Abstract
We present the case of a 26 year old HIV positive homosexual man who was managed for suspected Crohn's disease for over 1 year before lymphogranuloma venereum (LGV) was clinically diagnosed. He had presented with constipation, secondary to acute haemorrhagic proctitis, and subsequently had two chlamydia negative rectal smears, using direct fluorescent antibody (DFA) Chlamydia trachomatis staining. Positive chlamydial serology guided retrospective testing of an early rectal biopsy, which was found to have C trachomatis by polymerase chain reaction (Roche Cobas) and identified as LGV serovar L2 by the Sexually Transmitted Bacteria Reference Laboratory (STBRL), Health Protection Agency (HPA), Colindale, London. Chlamydial serology may have a role in identifying late stage LGV infection. Although no standardised test currently exists, consideration should be given to evaluating the role of chlamydial serology in establishing a diagnosis of LGV.
Keywords: lymphogranuloma venereum, chlamydia, Crohn's disease, serology, proctitis
A 26 year old HIV positive man presented, in February 2005, with anal ulceration and tenderness. He had a background history of rectal bleeding and constipation, unresponsive to medication for suspected Crohn's disease. His history also included HIV, treated secondary syphilis, and recurrent penile herpes (unconfirmed). He is homosexual and estimated he had 10–20 partners per month, often in a group setting, with inconsistent condom use.
He had first experienced abdominal pain and constipation, involving the passage of frequent hard blood coated motions, in December 2003 and presented to his GP who arranged referral to a gastroenterologist. Sigmoidoscopy examination revealed “florid proctitis to 15 cm above the anorectal verge…consistent with inflammatory bowel disease.” The histopathology of his rectal biopsy (fig 1) was debated, as it showed morphological features not entirely typical of either infective or inflammatory bowel disease. There was crypt destruction by a diffuse infiltrate of histiocytes and lymphoid cells, including blast forms. There were no crypt abscess or granulomata. He was provisionally diagnosed with Crohn's disease and commenced on daily prednisolone suppositories.
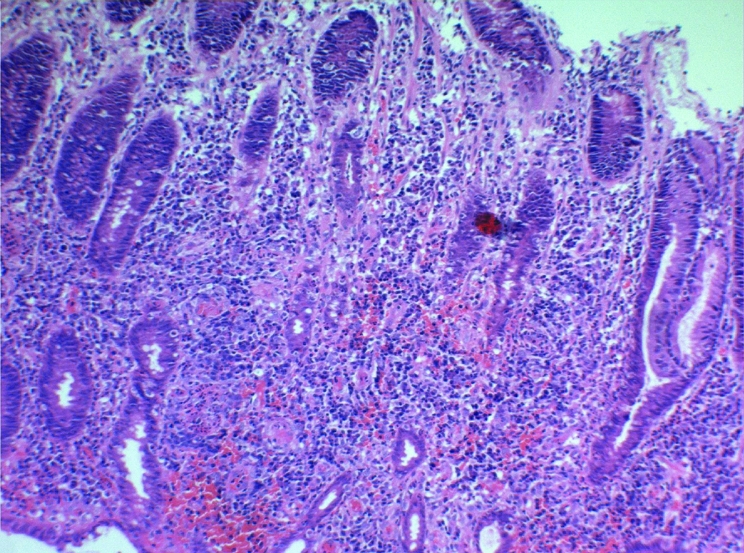
Figure 1 Histology of the rectal biopsy performed in January 2004.
By March 2004, his symptoms had worsened and in addition to rectal bleeding and constipation, he had begun to experience pain on defaecation, tenesmus, and fevers. Mesalazine and osalazine were added to his regimen. In October 2004, antiretroviral therapy of efavirenz, lamivudine, and didanosine was commenced, at a CD4 count of 373 ×106/l, with the view that his symptoms may have represented an HIV related enteropathy.
In November 2004, he presented to our department for routine testing and was diagnosed with rectal gonorrhoea. He did not report bowel or anal symptoms and no proctitis or intracellular diplococci were noted on rectal smear Gram stain (blind swab), but Neisseria gonorrhoeae was later cultured. Direct fluorescent antibody (DFA) testing for C trachomatis was negative (Syva Micro Trak, Trinity Biotech, Dublin, Ireland) and empirical treatment with cefixime 400 mg immediately and doxycycline 100 mg twice daily for 7 days was provided. Interestingly (he reported on retrospective questioning), he experienced a remission of his “proctitis” symptoms later in the month of November 2004, and medication for Crohn's disease was stopped in December 2004.
He next presented to our department, in February 2005, with a 2 week history of perianal tenderness and ulceration. He reported ongoing constipation and the recurrence of rectal bleeding over the preceding 3 months. External examination revealed marked perianal ulceration, precluding proctoscopy. Rectal sample Gram stain (blind swab) was clear but he was treated for presumed infective proctitis with cefixime 400 mg immediately, doxycycline 100 mg twice daily for 7 days, and aciclovir 200 mg five times a day for 5 days. Rectal sample DFA testing was C trachomatis negative, culture negative for N gonorrhoeae, and virus culture negative. There was no serological evidence of syphilis re‐infection and his CD4 count was 521 ×106/l.
Proctoscopy was performed, 2 weeks later, demonstrating the presence of intra‐anal warts and normal appearance of the rectal mucosa. However, within 10 seconds a large amount of pus appeared obscuring the mucosa and polymorphs were seen on Gram stain. Serology was performed, using the whole immunofluorescence test (WIF)1,2 and complement fixation test (CF).1 Antichlamydial antibodies were identified at dilutions of 4096 and 384 respectively. Our laboratory extracted DNA from the original rectal biopsy sample, from January 2004, and found it to be C trachomatis PCR positive (Roche Cobas PCR). This was identified as LGV serovar 2 by STBRL, HPA, Colindale, London. Electron microscopy revealed multiple intracellular inclusion bodies. 21 day course of doxycycline 100 mg twice daily was prescribed, in addition to a previous 6 days of azithromycin 500 mg daily and continuous aciclovir 400 mg twice daily. Anal herpes simplex virus type II was confirmed by viral culture of a repeat anal swab.
One month following the completion of treatment, he reported a return to normal bowel habit and his daily rectal bleeding had ceased. He is currently receiving cryotherapy for treatment of his intra‐anal warts and remains symptom free.
Discussion
Since 2003, there has been an ongoing outbreak of LGV among men who have sex with men (MSM) in Europe. Enhanced surveillance in the United Kingdom for this infection began in 2004, and 72 cases have been identified so far. The majority have presented with proctitis3 and some with constipation.4 LGV presenting as proctitis in a homosexual man is well recognised.5 If left untreated chronic inflammation, scarring, and the development of strictures and fistulae may occur.6
The patient in this case, despite being at risk, did not attribute his constipation to a sexually transmitted infection and thus presented to a gastroenterology department via his GP. It is also well recognised that the clinical and histological picture of early LGV proctocolitis is identical to that seen in inflammatory bowel disease.6 Although the sexual history was known to the gastroenterologists and an infective aetiology considered, LGV was, in early 2004, regarded a rare disease outside resource poor countries.5
The case definition for LGV in the United Kingdom (HPA)7 and Europe (Eurosurveillance),4 includes the detection of C trachomatis by nucleic acid amplification technique (NAAT) and molecular confirmation of L1, L2, or L3 genotypes.4,7 The sensitivity of NAATs for detection of C trachomatis, as evaluated in urogenital and urine specimens, is high and Roche PCR has good sensitivity for LGV.8 It is not possible to assess whether the patient would have been negative by NAAT testing, when he presented in 2005; however, this possibility would be consistent with the lymphotrophic nature of LGV serovars. During chronic disease, LGV infection resides in submucosal lymphoid tissue, which may form abscesses, and the proctitis can be patchy.6 Sampling of uninfected rectal mucosa may explain the two negative DFA tests, in this case. We speculate that there may be a “window period” for the mucosal detection of the lymphotrophic LGV serovars, after which time adequate specimen collection may become problematic.
Serological methods for detecting C trachomatis antibodies currently lack standardisation.1,9 A wide variety of assays exist with variable sensitivities and specificities. Of particular relevance is that C trachomatis shares antigenic determinants not only between serovars, but also with the common respiratory pathogen, C pneumoniae. The commonly accepted “gold standard” chlamydial serology test, micro‐immunofluorescence (MIF), uses serovar specific epitopes of the major outer membrane protein (MOMP) and the “genus specific” lipopolysaccharide (LPS) antigen, hence cross reactivity can occur.1,9 The test is qualitative, subjective, and not suitable for high throughput use.1,9 The WIF, performed at our laboratory, uses LGV serovar 2 infected cells as antigen and is the only test in which all chlamydial antigens are presented.1,2 While it suffers from the same problems as MIF, a stronger association with upper genital tract disease has been observed.2,10,11 The complement fixation test utilises chlamydial LPS, a T cell independent antigen, that elicits only poor memory; thus, raised antibody titres may well reflect active or recent disease.9
Since LGV is a systemic disease, antibody responses are stronger and may be similar to those observed in chlamydial pelvic inflammatory disease.2,10,11 While probably of limited use in early acute infection, chlamydial antibodies may be helpful in establishing a diagnosis in those with late disease, as demonstrated by this case. Serology is not included in the HPA case definition of LGV, possibly because currently used tests lack standardisation and specificity.1,5,9 We believe that evaluation of chlamydial serology, as a diagnostic tool in LGV disease, now requires urgent evaluation.
Acknowledgements
We thank Mr Ian Paul for performing PCR on the rectal biopsy sample.
Contributors
BF was responsible for clinically managing the patient, reviewing the literature and writing and revising the manuscript; PH supervised the management of the patient, provided great contribution to the both the initial manuscript and the review and revision process; JP wrote the histopathology comment in the case report and reviewed the manuscript.
Abbreviations
CF - complement fixation
DFA - direct fluorescent antibody
HPA - Health Protection Authority
LGV - lymphogranuloma venereum
LPS - lipopolysaccharide
MIF - micro‐immunofluorescence
MOMP - major outer membrane protein
NAAT - nucleic acid amplification technique
PCR - polymerase chain reaction
WIF - whole immunofluorescence test
Footnotes
Competing interests: none.
References
- 1.Persson K. The role of serology, antibiotic susceptibility testing and serovar determination in genital chlamydial infections. Best Pract Res Clin Obstet Gynaecol 200216801–814. [DOI] [PubMed] [Google Scholar]
- 2.Akande V A, Hunt L P, Cahill D J.et al Tubal damage in infertile women: prediction using chlamydial serology. Hum Reprod 2003181841–1847. [DOI] [PubMed] [Google Scholar]
- 3.Health Protection Agency Lymphogranuloma venereum in the UK—an update. Commun Dis Rep CDR Wkly [Serial online] 200515(20) [Google Scholar]
- 4.Götz H, Nieuwenhuis R, Ossewaarde T.et al Preliminary report of an outbreak of lymphogranuloma venereum in homosexual men in the Netherlands, with implications for other countries in western Europe. Eurosurveillance Weekly. 2004;8: 22 Jan, 2004
- 5.French P, Ison C A, Macdonald N. Lymphogranuloma venereum in the United Kingdom. Sex Trans infect 20058197–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perine P L, Osoba O A. Lymphogranuloma venereum. In: Holmes KK, Mardh P‐A, Sparling PF, et al, eds. Sexually transmitted diseases. 2nd ed. New York: McGraw Hill, 1990195–204.
- 7.Health Protection Agency Enhanced surveillance of lymphogranuloma venereum (LGV) in England. Commun Dis Rep CDR Wkly [serial online] 200414(41) [Google Scholar]
- 8.Chalker V J, Vaughan H, Patel P.et al External quality assessment for detection of Chlamydia trachomatis. J Clin Microbiol 2005431341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuuminen T, Palomaki P, Paavonen J. The use of serological tests for the diagnosis of chlamydial infections. J Microbiol Methods 200042265–279. [DOI] [PubMed] [Google Scholar]
- 10.Chernesky M, Luinistra K, Sellors J.et al Can serology diagnose upper genital tract Chlamydia trachomatis infection? Studies on women with pelvic pain, with or without chlamydial plasmid DNA in endometrial biopsy tissue. Sex Transm Dis 19982514–19. [DOI] [PubMed] [Google Scholar]
- 11.Land J, Evers J. Chlamydia infection and subfertility. Best Pract Res Clin Obstet Gynaecol 200216901–912. [DOI] [PubMed] [Google Scholar]


