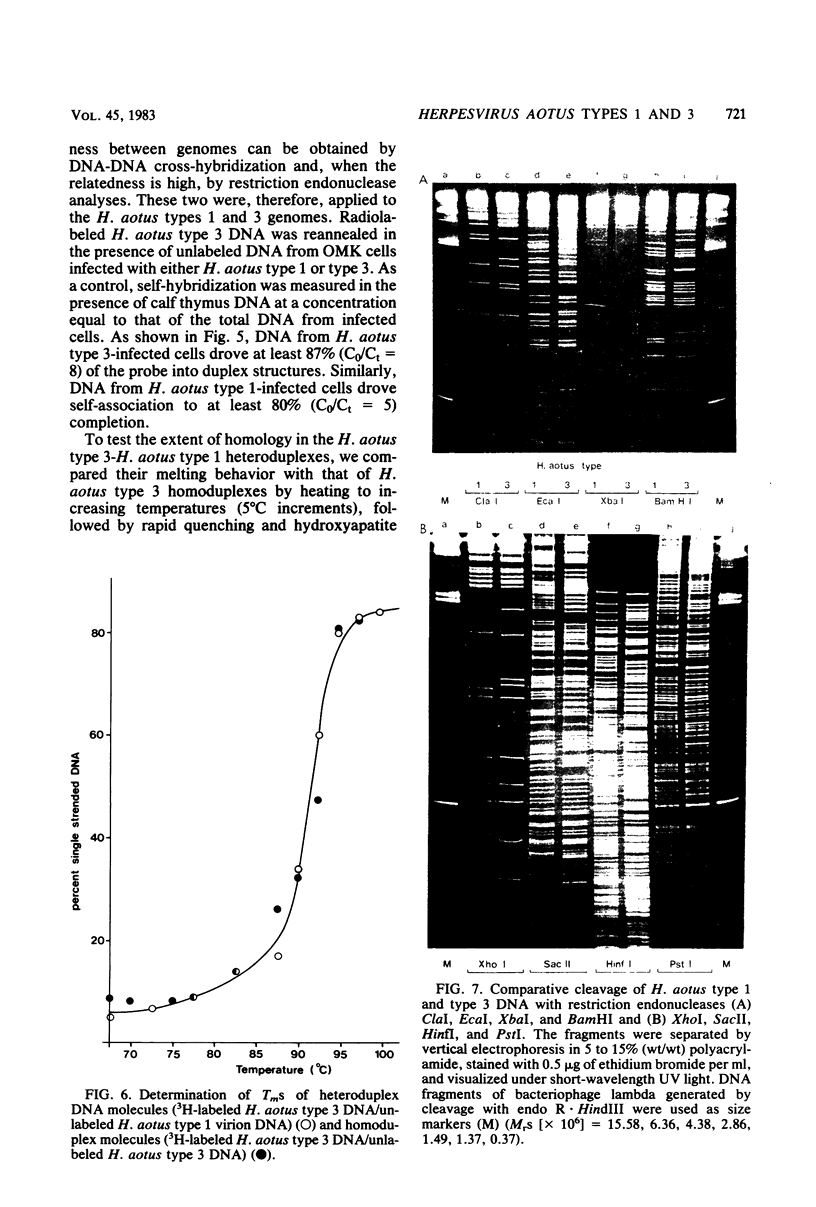
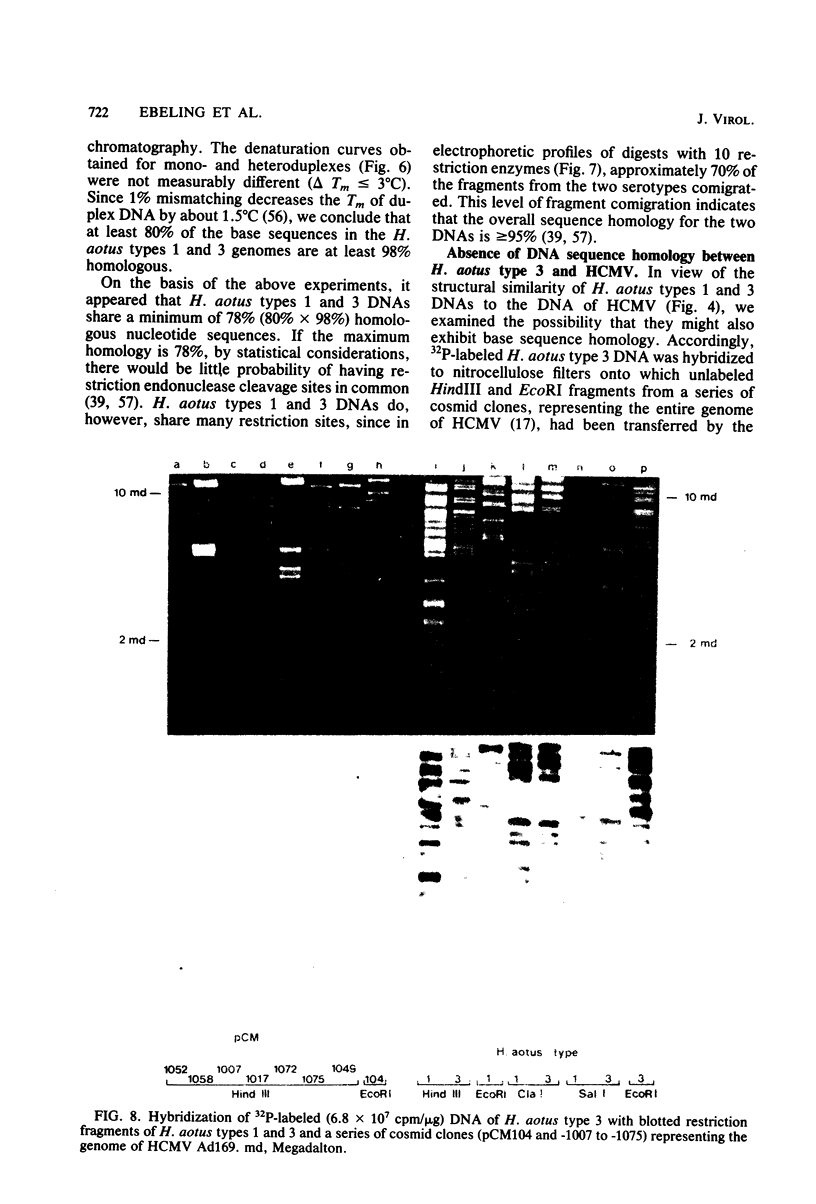
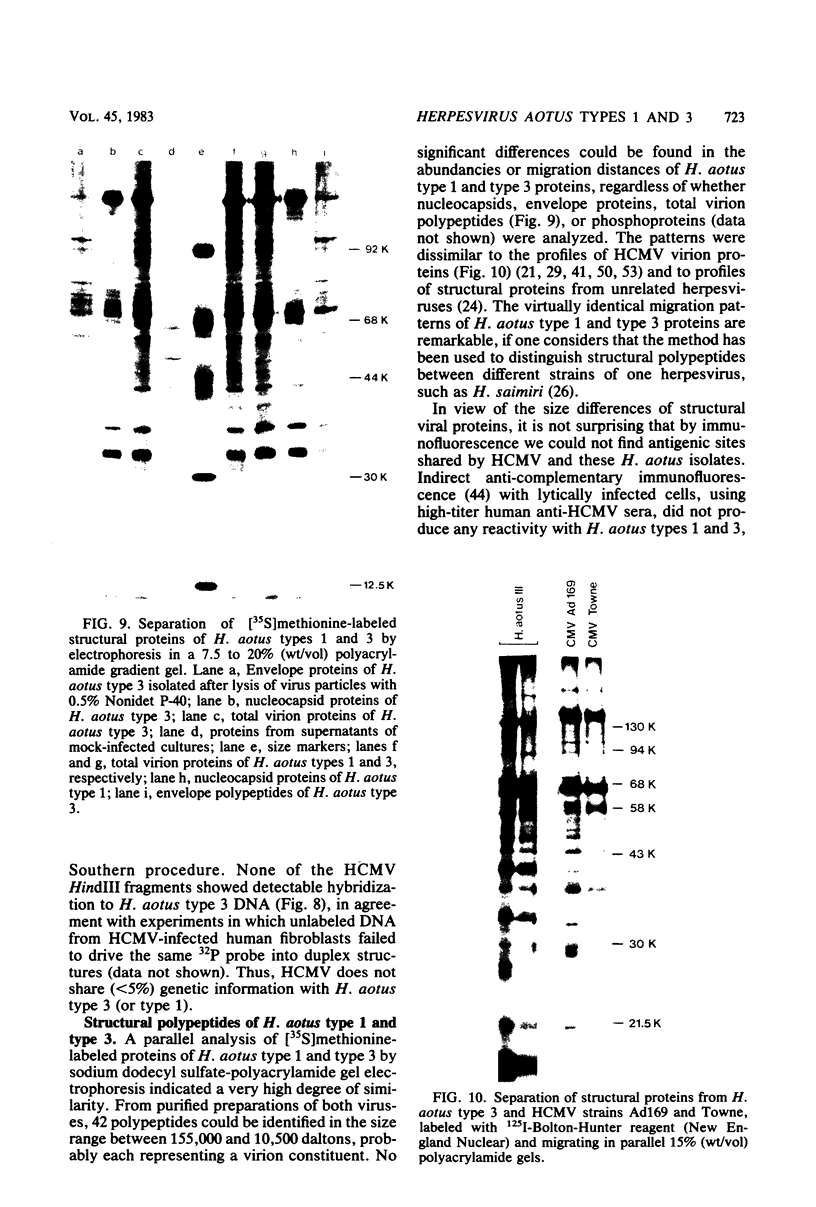
Abstract
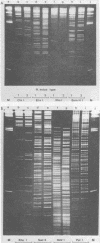
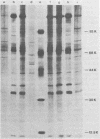
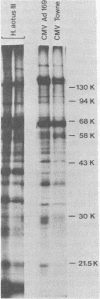
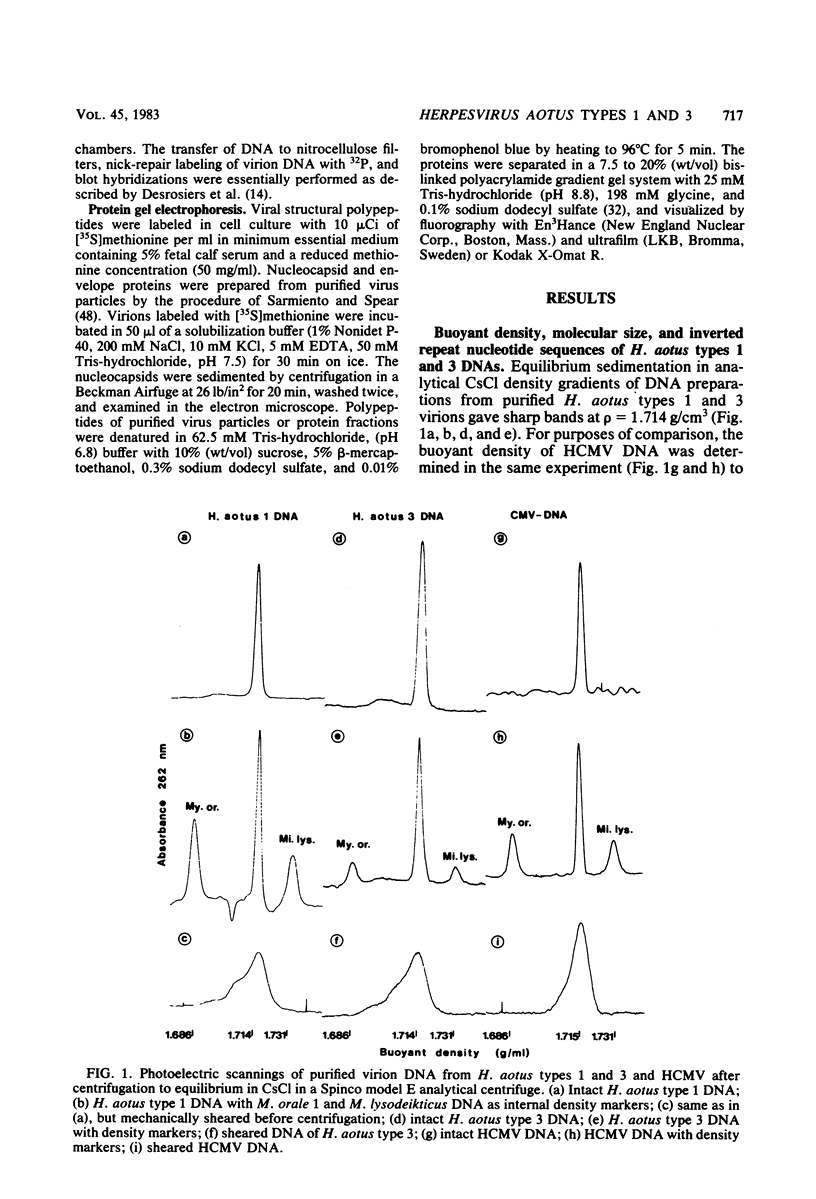
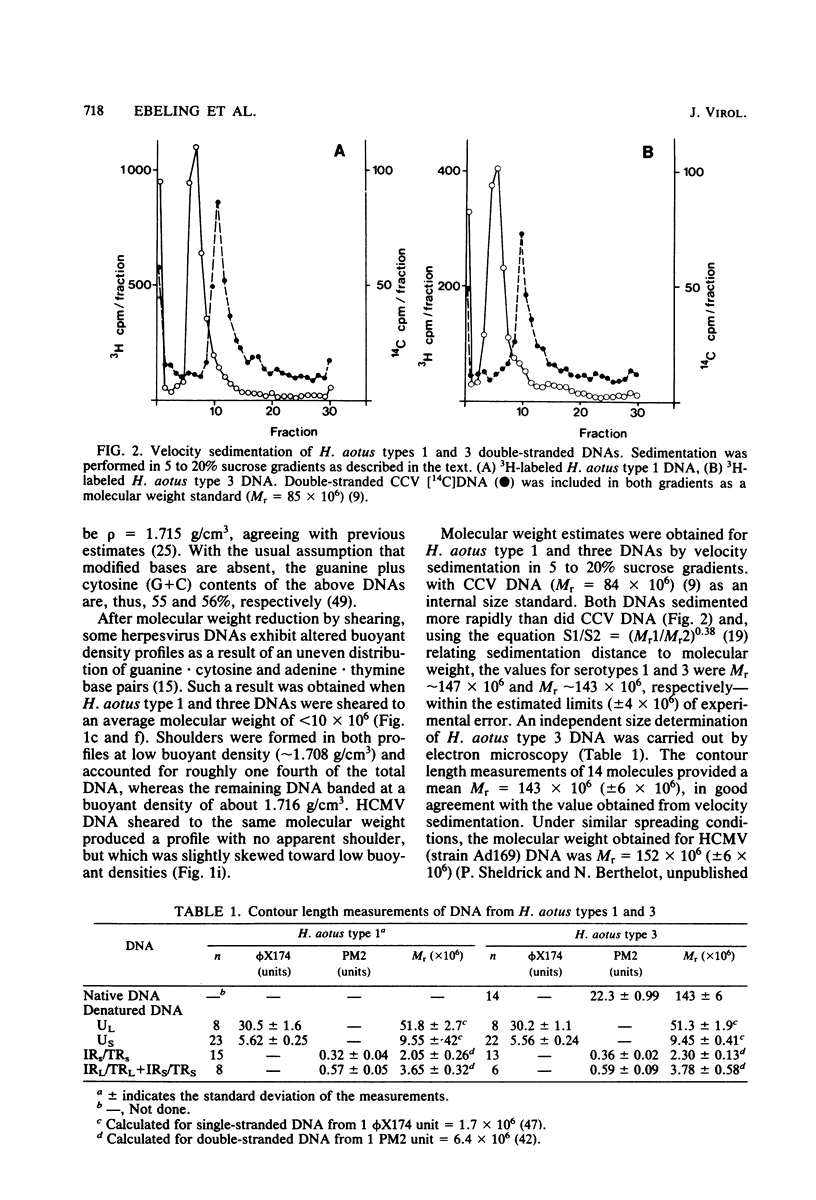
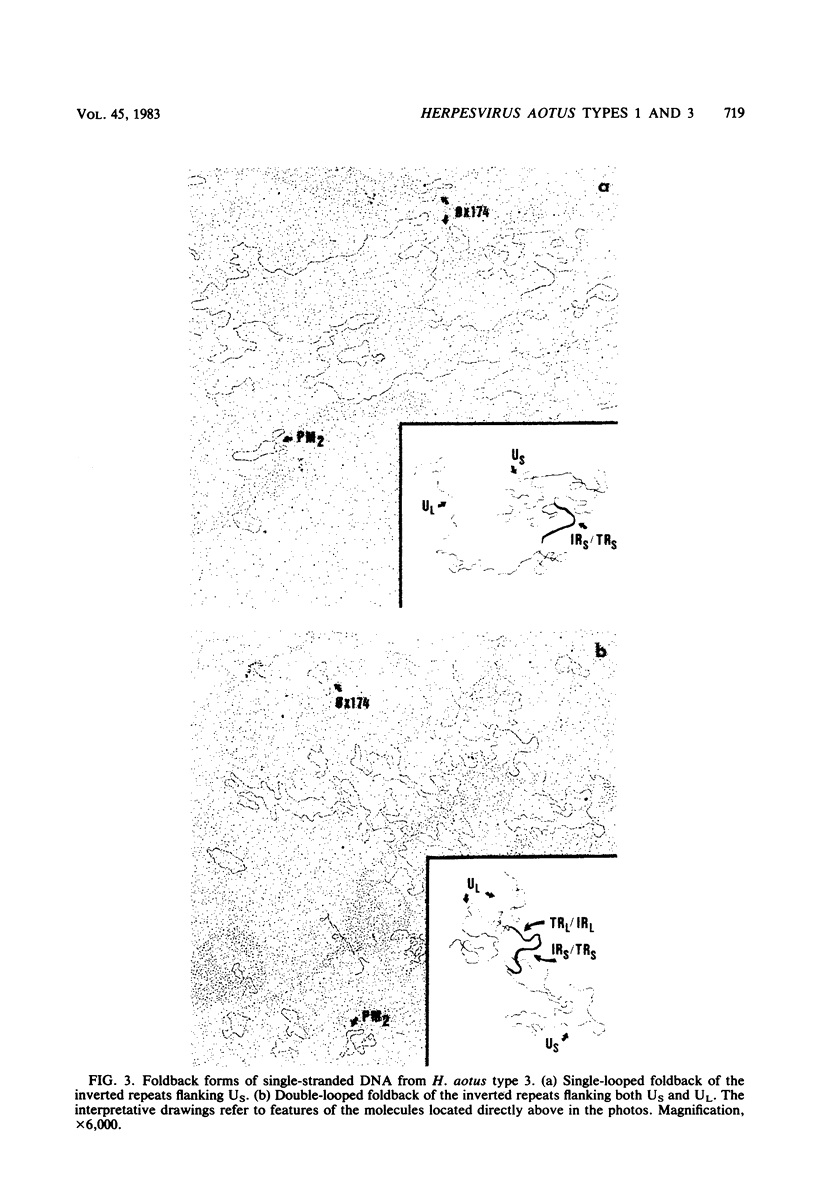
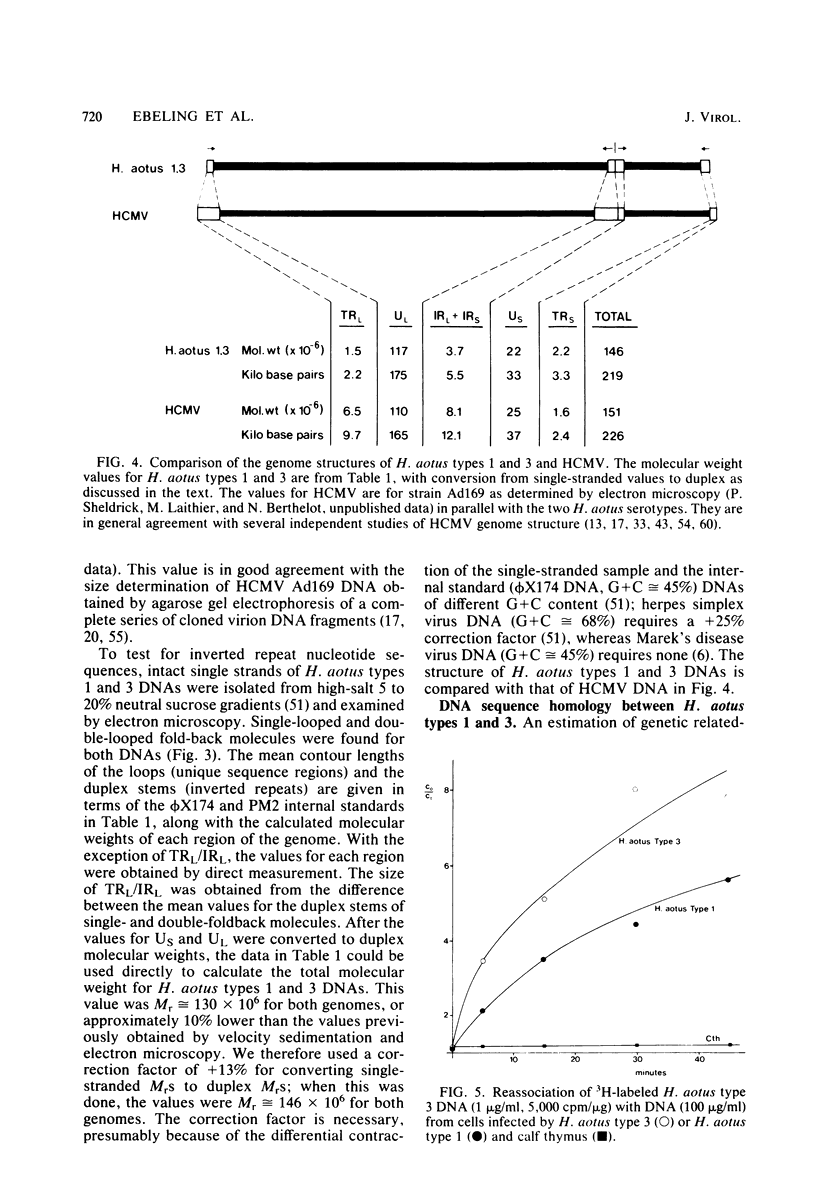
Two serologically distinguishable primate herpesviruses, Herpesvirus aotus type 1 and type 3, were examined with regard to their genomes and structural polypeptides. The duplex DNA genomes of these two viruses were found to be essentially identical in molecular weight (Mr approximately equal to 145 X 10(6)) and guanine plus cytosine composition (55%). Both contained unique and inverted repeat nucleotide sequences of the same size and arrangement, which, as judged by DNA-DNA hybridization and restriction enzyme analyses, were at least 95% homologous. In addition, no differences were observed in electrophoretic profiles of virion polypeptides. Because of their great similarity with respect to these criteria, the two viruses ought to be considered independent isolates (or strains) of a single virus, which should be designated H. aotus type 1. The elevated molecular weight and presence of two sets of inverted repeat sequences closely resemble the structure of the human cytomegalovirus genome. However, no sequence homology (less than 5%) nor similarity in virion polypeptides was detected between H. aotus type 1 and human cytomegalovirus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Chopra H. C., Armstrong G. R. A cytomegalovirus isolated from an owl monkey. Lab Anim Sci. 1972 Apr;22(2):190–195. [PubMed] [Google Scholar]
- Allen G. P., McGowan J. J., Randall C. C., Mancini W., Cheng Y. C., Gentry G. A. Purification and characterization of deoxythymidine kinase (dTK) induced in dTK- 3T3 mouse cells by equine herpesvirus type 1 (EHV-1). Virology. 1979 Jan 30;92(2):367–374. doi: 10.1016/0042-6822(79)90141-7. [DOI] [PubMed] [Google Scholar]
- Barahona H. H., Meléndez L. V., King N. W., Daniel M. D., Fraser C. E., Preville A. C. Herpesvirus aotus type 2: a new viral agent from owl monkeys (Aotus trivirgatus). J Infect Dis. 1973 Feb;127(2):171–178. doi: 10.1093/infdis/127.2.171. [DOI] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman T. G., Roizman B., Adams G., Stover B. H. Restriction endonuclease fingerprinting of herpes simplex virus DNA: a novel epidemiological tool applied to a nosocomial outbreak. J Infect Dis. 1978 Oct;138(4):488–498. doi: 10.1093/infdis/138.4.488. [DOI] [PubMed] [Google Scholar]
- Cebrian J., Kaschka-Dierich C., Berthelot N., Sheldrick P. Inverted repeat nucleotide sequences in the genomes of Marek disease virus and the herpesvirus of the turkey. Proc Natl Acad Sci U S A. 1982 Jan;79(2):555–558. doi: 10.1073/pnas.79.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Estes J. E., Huang E. S., Pagano J. S. Epstein-Barr virus-associated thymidine kinase. J Virol. 1978 Apr;26(1):203–208. doi: 10.1128/jvi.26.1.203-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Tsou T. Y., Hackstadt T., Mallavia L. P. Induction of thymidine kinase and DNase in varicella-zoster virus-infected cells and kinetic properties of the virus-induced thymidine kinase. J Virol. 1979 Jul;31(1):172–177. doi: 10.1128/jvi.31.1.172-177.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman S., Lacasa M., Sheldrick P. Physical Map of the Channel Catfish Virus Genome: Location of Sites for Restriction Endonucleases EcoRI, HindIII, HpaI, and XbaI. J Virol. 1979 Jul;31(1):73–85. doi: 10.1128/jvi.31.1.73-85.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Melendez L. V., King N. W., Barahona H. H., Fraser C. E., Garcia F. G., Silva D. Isolation and characterization of a new virus from owl monkeys: herpesvirus aotus type 3. Am J Phys Anthropol. 1973 Mar;38(2):497–500. doi: 10.1002/ajpa.1330380254. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Meléndez L. V., King N. W., Fraser C. E., Barahona H. H., Hunt R. D., Garcia F. G., Trum B. F. Herpes virus aotus: a latent herpesvirus from owl monkeys (Aotus trivirgatus) isolation and characterization. Proc Soc Exp Biol Med. 1971 Dec;138(3):835–845. doi: 10.3181/00379727-138-36002. [DOI] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Mulder C., Fleckenstein B. Methylation of Herpesvirus saimiri DNA in lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3839–3843. doi: 10.1073/pnas.76.8.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Ludwig H. Repetitive sequences in complete and defective genomes of Herpesvirus saimiri. J Virol. 1975 Feb;15(2):398–406. doi: 10.1128/jvi.15.2.398-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Mulder C., Werner F. J., Daniel M. D., Falk L. A., Delius H. Herpesvirus ateles DNA and its homology with Herpesvirus saimiri nucleic acid. J Virol. 1978 Jan;25(1):361–373. doi: 10.1128/jvi.25.1.361-373.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Collins J. Cloning of the complete human cytomegalovirus genome in cosmids. Gene. 1982 Apr;18(1):39–46. doi: 10.1016/0378-1119(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Werner J. The presence of Herpesvirus Saimiri genomes in virus-transformed cells. Int J Cancer. 1977 Apr 15;19(4):546–554. doi: 10.1002/ijc.2910190416. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Greenaway P. J., Oram J. D., Downing R. G., Patel K. Human cytomegalovirus DNA: BamHI, EcoRI and PstI restriction endonuclease cleavage maps. Gene. 1982 Jun;18(3):355–360. doi: 10.1016/0378-1119(82)90174-3. [DOI] [PubMed] [Google Scholar]
- Gupta P., St Jeor S., Rapp F. Comparison of the polypeptides of several strains of human cytomegalovirus. J Gen Virol. 1977 Mar;34(3):447–454. doi: 10.1099/0022-1317-34-3-447. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Honess R. W., O'Hare P., Young D. Comparison of thymidine kinase activities indiced in cells productively infected with herpesvirus saimiri and herpes simplex virus. J Gen Virol. 1982 Feb;58(Pt 2):237–249. doi: 10.1099/0022-1317-58-2-237. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelton W. H., Mandel M. Deoxyribonucleic acid base compositions of mycoplasma strains of avian orgin. J Gen Microbiol. 1969 May;56(2):131–135. doi: 10.1099/00221287-56-2-131. [DOI] [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S., Pagano J. S. Analysis of cytomegalovirus genomes with restriction endonucleases Hin D III and EcoR-1. J Virol. 1976 Jun;18(3):1095–1105. doi: 10.1128/jvi.18.3.1095-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural polypeptides of purified human cytomegalovirus. J Virol. 1976 Dec;20(3):604–611. doi: 10.1128/jvi.20.3.604-611.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Thymidine kinase isozymes of normal and virus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):703–715. doi: 10.1101/sqb.1974.039.01.084. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Viral-induced thymidine kinase isozymes. Prog Med Virol. 1975;21:13–34. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung W. C., Dubbs D. R., Trkula D., Kit S. Mitochondrial and herpesvirus-specific deoxypyrimidine kinases. J Virol. 1975 Sep;16(3):486–497. doi: 10.1128/jvi.16.3.486-497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. J., Allen G. P., Barnett J. M., Gentry G. A. Biochemical characterization of equine herpesvirus type 3-induced deoxythymidine kinase purified from lytically infected horse embryo dermal fibroblasts. J Virol. 1980 May;34(2):474–483. doi: 10.1128/jvi.34.2.474-483.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Hudson J. B. Thymidine kinase activity in mouse 3T3 cells infected by murine cytomegalovirus (MCV). Virology. 1977 Jul 15;80(2):430–433. doi: 10.1016/s0042-6822(77)80019-6. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Otsuka T., Takahashi M. Induction of deoxypyrimidine kinase activity in human embryonic lung cells infected with varicella-zoster virus. J Virol. 1977 Mar;21(3):1232–1235. doi: 10.1128/jvi.21.3.1232-1235.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Cremer N. Electrophoretic analysis of polypeptides immune precipitated from cytomegalovirus-infected cell extracts by human sera. Infect Immun. 1982 Jun;36(3):933–942. doi: 10.1128/iai.36.3.933-942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R. F. DNA nucleotide sequence heterogeneity between the Towne and AD169 strains of cytomegalovirus. J Virol. 1980 Oct;36(1):152–161. doi: 10.1128/jvi.36.1.152-161.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Roizman B., Carmichael L. E., Deinhardt F., de-The G., Nahmias A. J., Plowright W., Rapp F., Sheldrick P., Takahashi M., Wolf K. Herpesviridae. Definition, provisional nomenclature, and taxonomy. The Herpesvirus Study Group, the International Committee on Taxonomy of Viruses. Intervirology. 1981;16(4):201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Spear P. G. Membrane proteins specified by herpes simplex viruses. IV. Conformation of the virion glycoprotein designated VP7(B2). J Virol. 1979 Mar;29(3):1159–1167. doi: 10.1128/jvi.29.3.1159-1167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz H., Müller-Lantzsch N., Peteler G. Human immune response to proteins of cytomegalovirus. Intervirology. 1980;13(3):154–161. doi: 10.1159/000149120. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Laithier M., Lando D., Ryhiner M. L. Infectious DNA from herpes simplex virus: infectivity of double-stranded and single-stranded molecules. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3621–3625. doi: 10.1073/pnas.70.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. The relationship between mismatched base pairs and the thermal stability of DNA duplexes. II. Effects of deamination of cytosine. Biochim Biophys Acta. 1973 Feb 4;294(1):416–424. doi: 10.1016/0005-2787(73)90096-8. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyushin B. F., Belozersky A. N., Kokurina N. A., Kadirova D. X. 5-methylcytosine and 6-methylamino-purine in bacterial DNA. Nature. 1968 Jun 15;218(5146):1066–1067. doi: 10.1038/2181066a0. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Koprowski H., Lonsdale D. M., Brown S. M., Subak-Sharpe J. H. The polypeptide and the DNA restriction enzyme profiles of spontaneous isolates of herpes simplex virus type 1 from explants of human trigeminal, superior cervical and vagus ganglia. J Gen Virol. 1979 Apr;43(1):151–171. doi: 10.1099/0022-1317-43-1-151. [DOI] [PubMed] [Google Scholar]
- Weinmaster G. A., Misra V., McGuire R., Babiuk L. A., DeClercq E. Bovid herpesvirus type-1 (infectious bovine rhinotracheitis virus)-induced thymidine kinase. Virology. 1982 Apr 15;118(1):191–201. doi: 10.1016/0042-6822(82)90332-4. [DOI] [PubMed] [Google Scholar]
- Weststrate M. W., Geelen J. L., van der Noordaa J. Human cytomegalovirus DNA: physical maps for restriction endonucleases BglII, hindIII and XbaI. J Gen Virol. 1980 Jul;49(1):1–21. doi: 10.1099/0022-1317-49-1-1. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]
- Wolf H. A procedure for simultaneous preparation of large amounts of DNA and RNA by the use of potassium iodide gradients. Anal Biochem. 1975 Oct;68(2):505–511. doi: 10.1016/0003-2697(75)90645-4. [DOI] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]