Abstract
Background
Recent syphilis outbreaks have raised concern regarding the potential enhancement of HIV transmission. The incidence of syphilis and its association with HIV‐1 infection rates among a cohort of sexually transmitted infection (STI) clinic attendees was investigated.
Methods
2732 HIV‐1 seronegative patients attending three STI and one gynaecology clinic, were enrolled from 1993–2000 in an ongoing prospective cohort study of acute HIV‐1 infection in Pune, India. At screening and quarterly follow up visits, participants underwent HIV‐1 risk reduction counselling, risk behaviour assessment and HIV/STI screening that included testing for serological evidence of syphilis by RPR with TPHA confirmation. Patients with genital ulcers were screened with dark field microscopy.
Results
Among 2324 participants who were HIV‐1 and RPR seronegative at baseline, 172 participants were found to have clinical or laboratory evidence of syphilis during follow up (5.4 per 100 person years, 95% CI 4.8 to 6.5 per 100 person years). Independent predictors of syphilis acquisition based on a Cox proportional hazards model included age less than 20 years, lack of formal education, earlier calendar year of follow up, and recent HIV‐1 infection. Based on a median follow up time of 11 months, the incidence of HIV‐1 was 5.8 per 100 person years (95% CI 5.0 to 6.6 per 100 person years). Using a Cox proportional hazards model to adjust for known HIV risk factors, the adjusted hazard ratio of HIV‐1 infection associated with incident syphilis was 4.44 (95% CI 2.96 to 6.65; p<0.001).
Conclusions
A high incidence rate of syphilis was observed among STI clinic attendees. The elevated risk of HIV‐1 infection that was observed among participants with incident syphilis supports the hypothesis that syphilis enhances the sexual transmission of HIV‐1 and highlights the importance of early diagnosis and treatment of syphilis.
Keywords: sexually transmitted infections, HIV, genital ulcer disease, syphilis
Strong epidemiological evidence now exists to support the hypothesis that sexually transmitted infections (STIs), particularly genital ulcer disease (GUD), facilitate the sexual transmission of HIV.1,2,3,4,5,6 Numerous prospective studies have looked more specifically at the interaction between syphilis and the risk of HIV infection.6,7,8,9,10,11,12,13,14 The majority of studies have been limited by small numbers of syphilis cases and have only revealed a trend towards a higher risk of HIV infection among individuals with syphilis. Studies among men who have sex with men (MSM) have revealed a significant increased risk of HIV infection among men with previous syphilis but these studies were limited by the use of self reported rather than laboratory diagnoses.15,16,17 One study among STI clinic patients in Miami had a large number of syphilis cases and revealed a strong association between primary and secondary syphilis and the risk of HIV seroconversion; unfortunately no behavioural data were available to control for any possible confounding.18
Accurate figures on the incidence of syphilis are not available for most developing countries. Population based studies have shown the seroprevalence to vary widely depending on the group tested.19,20 Serological surveys in India have revealed high seroprevalence rates ranging from 9.07% among high risk STI patients in Himachal Pradesh to 21.9% in long distance truck drivers in central India.21,22 An earlier study by our group revealed that syphilis was an important cause of GUD among STI patients in Pune, India, Treponema pallidum DNA being isolated from 10% of genital ulcers.23
Our objective was to determine the incidence and identify risk factors for syphilis infection in India and any association with HIV‐1 infection. The identification of specific risk factors for syphilis will assist in developing cost effective interventions targeted to particular risk groups in this resource poor setting. A clear understanding of the relation between syphilis and HIV will also help guide focused HIV prevention strategies geared at STI treatment and control.
Methods
Study participants
HIV‐1 seronegative patients attending three referral STI clinics and a reproductive tract infection clinic were enrolled in a prospective study of HIV‐1 infection in Pune, India from May 1993 to April 2000. The study population represents a mixture of male STI patients, female partners of male STI patients, female sex workers, and women with reproductive tract infections.
Study design
The original study design was described previously.24 Briefly, all patients received pretest and post‐test counselling for HIV and other STIs. They were enrolled in the study after informed consent was obtained. At screening, participants were interviewed using a structured questionnaire regarding demographics, STD history, medical history, sexual behaviour, HIV/AIDS knowledge, and clinical symptoms. At the time of physical examination and interview, investigators and participants were unaware of participants' HIV antibody status. Participants were requested to return for quarterly follow up visits where they were examined for evidence of GUD, cervicitis, or urethritis and a follow up questionnaire on sexual behaviour was administered. Blood was drawn for HIV serology at enrolment and follow up visits. Unused sera were stored at −20°C for future testing.
HIV‐1 serology
Serum samples were screened with a commercially available enzyme linked immunosorbent assay (ELISA) kit for identification of HIV‐1 and HIV‐2 antibodies (Recombigen HIV‐1/HIV‐1‐2, Cambridge Biotech, Galway, Ireland). Specimens testing positive by ELISA were confirmed with a rapid test for HIV‐1 and HIV‐2 (Recombigen HIV‐1/HIV‐2 Rapid Test Device, Cambridge Biotech). Specimens with discrepant ELISA results were confirmed with a third different ELISA or western blot assay (Cambridge Biotech). Western blot assays were interpreted according to the CDC criteria.25
Syphilis serology
Participants were screened at follow up visits for serological evidence of syphilis using the rapid plasma reagin (RPR) (Serodia, Japan) non‐treponemal antibody test with confirmation by Treponema pallidum hemaglutination assay (TPHA) (Serodia). If a genital ulcer was found on physical examination, fluid from the lesion was collected and examined under dark field microscopy for evidence of typical spirochetes.
Case definitions
Incident syphilis infection was defined as TPHA seroconversion or a genital ulcer accompanied by either positive dark field microscopy examination or RPR seroconversion (titre >1:8). Incident syphilis cases were classified according to length of time since date of exposure to syphilis (within 6 months, or more than 6 months). The date of exposure to syphilis was estimated as 50 days before the visit where a primary syphilis case was identified, or for TPHA seroconversion, as the midpoint of the interval between the last visit with negative syphilis serology and the first visit with positive TPHA results.
Statistical analyses
Crude syphilis and HIV‐1 incidence rates were calculated as number of seroconversions divided by the summed person years of follow up. Ninety five per cent confidence intervals were calculated based on a Poisson distributed variable.26 Unadjusted rate ratios were computed as the ratio of the incidence rate in the category of interest divided by the rate in the referent category, with 95% confidence intervals based on exact Poisson methods.
Risk factors for incident HIV‐1 and syphilis infection were identified using Cox proportional hazards regression analysis with time invariant and time dependent covariates. Seroconverters were matched to the risk set by follow up time such that data collected at the seroconversion visit were compared with data collected from the risk set at the visit matching closest in terms of follow up time. Variables for the multivariate analyses were chosen based on previously identified risk factors for HIV‐1 infection in this cohort (listed in table 2, fig 1). Follow up time for incident syphilis cases was classified according to this time dependent criteria and used in the calculation of HIV‐1 incidence rates. All statistical analyses were performed with the use of SAS software (SAS Institute, Cary, NC, USA, release 8.02).
Table 2 Risk factors for incident syphilis infection among STD/RTI clinic patients, Pune, India, May 1993–April 2000.
| Characteristic | Incident syphilis | Person years* | Syphilis incidence rate (95% CI) | Unadjusted rate ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| Overall | 172 | 3166.2 | 5.4 (4.8 to 6.5) | – | – |
| Year | |||||
| 1993–6 | 104 | 1377.0 | 7.6 (6.2 to 9.2) | 1.00 (Referent) | <0.001 |
| 1997–2000 | 68 | 1789.2 | 3.8 (3.0 to 4.9) | 0.50 (0.37 to 0.69) | |
| Gender/risk group | |||||
| Male STI patient | 137 | 2690.8 | 5.1 (4.3 to 6.0) | 1.00 (Referent) | 0.51 |
| Hijra | 1 | 10.3 | 9.7 (0.3 to 54.0) | 1.91 (0.05 to 10.8) | 0.72 |
| Female STI patient | 10 | 223.5 | 4.5 (2.2 to 8.2) | 0.88 (0.41 to 1.67) | 0.005 |
| Female sex worker | 24 | 241.6 | 9.9 (6.4 to 14.7) | 1.95 (1.21 to 3.03) | |
| Age group (years) | |||||
| <20 | 19 | 140.3 | 13.5 (8.2 to 21.1) | 2.57 (1.46 to 4.33) | <0.001 |
| 20–24 | 51 | 934.4 | 5.5 (4.1 to 7.2) | 1.03 (0.70 to 1.51) | 0.85 |
| 25–29 | 36 | 840.2 | 4.3 (3.0 to 5.9) | 0.81 (0.53 to 1.24) | 0.32 |
| 30+ | 66 | 1251.3 | 5.3 (4.1 to 6.8) | 1.00 (Referent) | |
| Education | |||||
| None | 49 | 474.2 | 10.3 (7.6 to 13.7) | 2.40 (1.62 to 3.53) | <0.001 |
| <High school | 58 | 1182.9 | 4.9 (3.8 to 6.4) | 1.14 (0.78 to 1.65) | 0.48 |
| High school or more | 65 | 1508.2 | 4.3 (3.4 to 5.5) | 1.00 (Referent) | |
| Marital status | |||||
| Never married | 81 | 1466.3 | 5.5 (4.4 to 6.9) | 1.00 (Referent) | 0.71 |
| Married | 75 | 1440.0 | 5.2 (4.1 to 6.6) | 0.94 (0.68 to 1.31) | 0.67 |
| Separated† | 16 | 259.8 | 6.2 (3.5 to 10.0) | 1.11 (0.61 to 1.92) | |
| Living with family | |||||
| Yes | 131 | 2392.4 | 5.5 (4.6 to 6.5) | 1.00 (Referent) | 0.89 |
| No | 41 | 769.8 | 5.3 (3.8 to 7.2) | 0.97 (0.67 to 1.39) | |
| Number of recent sexual partners | |||||
| None/one | 127 | 2434.8 | 5.2 (4.4 to 6.2) | 1.00 (Referent) | 0.34 |
| Two or more | 45 | 731.3 | 6.2 (4.5 to 8.3) | 1.18 (0.82 to 1.67) | |
| Condom use | |||||
| No partners | 49 | 959.8 | 5.1 (3.8 to 6.8) | 1.00 (Referent) | 0.36 |
| Always used | 21 | 523.1 | 4.0 (2.5 to 6.1) | 0.79 (0.45 to 1.34) | 0.80 |
| Sometimes used | 21 | 386.4 | 5.4 (3.4 to 8.3) | 1.06 (0.61 to 1.81) | 0.27 |
| Never used | 81 | 1296.8 | 6.3 (5.0 to 7.8) | 1.22 (0.85 to 1.78) | |
| Urethritis/cervicitis at current visit | |||||
| No | 160 | 2981.7 | 5.4 (4.6 to 6.3) | 1.00 (Referent) | 0.51 |
| Yes | 12 | 184.5 | 6.5 (3.4 to 11.4) | 1.21 (0.61 to 2.18) | |
| Genital ulcer at current or previous visit | |||||
| None | 109 | 2572.2 | 4.2 (3.5 to 5.1) | 1.00 (Referent) | 0.42 |
| Previous visit only | 14 | 263.2 | 5.3 (2.9 to 8.9) | 1.26 (0.66 to 2.20) | <0.001 |
| Current visit only | 25 | 177.9 | 14.1 (9.1 to 20.8) | 3.32 (2.06 to 5.16) | <0.001 |
| Current and previous | 21 | 104.4 | 20.1 (12.5 to 30.8) | 4.75 (2.82 to 7.62) | |
| Recent medical injection‡ | |||||
| No | 127 | 2002.1 | 6.3 (5.3 to 7.6) | 1.00 (Referent) | 0.003 |
| Yes | 44 | 1152.4 | 3.8 (2.8 to 5.1) | 0.60 (0.42 to 0.85) | |
| Recent HIV‐1 infection | |||||
| No | 149 | 3023.1 | 4.9 (4.2 to 5.8) | 1.00 (Referent) | <0.001 |
| Yes | 23 | 142.7 | 16.1 (10.2 to 24.2) | 3.27 (2.01 to 5.09) | |
CI, confidence interval; CSW, commercial sex worker.
*Of 2324 individuals in the analysis, three were missing data on level of education, six were missing “living with family”; six were missing data on number of recent sexual partners at one of their follow up visits; two were missing genital lesion on examination at one visit; and one individual was missing HIV‐1 status at one follow up visit.
†Separated status included individuals who were separated from their spouse, divorced, or widowed.
¶‡Recent, within 6 months.
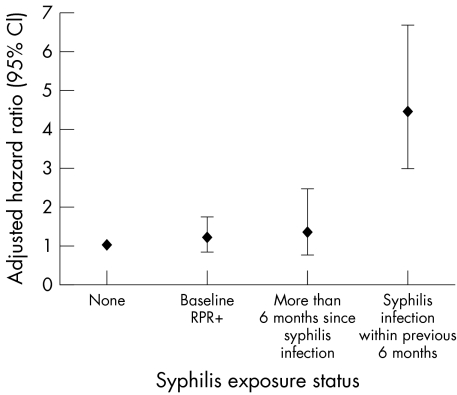
Figure 1 Risk of HIV acquisition by syphilis exposure status (HIV incidence per 100 person years. Adjusted for calendar year, gender/risk group, age, education, marital status, living with family, recent tattoo, recent medical injection, recent number of sexual or CSW partners, condom use, urethritis/cervicitis.)
Results
Baseline and follow up characteristics of the study participants
Of 2729 people enrolled, 2266 were male, eight were hijras (eunuchs), and 455 were female. Demographic characteristics of study patients are described in table 1. The median duration of follow up was 10.7 months (range 45 days–80.0 months), and the median number of follow up visits was three. The median interval between visits overall was 98 days (range 35 days–36.3 months), for those with no exposure to syphilis it was 98 days (range 49 days–36.1 months), for those with baseline RPR+ it was 100.5 days (range 46 days–35.8 months), for those with incident syphilis >6 months it was 105 days (range 35 days–33.0 months), and for those with recent syphilis within months it was 97 days (range 44 days–26.0 months). Participants with more lifetime sex partners (10–99) tended to have a longer duration of follow up (median 13.1 months) but were less likely to report for their regularly scheduled quarterly follow up visit. Participants with high school level education tended to have both a longer duration of follow up (median 12.9 months) and were more likely to report for their regular quarterly follow up visit (data not shown).
Table 1 Baseline characteristics of HIV‐1 seronegative cohort study participants.
| Characteristic | Number* | ||
|---|---|---|---|
| Total | 2729 (100%) | ||
| Gender/risk group | |||
| Male STI patient | 2266 (83.0%) | ||
| Hijra | 8 (0.3%) | ||
| Female STD patient | 151 (5.5%) | ||
| Female RTI patient | 116 (4.3%) | ||
| Female sex worker | 188 (6.9%) | ||
| Age group | |||
| <20 | 329 (12.1%) | ||
| 20–24 | 901 (33.0%) | ||
| 25–29 | 623 (22.8%) | ||
| 30+ | 876 (32.1%) | ||
| Marital status | |||
| Never married | 1357 (49.7%) | ||
| Married | 1215 (44.5%) | ||
| Separated† | 157 (5.8%) | ||
| Living away from spouse/family | |||
| No | 2057 (75.5%) | ||
| Yes | 668 (24.5%) | ||
| Level of education | |||
| None | 456 (16.7%) | ||
| Primary/middle school | 1078 (39.5%) | ||
| High School | 1193 (43.8%) | ||
| Lifetime sex partners | |||
| 1 | 639 (24.6%) | ||
| 2–9 | 1339 (51.6%) | ||
| 10+ | 618 (23.8%) | ||
| Condom use | |||
| No recent partners | 1059 (38.8%) | ||
| Never | 1104 (40.5%) | ||
| Sometimes | 325 (11.9%) | ||
| Always | 241 (8.8%) | ||
| History of genital ulcer‡ | |||
| No | 1533 (56.3%) | ||
| Yes | 1188 (43.7%) | ||
| Genital ulcer on examination | |||
| No | 1757 (65.2%) | ||
| Yes | 939 (34.8%) | ||
| Genital discharge on examination | |||
| No | 2132 (78.9%) | ||
| Yes | 570 (21.1%) | ||
*Data on “living away from spouse/family” were missing for four participants; two were missing level of education; 133 were missing “lifetime sex partners”; eight were missing “history of genital ulcer”; 33 were missing “genital ulcer on examination” at baseline visit; 27 were missing “genital discharge on examination” at baseline visit.
†Separated status included individuals who were separated from their spouse, divorced, or widowed.
‡Ever having reported a genital ulcer.
Incidence and risk factors for syphilis
At screening, three participants were missing RPR results, and 405 participants had a positive RPR (14.8%), and were not included in the syphilis incidence analysis. Of the remaining 2324 participants, 172 were found to have clinical or laboratory evidence of incident syphilis during follow up resulting in a crude syphilis incidence rate of 5.4 cases per 100 person years (95% CI, 4.8 to 6.5 per 100 person years). Risk factors for incident syphilis identified in the unadjusted analysis included earlier calendar year of follow up, sex work among females, younger age, lower level of education, having a genital ulcer (any cause) at current or previous visit, lack of a recent medical injection, and recent HIV‐1 infection (table 2). In the multivariate analysis, the risk of incident syphilis was highest in those <20 years old with a RR 2.79 (95% CI 1.47 to 5.28, p = 0.002) compared to participants older than 30 years. Other independent predictors of syphilis acquisition included a lack of formal education, RR 2.31 (95% CI 1.49 to 3.60, p<0.001), and recent HIV‐1 infection, RR 1.89 (95% CI 1.22 to 2.93, p = 0.004). Syphilis incidence was also higher in the earlier years of the study (1993–6), incidence 7.6 per 100 person years (95% CI 6.5 to 9.2 per 100 person years).
Syphilis and the risk of HIV‐1 infection
The unadjusted rate ratio of HIV 1 acquisition within 6 months of becoming infected with syphilis was 6.26 (95% CI, 4.08 to 9.32). The relation between incident syphilis and HIV was investigated in a Cox proportional hazards model (fig 1). The greatest risk of HIV‐1 infection was found among participants within 6 months of syphilis infection (RR 4.44, 95% CI; 2.96 to 6.65, p<0.001) compared to those with no evidence of syphilis at baseline or follow up. The risk of HIV‐1 infection decreased significantly more than 6 months after syphilis infection (RR 1.34, 95% CI 0.74 to 2.43, p = 0.34). Participants with a reactive RPR at baseline screening and no clinical or laboratory evidence of primary syphilis did not have significantly increased risk of HIV‐1 acquisition during follow up (RR 1.19, 95% CI 0.82 to 1.73, p = 0.35).
Discussion
The findings of this study demonstrate that individuals who acquire syphilis during follow up are at increased risk of also acquiring HIV‐1 infection. The first 6 months following the estimated time of exposure to syphilis represents the period with the greatest risk of HIV‐1 infection compared to individuals who have either never had syphilis or who have had syphilis but more than 6 months have passed since the estimated date of syphilis exposure. The increased risk of HIV infection among participants with incident syphilis remained strong (adjusted RR 4.44) even after controlling for other sexual risk behaviours, which illustrates that syphilis infection is independently associated with HIV‐1 infection.
There are no prospective studies of syphilis incidence in India with which to compare our incidence rates. Few prospective studies have reported syphilis incidence rates as most have reported active syphilis (any stage) based on reactive serological testing. Syphilis incidence was measured in a population based cohort in Mwanza, Tanzania using TPHA serology.27 This study found an overall syphilis incidence of 1.8% with slightly higher rates observed among men. Similar incidence rates were measured in a multiethnic San Francisco neighbourhood (1.5%) and among female sex workers in Mexico City (2.4%).28,29 The higher overall syphilis incidence rate (5.4 per 100 person years) observed in our study is explained by the cohort characteristics; most participants were high risk STI patients referred to our clinics for follow up. Our results are consistent with the World Health Organization estimates for overall syphilis rates, south Asia having the highest number of syphilis cases in the world.30 This study was conducted during a period when aggressive HIV awareness campaigns were implemented in India. The observed decline in syphilis incidence rates over time is consistent with similarly observed declines in HIV‐1 and herpes simplex virus type 2 incidence rates over this period which may be explained by a greater proportion of lower risk individuals (the worried well) presenting for screening as a result of successful HIV awareness campaigns.31
The majority of studies examining the specific role of syphilis in HIV acquisition and transmission have been limited either by small numbers of syphilis cases or by self reported rather than laboratory defined end points. Confounding by sexual risk behaviour remains an important unmeasured factor in many of these studies. This study of syphilis and HIV rates in India is unique owing to the high rates of both infections measured in this population, the frequent follow up schedule allowing time dependent exposure modelling of syphilis, and the ability to control for many important HIV risk behaviours.
The enhancement of HIV transmission by ulcerative STIs may occur by a variety of biological mechanisms, which probably affect both HIV infectiousness and susceptibility. The biological explanation for enhanced susceptibility to HIV among individuals infected with syphilis is based on the theory that the breakdown in mucosal integrity due to ulceration provides a portal of entry for the HIV virus. In addition, this ulceration may result in an influx of CD4+ lymphocytes locally, increasing the number of HIV target cells, which has been observed in the setting of chancroid and recurrent genital herpes infections.32,33,34 Syphilis may also increase HIV infectiousness and transmission among HIV seropositive individuals dually infected with syphilis by increasing the amount of HIV viral shedding.35,36,37,38 In our study, the majority of cases where both syphilis and HIV were acquired during follow up were detected at the same follow up visit. We propose that the increased risk of HIV‐1 infection among participants with incident syphilis in this study probably represents a combination of both the effects of syphilis on susceptibility among HIV‐1 uninfected cohort participants and also an effect on transmission with individuals being exposed to dually infected (both HIV‐1 and syphilis) partners and acquiring both infections simultaneously.
The use of time dependent exposure modelling allowed us to approximate the period where an individual exposed to syphilis could naturally progress through both the primary and secondary stages if untreated. Although all participants received treatment for syphilis, the majority did not present during the primary stage and were only detected serologically in the absence of clinical findings. The 6 month exposure window was chosen based on the natural history of untreated syphilis corresponding to the period of primary and secondary syphilis when an individual could have genital ulceration.39 Genital ulceration is not a feature of the latent period of syphilis (beyond 6 months following syphilis acquisition) and we hypothesised that this period would not increase the relative risk of HIV‐1 infection. Our results confirm this hypothesis with no significant increased risk of HIV‐1 infection observed among cohort participants with syphilis exposure modelled as more than 6 months following estimated acquisition date of syphilis. Interestingly, participants who had a reactive baseline RPR and no clinical or laboratory evidence of primary syphilis were also not found to be at increased risk of HIV‐1 acquisition (RR 1.19, 95% CI 0.82 to 1.73, p = 0.35). This finding is also consistent with our hypothesis as the majority of these participants did not have a genital ulcer on examination.
Residual confounding by sexual risk behaviour remains a major limitation of any study examining the relation between STIs and the risk of HIV infection. Our finding that only the initial 6 months following syphilis exposure increased the risk of HIV‐1 infection in this cohort would support that our statistical models adequately controlled for sexual risk behaviour, as one would not expect an increased biological risk of HIV‐1 acquisition during the latent phases of syphilis and an increased risk during this period (>6 months) may represent unmeasured differences in risk behaviour among syphilis patients.
There are a number of potential limitations to our observed association between syphilis infection and HIV‐1 infection. The use of quantitative survey methods to assess sexual risk behaviour relies on the assumption that participants will accurately report on topics that are not openly discussed in India. The counsellors conducting the interviews in this study had extensive training in counselling methods, in an attempt to minimise error in the measurement of sexual risk behaviours. Unmeasured confounding by sexual behaviour of the sexual partners of participants in our study could also explain in part our findings and could not be evaluated in the current study design. Potential confounding by multi‐colinearity was assessed by incorporating data on herpes simplex virus type 2 serostatus (incident of prevalent infection) into our Cox proportional hazards models. No significant change was found in the risk estimates shown in figure 1. Temporality is also difficult to establish in studies with moderate time periods between visits. The majority of dual infections (syphilis and HIV‐1) observed during follow up were detected at the same clinic visit (n = 22/27) which leaves open the interpretation that acute HIV‐1 infection increased the risk of syphilis infection in this cohort. We think the biological and epidemiological literature is more supportive of the alternative hypothesis that syphilis and the resultant mucosal ulceration increase the risk of HIV‐1 acquisition and transmission.
The ability to demonstrate that treatment and control of STIs can reduce HIV transmission has been complex and challenging. Two large population based studies have yielded mixed results on HIV transmission, the results of which have been recently reviewed.40,41,42,43 The continued controversy over the role in STI treatment in HIV prevention resulting from these discrepant findings may result in an erosion of this key prevention strategy. Our study adds important data to highlight the role of appropriate treatment of syphilis in HIV prevention where this STI is commonly transmitted. HIV prevention strategies in developing countries have focused on the control and treatment of STIs at the population level. Future public health interventions aimed at controlling the spread of HIV through STI treatment may need to be targeted more closely to the populations under study with particular attention paid to the rates of various STIs in the target population.
The high rates of syphilis observed in this cohort are consistent with earlier cross sectional data from India, which revealed high rates of syphilis among truck drivers and STI clinic attendees.21,22 Public health interventions to promote awareness of syphilis among physicians and populations at risk in India are urgently needed to avoid the adverse consequences which could result from missed diagnoses or improper treatment. In addition to contributing to the spread of HIV in India, untreated syphilis could also contribute to poor health outcomes resulting from the consequences of latent stages of the disease and maternal infant transmission with resultant congenital syphilis.
A resurgence of syphilis has been observed beginning in 1997 across North America and Europe with outbreaks being reported among men who have sex with men (MSM) groups in urban settings.44 The potential amplification of HIV transmission in the setting of active syphilis infection suggested by our results and others raises concern that a rise in HIV rates among these high risk populations could be on the horizon. Public health authorities have responded to this concern proactively by mounting aggressive syphilis awareness campaigns to promote prompt and appropriate access to treatment. Our study reveals that syphilis is also an important cofactor contributing to the spread of HIV among high risk individuals in India and should be targeted in the current Government of India strategy to control the spread of HIV.
Key messages
This paper provides the first syphilis incidence data in India documenting high incidence rates when compared to other populations
The risk of incident HIV‐1 is shown to be considerably higher among participants with recent syphilis infection contributing to the spread of HIV‐1 in India
The importance of appropriate treatment and control of syphilis as an HIV‐1 prevention strategy is discussed given these important findings
Contributors
ARR, MVG, SVG, SNJ, ADD, RRG, RCB, and SMM contributed to study design, participant enrolment, data collection, statistical analysis, and manuscript preparation; SJR, AR, and MES contributed to study design, data collection, statistical analysis, and manuscript preparation.
Abbreviations
ELISA - enzyme linked immunosorbent assay
GUD - genital ulcer disease
MSM - men who have sex with men
RPR - rapid plasma reagin
STI - sexually transmitted infections
TPHA - Treponema pallidum hemaglutination assay
Footnotes
Funding/support: This work was supported by the Indian Council of Medical Research (ICMR) and by grants from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (AI 41369, AI 01633), through a contract from the NIAID, NIH through Family Health International (FHI) (AI 35173), in part by a fellowship from Fogarty International Center/USNIH (5 D43 Tw00010‐AITRP). SJ Reynolds was supported by a grant from the R Samuel McLaughlin Foundation. The views expressed do not necessarily reflect the view of the ICMR, NIH, or FHI.
Previous presentations: Presented in part at the 15th Biennial Congress of the International Society for Sexually Transmitted Diseases Research (ISSTDR), 2003.
Informed consent: Informed consent was obtained from all patients participating in the study. Human experimentation guidelines of the US Department of Health and Human Services and those participating institutions were followed in the conduct of this research.
References
- 1.Fleming D T, Wasserheit J N. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999753–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreiss J K, Coombs R, Plummer F.et al Isolation of human immunodeficiency virus from genital ulcers in Nairobi prostitutes. J Infect Dis 1989160380–384. [DOI] [PubMed] [Google Scholar]
- 3.Plummer F A, Wainberg M A, Plourde P.et al Detection of human immunodeficiency virus type 1 (HIV‐1) in genital ulcer exudate of HIV‐1‐infected men by culture and gene amplification. J Infect Dis 1990161810–811. [DOI] [PubMed] [Google Scholar]
- 4.Plummer F A, Simonsen J N, Cameron D W.et al Cofactors in male‐female sexual transmission of human immunodeficiency virus type 1. J Infect Dis 1991163233–239. [DOI] [PubMed] [Google Scholar]
- 5.Quinn T C, Cannon R O, Glasser D.et al The association of syphilis with risk of human immunodeficiency virus infection in patients attending sexually transmitted disease clinics. Arch Intern Med 19901501297–1302. [PubMed] [Google Scholar]
- 6.Telzak E E, Chiasson M A, Bevier P J.et al HIV‐1 seroconversion in patients with and without genital ulcer disease. A prospective study. Ann Intern Med 19931191181–1186. [DOI] [PubMed] [Google Scholar]
- 7.Bakari M, Lyamuya E, Mugusi F.et al The prevalence and incidence of HIV‐1 infection and syphilis in a cohort of police officers in Dar es Salaam, Tanzania: a potential population for HIV vaccine trials. AIDS 200014313–320. [DOI] [PubMed] [Google Scholar]
- 8.Celentano D D, Nelson K E, Suprasert S.et al Risk factors for HIV‐1 seroconversion among young men in northern Thailand. JAMA 1996275122–127. [PubMed] [Google Scholar]
- 9.Deschamps M M, Pape J W, Hafner A.et al Heterosexual transmission of HIV in Haiti. Ann Intern Med 1996125324–330. [DOI] [PubMed] [Google Scholar]
- 10.Kapiga S H, Lyamuya E F, Lwihula G K.et al The incidence of HIV infection among women using family planning methods in Dar es Salaam, Tanzania. AIDS 19981275–84. [DOI] [PubMed] [Google Scholar]
- 11.Kilmarx P H, Limpakarnjanarat K, Mastro T D.et al HIV‐1 seroconversion in a prospective study of female sex workers in northern Thailand: continued high incidence among brothel‐based women. AIDS 1998121889–1898. [DOI] [PubMed] [Google Scholar]
- 12.Laga M, Manoka A, Kivuvu M.et al Non‐ulcerative sexually transmitted diseases as risk factors for HIV‐1 transmission in women: results from a cohort study. AIDS 1993795–102. [DOI] [PubMed] [Google Scholar]
- 13.Nelson K E, Eiumtrakul S, Celentano D.et al The association of herpes simplex virus type 2 (‐2), Haemophilus ducreyi, and syphilis with HIV infection in young men in northern Thailand. J Acquir Immune Defic Syndr Hum Retrovirol 199716293–300. [DOI] [PubMed] [Google Scholar]
- 14.Rakwar J, Lavreys L, Thompson M L.et al Cofactors for the acquisition of HIV‐1 among heterosexual men: prospective cohort study of trucking company workers in Kenya. AIDS 199913607–614. [DOI] [PubMed] [Google Scholar]
- 15.Craib K J, Meddings D R, Strathdee S A.et al Rectal gonorrhoea as an independent risk factor for HIV infection in a cohort of homosexual men. Genitourin Med 199571150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darrow W W, Echenberg D F, Jaffe H W.et al Risk factors for human immunodeficiency virus (HIV) infections in homosexual men. Am J Public Health 198777479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuiken C L, van Griensven G J, de Vroome E M.et al Risk factors and changes in sexual behavior in male homosexuals who seroconverted for human immunodeficiency virus antibodies. Am J Epidemiol 1990132523–530. [DOI] [PubMed] [Google Scholar]
- 18.Otten M W, Jr, Zaidi A A, Peterman T A.et al High rate of HIV seroconversion among patients attending urban sexually transmitted disease clinics. AIDS 19948549–553. [DOI] [PubMed] [Google Scholar]
- 19.Aral S O, Holmes K K. Sexually transmitted diseases in the AIDS era. Sci Am 199126462–69. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization An overview of selected curable STDs. Syphilis estimates, 1995. WHO Office of HIV/AIDS and STDs. Geneva: WHO, 1995
- 21.Gawande A V, Vasudeo N D, Zodpey S P.et al Sexually transmitted infections in long distance truck drivers. J Commun Dis 200032212–215. [PubMed] [Google Scholar]
- 22.Thakur T S, Sharma V, Goyal A.et al Seroprevalence of HIV antibodies, Australia antigen and VDRL reactivity in Himachal Pradesh. Indian J Med Sci 199145332–335. [PubMed] [Google Scholar]
- 23.Risbud A, Chan‐Tack K, Gadkari D.et al The etiology of genital ulcer disease by multiplex polymerase chain reaction and relationship to HIV infection among patients attending sexually transmitted disease clinics in Pune, India. Sex Transm Dis 19992655–62. [DOI] [PubMed] [Google Scholar]
- 24.Mehendale S M, Rodrigues J J, Brookmeyer R S.et al Incidence and predictors of human immunodeficiency virus type 1 seroconversion in patients attending sexually transmitted disease clinics in India. J Infect Dis 19951721486–1491. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control Interpretation and use of the western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR 1989381–7. [PubMed] [Google Scholar]
- 26.Breslow N E, Day N E.Statistical methods in cancer research. Vol 2. The design and analysis of cohort studies. New York, Oxford: Oxford University Press, 1987 [PubMed]
- 27.Todd J, Munguti K, Grosskurth H.et al Risk factors for active syphilis and TPHA seroconversion in a rural African population. Sex Transm Infect 20017737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel D, Larsen S A, Golden E.et al Prevalence, incidence, and correlates of syphilis seroreactivity in multiethnic San Francisco neighborhoods. Ann Epidemiol 19944460–465. [DOI] [PubMed] [Google Scholar]
- 29.Uribe‐Salas F, Rio‐Chiriboga C, Conde‐Glez C J.et al Prevalence, incidence, and determinants of syphilis in female commercial sex workers in Mexico City. Sex Transm Dis 199623120–126. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates 2001. Geneva: WHO ( www.who.int/csr )
- 31.Reynolds S J, Risbud A R, Shepherd M E.et al Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis 20031871513–1521. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham A L, Turner R R, Miller A C.et al Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Invest 198575226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koelle D M, Abbo H, Peck A.et al Direct recovery of herpes simplex virus ()‐specific T lymphocyte clones from recurrent genital ‐2 lesions. J Infect Dis 1994169956–961. [DOI] [PubMed] [Google Scholar]
- 34.Spinola S M, Orazi A, Arno J N.et al Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis 1996173394–402. [DOI] [PubMed] [Google Scholar]
- 35.Ghys P D, Fransen K, Diallo M O.et al The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d'Ivoire. AIDS 199711F85–F93. [DOI] [PubMed] [Google Scholar]
- 36.Gadkari D A, Quinn T C, Gangakhedkar R R.et al HIV‐1 DNA shedding in genital ulcers and its associated risk factors in Pune, India. J Acquir Immune Defic Syndr Hum Retrovirol 199818277–281. [DOI] [PubMed] [Google Scholar]
- 37.Mbopi‐Keou F X, Gresenguet G, Mayaud P.et al Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis 20001821090–1096. [DOI] [PubMed] [Google Scholar]
- 38.Schacker T, Ryncarz A J, Goddard J.et al Frequent recovery of HIV‐1 from genital herpes simplex virus lesions in HIV‐1‐infected men. JAMA 199828061–66. [DOI] [PubMed] [Google Scholar]
- 39.Singh A E, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev 199912187–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosskurth H, Mosha F, Todd J.et al Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet 1995346530–536. [DOI] [PubMed] [Google Scholar]
- 41.Grosskurth H, Gray R, Hayes R.et al Control of sexually transmitted diseases for HIV‐1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet 20003551981–1987. [DOI] [PubMed] [Google Scholar]
- 42.Hitchcock P, Fransen L. Preventing HIV infection: lessons from Mwanza and Rakai. Lancet 1999353513–515. [DOI] [PubMed] [Google Scholar]
- 43.Wawer M J, Sewankambo N K, Serwadda D.et al Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet 1999353525–535. [DOI] [PubMed] [Google Scholar]
- 44.Ciesielski C A. Sexually transmitted diseases in men who have sex with men: an epidemiologic review. Curr Infect Dis Rep 20035145–152. [DOI] [PubMed] [Google Scholar]