58 to 1.00 | 0.0485 |
| Male sex | 1.10 | 0.72 to 1.69 | 0.6453 | 1.19 | 0.74 to 1.92 | 0.4751 |
McGill University Study on the Natural History of Nicotine Dependence (NDIT Study), Montreal, Quebec, 1999–2004.
IRR, incidence rate ratio; CI, confidence interval.
Analysis of prevalence of positive TD status
Among the 2592 person‐surveys available in the source data base for the prevalence analysis, those with missing values for TD status six months ago (n = 606, 23.4%), cigarette consumption 3–6 months ago (n = 294, 11.3%), and depression symptoms score (n = 19, 0.7%) were excluded. Among the 1673 person‐surveys contributed by 429 smokers during follow‐up, which were retained for the analysis, 502 were positive for TD status, thus producing an overall prevalence rate of 30.0%.
The distribution of selected characteristics in the study base series for the prevalence analyses are provided in table 1.
The results of the logistic regression model with the occurrence of the state of TD as the dependent variable are presented in table 3. Recent cigarette consumption increased the prevalence of the TD state considerably, while the association with cigarette consumption in the more distant past was weak and not statistically significant. Slowest CYP2A6 activity status and higher depression levels in the past three months were associated with significant increases in the risk of TD. The results for the associations with time since cigarette use onset, age, and sex were non‐significant.
Table 3 Crude and adjusted prevalence odds‐ratios for tobacco dependence (TD).
| Characteristic | Crude | Adjusted | ||||
|---|---|---|---|---|---|---|
| POR | 95% CI | p Value | POR | 95% CI | p Value | |
| Average monthly cigarette consumption in the past three months (per 100 cigarettes) | 1.40 | 1.30 to 1.51 | <0.0001 | 1.35 | 1.23 to 1.48 | <0.0001 |
| Average monthly cigarette consumption in the past 3–6 months (per 100 cigarettes) | 1.23 | 1.16 to 1.32 | <0.0001 | 1.08 | 0.99 to 1.18 | 0.0825 |
| Slowest CYP2A6 activity status | 1.96 | 0.98 to 3.92 | 0.0571 | 2.30 | 1.29 to 4.09 | 0.0047 |
| Positive TD status six months ago | 5.37 | 3.76 to 7.65 | <0.0001 | 3.53 | 2.41 to 5.19 | <0.0001 |
| Depression symptoms score | 1.29 | 1.09 to 1.53 | 0.0037 | 1.47 | 1.22 to 1.75 | <0.0001 |
| Age (per year) | 1.14 | 1.03 to 1.26 | 0.0096 | 0.92 | 0.82 to 1.04 | 0.1830 |
| Time since the onset of cigarette use (per year) | 1.26 | 1.10 to 1.45 | 0.0011 | 1.01 | 0.84 to 1.21 | 0.9024 |
| Male sex | 0.91 | 0.60 to 1.39 | 0.6626 | 1.05 | 0.71 to 1.56 | 0.7880 |
McGill University Study on the Natural History of Nicotine Dependence (NDIT Study), Montreal, Quebec, 1999–2004.
POR, prevalence odds ratio; CI, confidence interval.
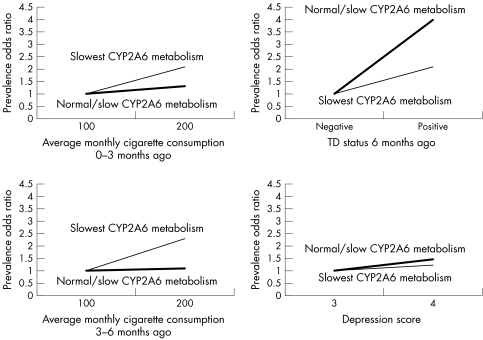
Figure 1 shows the adjusted prevalence odds ratios obtained from four regression models with the occurrence of the TD state as the dependent variable and with product‐terms between CYP2A6 activity status and other variables (p values for the product terms with CYP2A6 activity status in the corresponding models were 0.1881 (with amount of cigarette consumption 0–3 months ago), 0.1245 (with amount of cigarette consumption 3–6 months ago), 0.5789 (with positive TD status 6 months ago), and 0.8011 (with the depression symptom score), respectively). Although none of the product terms was statistically significant at the α level of 0.05, the results suggest that there might be a positive multiplicative interaction between CYP2A6 activity and cigarette consumption in the recent and intermediate past. For example, a 100‐cigarette increase in monthly consumption 0–3 and 3–6 months ago in respondents with normal/slower CYP2A6 activity increased the odds of being tobacco dependent by 31% and 9%, respectively. In respondents with slowest CYP2A6 activity, a 100‐cigarette increase in cigarette consumption increased the odds by 107% and 127%, respectively. There was a potential negative multiplicative interaction between CYP2A6 activity and the depression symptom score and positive TD status six months ago. For example, a 1‐unit change in the depression symptom score in respondents with normal/slower CYP2A6 activity increased the odds of being tobacco dependent by 48%, while in respondents with slowest CYP2A6 activity the difference in the depression symptom score increased the odds by 22%. A positive TD status six months ago in respondents with normal/slower CYP2A6 activity increased the odds of being tobacco dependent by 299%, while in subjects with slowest CYP2A6 activity a positive TD status six months ago increased the odds by 113%.
Figure 1 Plots of estimated adjusted prevalence odds ratios associated with four determinants of tobacco dependence (TD) in subjects with slowest (thin line) and normal/slow (thick line) CYP2A6 metabolism status. McGill University Study on the Natural History of Nicotine Dependence (NDIT Study), Montreal, Quebec, 1999–2004.
DISCUSSION
Our study identified determinants of the onset of TD, as well as of the state of TD. Among the more important findings is that recent cigarette consumption and not smoking in the intermediate or distant past is a strong risk factor for conversion. In other words, our results suggest that the TD‐inducing effects of cigarette consumption are relatively acute, while past smoking may not have strong long‐lasting or cumulative effects in terms of affecting the risk of TD. This finding corroborates recent reports that TD symptoms in adolescents can begin very soon after the onset of cigarette use,7,8,9 and suggests that, for the purpose of prevention, cigarette use and the development of TD are probably concomitant, at least in some subjects.
CYP2A6 genotype conferring slow nicotine metabolism considerably increases the risk of TD conversion and maintaining the state of TD. Because the CYP2A6 enzyme mediates over 90% of the conversion of nicotine to cotinine,25,26 the major route of elimination of nicotine, it is not surprising that CYP2A6 activity is an important indicator of susceptibility to TD. Although studies have found that slowest CYP2A6 activity may be protective for being a current adult smoker,17,27 suggesting a possible TD‐averting effect of the slow CYP2A6‐mediated nicotine metabolism on acquisition, this was not found in our acquisition study,18 suggesting alternative explanations are responsible for the increased risk of acquisition in adolescents and decreased risk for being a smoker in adults. The biologic plausibility of our findings stems from the reasoning that slow rate of nicotine conversion into cotinine results in a prolonged presence of higher nicotine concentrations in the bloodstream, thus increasing the exposure of nicotinic acetylcholine receptors in the brain to nicotine.
Another important finding is the observed positive association between symptoms of depression and the risk of TD. While others have reported this association,28,29,30,31 including in young people,32,33,34 our study is the first to provide empirical evidence that even after controlling for smoking intensity and other risk factors, higher depression levels are associated with a higher risk of TD. Depression is characterised by disruption of dopamine metabolism and dysfunction of the dopaminergic reward systems in the brain,35 and there is now evidence that nicotinic acetylcholine receptors, which mediate the reinforcing effects of nicotine,36 are expressed on dopaminergic neurons37 in key brain regions.38 Still, while it is plausible that depression could predispose individuals to develop and maintain TD by enhancing “rewarding” properties of nicotine (or through some other mechanism),35 it remains possible that the association could merely reflect a genetic link between susceptibility to depression and TD.39
We have found that the incidence rate of conversion to TD decreases with time since the onset of cigarette use. The negative association is most likely due to “depletion of susceptibles”: among subjects with the same relevant aspects of cigarette use profile (as well as the other covariates controlled for in our analyses), those with increased susceptibility to TD could be expected to tend to convert earlier and thus gradually leave the pool of candidates for TD conversion so that the remaining subjects would be comprised of the relatively less susceptible individuals who experience conversion to TD at a lower rate.
The overall prevalence of a positive TD status in the study population was 30%; the prevalence varied considerably across population subgroups. For example, according to the fitted multivariable logistic regression prevalence function, among 15‐year‐old girls one year after the onset of cigarette use, with the monthly smoking consumption of 10 cigarettes in the past six months, depression symptom score of 1, normal/slower CYP2A6 activity status, and negative TD status six months ago, the expected prevalence of TD is approximately 10%, while among the subjects of the same age, sex, and time since the onset of cigarette use, but with the monthly smoking consumption of 300 cigarettes in the past six months, depression symptom score of 3, slowest CYP2A6 activity status, and positive TD status six months ago, the expected prevalence of TD is 83%.
Although our analysis of effect‐modification by CYP2A6 activity status was “hypothesis‐generating”, the results are potentially highly interesting. They suggest that TD‐inducing effects of smoking are particularly evident in slow nicotine metabolisers. At the same time, depression was strongly associated with increased TD risk among normal nicotine metabolisers while the association appeared more modest among slow metabolisers. Similarly, a positive history of TD six months ago was associated with a particularly elevated risk of positive current TD status among normal nicotine metabolisers.
What this paper adds
Previous research has shown that symptoms of tobacco dependence can occur in novice smokers soon after onset of cigarette use. However, current knowledge of the aetiology of tobacco dependence in adolescents is lacking. To our knowledge, this is the first study to investigate risk factors for both initial conversion to tobacco dependence, and for being in the tobacco dependent state among adolescent smokers. The study shows that the risk of tobacco dependence in adolescents is associated with intensity of recent cigarette consumption, while the role of more distant cigarette consumption appears to be negligable; subjects with genetically‐determined slow nicotine metabolism and those with more depression symptoms are at increased risk of developing tobacco dependence. The risk of being tobacco dependent is considerably higher in subjects who had previously developed the tobacco dependent state.
Strengths of our study include a well‐defined source base, thorough follow‐up of the source population over a long period of time, and use of standardised and validated measurement instruments for data collection. In addition, we used a clinically relevant definition of TD as the outcome, in contrast with smoking behaviour, which, especially in adolescents, is driven by multiple constitutional, social, and environmental factors, of which TD is but one.2,3,5
Limitations include that self‐reports of cigarette consumption and TD symptoms are subject to misclassification, which could attenuate observed associations with cigarette consumption and explain the absence of observed effect of cigarette consumption in the more distant past. In addition, CYP2A6 genotyping can be subject to misspecification,40 which could result in attenuation of the observed association between CYP2A6 activity status and TD. Secondly, although at least for some determinants examined, stronger associations could be expected to be found in a domain of high risk of TD, the limited sample size prevented us from restricting our study to a domain of more intensive smoking. Nevertheless, even in the relatively low‐smoking‐intensity domain (namely, smoking at least one cigarette per month in the previous three months) we were able to demonstrate several important associations with TD. Finally, although the response proportion in our study was relatively low, the reasons for this (namely, the need to take blood samples, and labour dispute issues among the teachers) were likely independent of the associations investigated in our study, and thus no or little systematic error should have been introduced into the results. In addition to the low response rate, some participants dropped out of the study or failed to provide information on all the relevant study variables, and were thus excluded from the analysis. Here too, however, a bias would have been introduced into our results only if the reasons for dropping out and/or unavailability of data influenced the associations of interest.
In conclusion, our study suggests that the occurrence of TD, as indicated by ICD‐10 criteria,20 in adolescents is determined by recent cigarette consumption. Furthermore, subjects with genetically determined slow nicotine metabolism, which increases the duration of nicotine exposure during smoking, and those with more symptoms of depression are at increased risk of becoming tobacco dependent. The risk of being tobacco dependent is considerably higher in subjects who had previously developed the TD state. Future research should investigate the likely role of gene–environment interactions in determining the risk of TD.
Our results point to the importance of early intervention among youth who experiment with cigarettes, to prevent the occurrence of TD. Both the high risk of conversion to TD among recent smokers, and the importance of past TD as a determinant of current TD, suggest that considerable efforts should be devoted to the development of cessation programmes for novice smokers within the first few months of initiation. In addition, the finding that slow metabolisers of nicotine have significantly higher risks of TD suggests that the length of time the brain is exposed to nicotine should be minimised, to reduce the likelihood of conversion to TD. Hence, our results support the need for early and intensive interventions aimed at reducing exposure to cigarettes in secondary school, although the impact of cessation programmes for youth remains to be documented. Finally, our results in combination with those of other investigators suggest a strong link between depressive symptoms and TD in youth. Clinicians should be aware of this association to enable provision of relevant cessation and prevention counselling among youth with symptoms of depression, and to assess and treat co‐occurrence of depression and cigarette use among novice smokers.
ACKNOWLEDGEMENTS
This research was funded by the National Cancer Institute of Canada, with funds from the Canadian Cancer Society. Jennifer O'Loughlin holds a Canada Research Chair in the Childhood Determinants of Adult Chronic Disease.
Abbreviations
aIRR - adjusted incidence rate ratio
aPOR - adjusted prevalence odds ratio
CI - confidence interval
GEE - generalised estimating equation
OR - odds ratio
NDIT - Natural History of Nicotine Dependence Study
SE - standard error
TD - tobacco dependence
Footnotes
Competing interests: Rachel F Tyndale is a shareholder in Nicogen Research Inc, a company focused on the development of novel smoking cessation treatments.
References
- 1.US Department of Health and Human Services The health consequences of smoking: nicotine addiction. A report of the Surgeon General, 1988. Rockville, Maryland: Public Health Service, Centers for Disease Control, Office on Smoking and Health, 1988, (DHHS Publication No (CDC) 88‐8406. )
- 2.Pomerleau O F, Collins A, Shiffman S.et al Why some people smoke and others do not: new perspectives. J Consult Clin Psychol 199361723–731. [DOI] [PubMed] [Google Scholar]
- 3.Pomerleau O F. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet 199525161–177. [DOI] [PubMed] [Google Scholar]
- 4.Lerman C, Audrain J, Orleans C T.et al Investigation of mechanisms linking depressed mood to nicotine dependence. Addict Behav 1996219–19. [DOI] [PubMed] [Google Scholar]
- 5.Bergen A W, Caporaso N. Cigarette smoking. J Natl Cancer Inst 1999911365–1375. [DOI] [PubMed] [Google Scholar]
- 6.Horn K, Fernandes A, Dino G.et al Adolescent nicotine dependence and smoking cessation outcomes. Addict Behav 200328769–776. [DOI] [PubMed] [Google Scholar]
- 7.DiFranza J R, Savageau J A, Rigotti N A.et al Development of symptoms of tobacco dependence in youths: 30 months of follow up data from the DANDY study. Tob Control 200211228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiFranza J R, Savageau J A, Fletcher K.et al Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (development and assessment of nicotine dependence in youths) study. Arch Pediatr Adolesc Med 2002156397–403. [DOI] [PubMed] [Google Scholar]
- 9.O'Loughlin J, DiFranza J, Tyndale R.et al Nicotine dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med 200325219–225. [DOI] [PubMed] [Google Scholar]
- 10.Colby S M, Tiffany S T, Shiffman S.et al Measuring nicotine dependence among youth: a review of available approaches and instruments. Drug Alcohol Depend 200059(suppl 1)S23–S39. [DOI] [PubMed] [Google Scholar]
- 11.Breslau N. The idiosyncratic definition of nicotine dependence. Arch Gen Psychiatry 199754973–974. [DOI] [PubMed] [Google Scholar]
- 12.Johnson E O, Breslau N, Anthony J C. The latest dimensionality of DIS/DSM‐III‐R nicotine dependence: exploratory analyses. Addiction 199691583–588. [PubMed] [Google Scholar]
- 13.O'Loughlin J, DiFranza J, Tarasuk J.et al Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob Control 200211354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenstein E, Mermelstein R J. Some methodological cautions in the use of the Tolerance Questionnaire. Addict Behav 198611439–442. [DOI] [PubMed] [Google Scholar]
- 15.Etter J F, Le Houezec J, Perneger T V.et al A self‐administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology 200328359–370. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen O S, Flegel K M. Elementary concepts of medicine: XI. Illness in a community: morbidity, epidemiology, J Eval Clin Pract 20039345–348. [DOI] [PubMed] [Google Scholar]
- 17.Schoedel K A, Hoffmann E B, Rao Y.et al Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics 200414615–626. [DOI] [PubMed] [Google Scholar]
- 18.O'Loughlin J, Paradis G, Kim W.et al Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of young adolescents. Tob Control 200413422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miettinen O S.Theoretical epidemiology. Principles of occurrence research in medicine. New York: John Wiley and Sons, 1985
- 20.World Health Organization International statistical classification of diseases and related health problems, 10th revision. Geneva: WHO, 1992
- 21.Kandel D B, Davies M. Epidemiology of depressive mood in adolescents. Arch Gen Psychiatry 1982391205–1212. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen O S. Epidemiology: Quo vadis? Eur J Epidemiol 200419713–718. [DOI] [PubMed] [Google Scholar]
- 23.Zeger S L, Liang K Y. Models for longitudinal data: a generalized estimating equations approach. Biometrics 1988441049–1060. [PubMed] [Google Scholar]
- 24.Jennrich R I, Schluchter M D. Unbalanced repeated‐measures models with structured covariance matrices. Biometrics 198642805–820. [PubMed] [Google Scholar]
- 25.Nakajima M, Yamamoto T, Nunoya K.et al Role of human cytochrome P4502A6 in C‐oxidation of nicotine. Drug Metab Dispos 1996241212–1217. [PubMed] [Google Scholar]
- 26.Messina E S, Tyndale R F, Sellers E M. A major role of CYP2A6 in nicotine C‐oxidation by human liver microsomes. J Pharmacol Exp Ther 19972821608–1614. [PubMed] [Google Scholar]
- 27.Pianezza M, Sellers E M, Tyndale R F. A common genetic defect in nicotine metabolism decreases smoking. [Letter]. Nature 1998393750 [Google Scholar]
- 28.Lerman C, Berrettini W. Elucidating the role of genetic factors in smoking behaviour and nicotine dependence. Am J Med Genet 2003118B48–54. [DOI] [PubMed] [Google Scholar]
- 29.Glassman A H, Helzer J E, Covey L S.et al Smoking, smoking cessation, and major depression. JAMA 19902641546–1549. [PubMed] [Google Scholar]
- 30.Anda R F, Williamson D F, Escobedo L G.et al Depression and dynamics of smoking. A national perspective. JAMA 19902641541–1545. [PubMed] [Google Scholar]
- 31.Covey L S, Glassman A H, Stetner F. Cigarette smoking and major depression. J Addict Res 19981735–46. [DOI] [PubMed] [Google Scholar]
- 32.Fergusson D M, Lynskey M T, Horwood L J. Comorbidity between depressive disorders and nicotine dependence in a cohort of 16‐years‐olds. Arch Gen Psychiatry 1996531043–1047. [DOI] [PubMed] [Google Scholar]
- 33.Breslau N, Kilber M M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry 1991481069–1074. [DOI] [PubMed] [Google Scholar]
- 34.Breslau N, Kilber M M, Andreski P. Nicotine dependence and major depression. Arch Gen Psychiatry 19935031–35. [DOI] [PubMed] [Google Scholar]
- 35.Cardenas L, Tremblay L K, Naranjo C A.et al Brain reward system activity in major depression and comorbid nicotine dependence. J Pharmocol Exp Ther 20023021265–1271. [DOI] [PubMed] [Google Scholar]
- 36.Dani J, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron 199616905–908. [DOI] [PubMed] [Google Scholar]
- 37.Pidoplichko V, DeBiasi M, Williams J.et al Nicotine activates and desensitizes midbrain dopamine neurons. Nature 1997390401–404. [DOI] [PubMed] [Google Scholar]
- 38.Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol 199753199–237. [DOI] [PubMed] [Google Scholar]
- 39.Janowsky D S, Overstreet D H, Nurnberger J I., Jr Is cholinergic sensitivity a genetic marker for the affective disorders? Am J Med Genet 199415335–344. [DOI] [PubMed] [Google Scholar]
- 40.Oscarson M, Gullsten H, Rautio A.et al Genotyping of human cytochrome P450 2A6 (CYP2A6), nicotine C‐oxidase. FEBS Letters 1998438201–205. [DOI] [PubMed] [Google Scholar]