Abstract
Background/objectives
Testing for Chlamydia trachomatis (Ct) is less accepted in people of non‐Dutch ethnicity than Dutch people. We offered additional Ct and gonorrhoea testing through our outreach sexually transmitted infections (STI) prevention programme to determine whether this intervention strategy is feasible and efficient.
Methods
Outreach workers offered test kits to women and men aged 15–29 years, in group and street settings and in a vocational training school. Demographic and behavioural data and characteristics of non‐responders were assessed. DNA was isolated (using the MagNA Pure LC system) from urine and tested using the Cobas Amplicor test.
Results
Among sexually active people, the test rate differed by venue (groups 80% (74/93), school 73% (49/67), street 17% (49/287); p<0.001). There was no difference in test rate between group and school settings by gender or ethnicity. Ct positivity was 14.5% (25/172); women 20.2% (20/99) versus men 6.8% (5/73); p = 0.01. Ct positivity was highest at school (24.5% (12/49)) and among Surinamese/Antillean people (17.5% (14/80)). Treatment rate of index cases and current partners was 100% and 78%, respectively.
Conclusions
We found a high acceptance of chlamydia testing in group and school settings in both men and women of non‐Dutch ethnicity. The prevalence indicates that we have accessed high risk people. Outreach testing and is feasible and most efficient in school and group settings. School screening may have an impact on community prevalence of Ct infections.
Keywords: adolescent, adult, Chlamydia trachomatis , ethnic minorities, high risk groups
The most prevalent bacterial sexually transmitted infections (STI) in developed countries, Chlamydia trachomatis (Ct) infection, can progress to pelvic inflammatory diseases (PID) in women with sequelae such as ectopic pregnancy, infertility, and chronic pelvic pain.1 Improved detection methods of Ct in urine allow community based testing in both sexes, including home based testing,2,3,4,5,6,7,8,9,10 school based screening,11,12 and tailored community outreach testing.13,14,15,16,17,18,19,20,21
In a home based Ct screening project targeting 25–29 year olds, we found non‐Dutch ethnicity, particularly Surinamese/Antillean origin, a predictor for infection.9,22 Participation in screening was less in people of non‐Dutch ethnicity than of Dutch ethnicity (31% versus 42%, p<0.001).9,22
Rotterdam is a multiethnic city; 45% of the inhabitants are of non‐Dutch origin, and 27% of these are of Surinamese/Antillean origin. We were therefore interested to develop alternative strategies to reach people of non‐Dutch ethnicity for chlamydia testing, and conducted a pilot screening project for 15–29 year old youths as part of our community STI prevention programme in Rotterdam. This “STI prevention PLUS” project also included gonorrhoea testing. We aimed to determine whether the intervention strategy of outreach testing is feasible, and technically efficient (response rate and Ct positivity) in various outreach settings.
Methods
The municipal health service (MHS) in Rotterdam has outreach projects targeting populations at high risk for STI/HIV. Youths, particularly of non‐Dutch ethnicity, are approached by outreach workers in three separate venues: group settings (for example, projects for Surinamese/Antillean immigrants, Surinamese/Antillean and African women, teenage dropouts of all ethnicities), street settings (for example, street corners, parks and underground stations; majority are men and of non‐Dutch ethnicity), and sessions at vocational training schools. Outreach workers belonging to the ethnic groups concerned discuss STIs and distribute prevention materials such as condoms in group and street settings. STI nurses provide STI education at the schools.
Project design and data collection
In this project additional confidential testing for gonorrhoea and Ct infection was offered to sexually active people, but others were not excluded. In addition to a verbal explanation, a leaflet was provided about chlamydia and gonorrhoea and the procedure of testing, including a waiver consent form and safe sex information. Participants received a urine sampling kit, a 13 item questionnaire concerning demographic characteristics (age, gender, education, self assigned ethnicity), sexual behaviour, symptoms of STI and history of STI testing, and risk perception for having an STI. As an incentive, all participants received a small backpack to carry the test material home. Participants could either provide first void urine on location, or mail urine later in a postage free plastic envelope. The coded questionnaire and a card with ID number, personal data (at least name and telephone number), and preferred way of receiving the result (mail, email, or SMS) were left with the outreach worker. Information was collected systematically to assess participation and reasons for refusal. The time spent to approach all people, including those who were sexually non‐active, was registered. Technical efficiency, the optimal utilisation of given resources,23 was assessed by the time required per person approached, to reach one person to discuss testing, to obtain one urine sample, and to detect one infected person.
Detection of Chlamydia trachomatis and gonorrhoea
Urine specimens were collected by the outreach workers and kept at room temperature until transport the next working day (maximum 60 hours) to the laboratory. Specimens collected at home were mailed to the laboratory, where they were stored refrigerated. DNA was isolated from urine specimens using the MagNA Pure LC system and tested using the Cobas Amplicor test (Roche Diagnostics, Almere, Netherlands).24
Notification of results and treatment
ID coded results were linked to personal data at the MHS. Non‐infected subjects received their result according to their choice, together with a telephone number for inquiries. Those tested positive for Ct were phoned by the nurse, and offered the choice to be treated by the MHS or by their general practitioner (GP), and were advised to bring their current partner along. Directly observed treatment was according to current standards (Ct: single dose azithromycin 1 g; gonorrhoea: ciproxin 750 mg single dose) and provided free of charge. Partners in the last 6 months were assessed, and offered assistance in partner notification.
Data analysis
Participation rate was defined as the number of people who accepted a test kit, and test rate as the actual number who supplied urine of those who discussed testing with the outreach worker. Non‐response was analysed by comparing participants with those who declined a test kit. Univariate logistic regression analyses for sexually active participants were performed with screening venue and demographic factors as independent variables and participation and test rate as well as diagnosis of Ct as the dependent variable. The 95% confidence intervals (CI) were calculated. The χ2 statistic was used to compare proportions. Statistical significance was considered to be p<0.05. Data were analysed with SPSS statistical software version 10.0 (SPSS, Inc, Chicago, IL, USA).
The medical ethics committee of the Erasmus MC stated that there was no objection to the study.
Results
From September to December 2004, 28 street outreach sessions were held (average 10, range 1–27 people per session), 14 group sessions (average 10, range 4–20 people), and 13 school sessions (average 10, range 3–15 people). In street outreach, approximately 30% of those approached discussed testing. The study population included 556 individuals.
Participation and test rate
The overall participation rate was 43% (239/556) and the test rate 34% (190/556). Determinants for participation were sexual activity (47% versus 16%, p<0.001) and venue (street 27%, group 79%, school 52%). In street outreach, 16% (14/88) of the interested people delivered urine at the venue, and 51% (38/74) of those who took the kit home were tested. In the groups and at the school 90% (135/151) used the urine kit at the venue and 19% (3/16) of those who intended to mail the specimen did so.
Among those who indicated they were sexually active, 38% (172/447) were actually tested (table 1). The test rate was determined mainly by venue (street 17%, groups 80%, school 73%). The test rate by gender and ethnicity differed significantly only in the street setting (female 23% versus male 15% (p = 0.02); Dutch 44%, Surinamese/Antillean 13%, other 19% (p = 0.002)). There was no significant difference in test rate by gender and ethnicity in the other settings, nor was there a gender difference within ethnicities (data not shown).
Table 1 Participation rate, test rate, and Ct positivity among 447 sexually active participants at outreach testing by demographic determinants and venue.
| No | Participants | Participation rate | p Value | Urine collection | Test rate | p Value | No of cases | % positive | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Street | |||||||||||
| Total | 287 | 79 | 28% | 49 | 17% | 6 | 12.2% | 5.7% to 24.2% | |||
| Sex | |||||||||||
| Male | 205 | 47 | 23% | 0.006 | 30 | 15% | 0.02 | 2 | 6.7% | 1.8% to 21.3% | |
| Female | 82 | 32 | 39% | 19 | 23% | 4 | 21.1% | 9.8% to 39.5% | |||
| 5 years age group | |||||||||||
| 15–19 | 136 | 38 | 28% | ns | 23 | 17% | ns | 3 | 13.0% | 4.5% to 32.1% | |
| 20–24 | 110 | 33 | 30% | 20 | 18% | 1 | 5.0% | 0.8% to 23.6% | |||
| 25–29 | 38 | 7 | 18% | 5 | 13% | 2 | 40.0% | 3.6% to 62.4% | |||
| Ethnicity | |||||||||||
| Dutch | 25 | 15 | 60% | 0.001 | 11 | 44% | 0.002 | 2 | 18.2% | 5.1% to 47.7% | |
| Sur/Ant | 175 | 42 | 24% | 23 | 13% | 3 | 13.0% | 4.5% to 32.1% | |||
| Other | 77 | 22 | 29% | 15 | 19% | 1 | 6.7% | 1.2% to 29.8% | |||
| Group | |||||||||||
| Total | 93 | 76 | 82% | 74 | 80% | 7 | 9.5% | 4.6% to 18.3% | |||
| Sex | |||||||||||
| Male | 47 | 37 | 79% | ns | 37 | 79% | ns | 3 | 8.1% | 2.8% to 21.3% | |
| Female | 46 | 39 | 85% | 37 | 80% | 4 | 10.8% | 4.3% to 24.7% | |||
| 5 years age group | |||||||||||
| 15–19 | 37 | 33 | 89% | ns | 33 | 89% | ns | 2 | 6.1% | 1.7% to 19.6% | |
| 20–24 | 42 | 30 | 71% | 29 | 69% | 4 | 13.8% | 5.5% to 30.6% | |||
| 25–29 | 12 | 11 | 92% | 10 | 83% | 1 | 10.0% | 1.8% to 40.4% | |||
| Ethnicity | |||||||||||
| Dutch | 17 | 14 | 82% | ns | 14 | 82% | ns | 1 | 7.1% | 1.3% to 31.5% | |
| Sur/Ant | 53 | 44 | 83% | 42 | 79% | 3 | 7.1% | 2.5% to 19.0% | |||
| Other | 23 | 18 | 78% | 18 | 78% | 3 | 16.7% | 5.8% to 39.2% | |||
| School | |||||||||||
| Total | 67 | 53 | 79% | 49 | 73% | 12 | 24.5% | 14.6% to 38.0% | |||
| Sex | |||||||||||
| Male | 8 | 6 | 75% | ns | 6 | 75% | ns | 0 | 0.0% | 0% to 39.0% | |
| Female | 59 | 47 | 80% | 43 | 73% | 12 | 27.9% | 16.7% to 42.6% | |||
| 5 years age group | |||||||||||
| 15–19 | 59 | 46 | 78% | ns | 43 | 73% | ns | 12 | 27.9% | 16.7% to 42.6% | |
| 20–24 | 7 | 6 | 86% | 6 | 86% | 0 | 0.0% | 0% to 39.0% | |||
| 25–29 | 0 | 0 | % to | 0 | – | 0 | – | – | |||
| Ethnicity | |||||||||||
| Dutch | 28 | 22 | 79% | ns | 19 | 68% | ns | 2 | 10.5% | 2.9% to 31.4% | |
| Sur/Ant | 23 | 16 | 70% | 15 | 65% | 7 | 46.7% | 24.8% to 69.8% | |||
| Other | 15 | 14 | 93% | 14 | 93% | 2 | 14.3% | 4.0% to 39.9% | |||
| All | |||||||||||
| Total | 447 | 208 | 47% | 172 | 38% | 25 | 14.5% | 10.0% to 20.6% | |||
| Sex | |||||||||||
| Male | 260 | 90 | 35% | <0.001 | 73 | 28% | <0.001 | 5 | 6.8% | 3.4% to 13.4% | |
| Female | 187 | 118 | 63% | 99 | 53% | 20 | 20.2% | 14.4% to 27.6% | |||
| 5 years age group | |||||||||||
| 15–19 | 232 | 117 | 50% | 0.039 | 98 | 42% | 0.036 | 17 | 17.3% | 11.1% to 26.0% | |
| 20–24 | 159 | 69 | 43% | (trend) | 55 | 35% | 5 | 9.1% | 3.9% to 19.6% | ||
| 25–29 | 50 | 18 | 36% | 15 | 30% | 3 | 20.0% | 7.0% to 45.2% | |||
| Ethnicity | |||||||||||
| Dutch | 70 | 51 | 73% | <0.001 | 44 | 63% | <0.001 | 5 | 11.4% | 4.9% to 23.9% | |
| Sur/Ant | 251 | 102 | 41% | 80 | 32% | 14 | 17.5% | 10.7% to 27.3% | |||
| Other | 115 | 54 | 47% | 47 | 41% | 6 | 12.8% | 5.9% to 25.2% | |||
Reasons for refusal
Of 316 people declining a test kit, 19% stated they have never been sexually active, 20% perceived they would not have any or only a small risk, 37% was not interested or did not have time, 5% was tested for chlamydia in the past months, and 19% stated other reasons. Of the 180 declining men, 50% said they were not interested. Among the 136 women who declined, sexual non‐activity was the most common reason (42%).
Characteristics of the sexually active participants
In the sexually active group, 57% of the women had two to five, and 10% more than five lifetime partners; for men these percentages were 27% and 66%, respectively. These distributions were also found in the 15–19 year olds. Recent partner change in the last 2 months was reported by 26% of the women and 53% of the men. More women than men had ever been tested for STIs (31% versus 17%; p = 0.037), and of those tested, 28% of the women and 36% of the men reported a history of STI (Ct and gonorrhoea). In total 48% of the women and 13% of the men indicated having symptoms compatible with an STI.
Infection rates
With 25 cases among 172 sexually active participants, Ct positivity was 14.5 % (95% CI: 10.0% to 20.6%), and higher in women (20.2%) than in men (6.8%; p = 0.01). Ct positivity was highest in schools (24.5%), and among Surinamese/Antillean participants (17.5%) (table 1). We found two cases of gonorrhoea (1.2%; 95% CI: 0.3% to 4.1%); one Ct co‐infected female and one male.
Notification of results, treatment, and partner notification
All but two non‐infected participants could be notified of their results; 88% by SMS, 4% by telephone, 4% by email, and 3% by mail. After receiving the SMS message, six participants called the STI nurse for further information. All 26 infected participants were informed by phone and 25 were treated at the Municipal Health Service. Of the infected people, five (19%) said they did not have a GP, nine (35%) consented, and 11 (42%) withheld consent for the Municipal Health Service to inform their GP.
The 26 infected people named 45 partners (mean 1.7) in the previous 6 months. The median number of partners of infected men during the last 6 months was 1.5 (1–3), while for women this was 1.0 (1–6). Notably, of 20 infected women (five of them native Dutch), 19 (95%) had partners of non‐Dutch ethnicity.
Ten partners were anonymous and 35 partners could be notified. Eighteen index cases (69%) had a current partner at the time of testing; of those two (11%) who tested negative, 12 (67%) were treated and four were referred by the index to their physician.
Efficiency
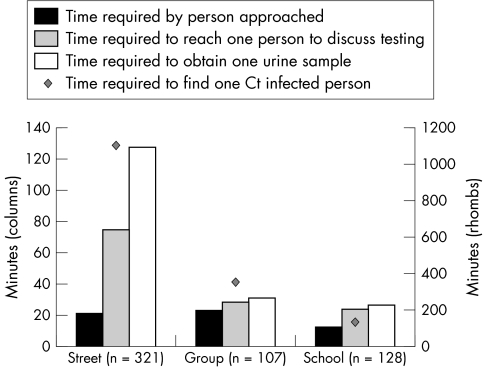
From the response rates at different venues it shows that the efficiency of community based testing varies considerably by venue. Figure 1 shows the time required (in minutes) to approach one person, to find a person who is willing to discuss testing, to ultimately obtain one urine specimen, and to find one infected person by venue.
Figure 1 Efficiency in the STI outreach testing project depicted at the time required by person approached, to reach one person to discuss testing, to obtain one urine sample, and to detect one infected person. (Time required includes approaching all, including sexually non‐active people (n = 556).)
Discussion
The test rate of Chlamydia trachomatis was high in the group and school settings (73–80%) compared to the street setting (17%). A crude Ct prevalence of 14.5% indicates reaching a high risk group for Ct infection, with the highest Ct prevalence (24.5%) at the school.
To our knowledge this is the first report of a study combining health education and outreach testing in Europe. An additional feature is the innovative use of SMS as a communication tool. The main determinant of test uptake was the setting. The low street based test rate (17%) is in line with field based projects elsewhere15,17 and can be explained primarily by the unexpected approach of people. The test rate at the school was similar to the comparable school based study of Cohen et al (59–67%).12 A higher test rate of women compared to men was found only in the street setting. Notably, in the group and school settings the test rate among individuals of non‐Dutch ethnicity was similar for both men and women to individuals of ethnic Dutch (65–80%). This is contrary to population based postal screening, where the test rate for men and those of non‐Dutch ethnicity was lower than in women and those of Dutch ethnicity.9,25 It should be kept in mind, however, that our data have the limitation of a small sample size and short study duration.
Compared to the Ct prevalence found in Rotterdam during a population based screening, Ct rates were higher in the present study in Dutch participants (11.4% versus 3.1%), Surinamese/Antillean participants (17.5% versus 12.6%), and other ethnic groups (12.8% versus 3.7%).9,25 This indicates that we have succeeded in finding a high risk group. The Ct prevalence is comparable to that of the Rotterdam STI clinic in 2004 (overall 10%, and 16% in Surinamese/Antillean visitors). The most striking Ct positivity level was 24.5% at the vocational training school. Whether such infection rates would be found repeatedly in school screening on a larger scale remains to be seen; in the United States in routine school based Ct screening the rates were between 8% and 20%.11,12,26,27,28,29 Our gonorrhoea rate was lower (1.2%) than in field based testing in the United States (2.5–4.9%).16,17,30 The rate of Ct gonorrhoea co‐infection was 4% (1/25). This rate suggests that chlamydia infected people should also be advised to be tested for other STIs.
Community based testing includes challenges such as motivation of people who do not actively seek care, confidentiality, communication of results, and treatment of those infected. Maintaining privacy is important, especially for adolescents living with their parents. We therefore asked the participants how they would like to receive results. Most preferred SMS. The treatment rate in outreach testing varies from 61% to 100%13,15,16,17,18,19,30 and only two studies from the United States reported partner treatment rates (56 and 77%).15,18 Our treatment rate of index cases was 100%. In total 78% (14/18) of the current partners either tested negative or were treated at the Municipal Health Service. Participants preferred not to consult their GP. This suggests lack of trust in confidentiality during STI consultation, and the need of youth friendly sexual health care.
Key messages
Offering STI testing to high risk groups during STI prevention activities is feasible in groups, school and in street setting
Acceptance of testing in difficult to reach people can be increased by a personal approach in groups, and is particularly effective in people of non‐Dutch ethnicity
Behavioural factors and a high Ct prevalence indicate that a high risk group for transmission could be reached
Outreach testing can be offered as individual care, and serves as an alternative for hard to reach populations
The pilot study showed that efficiency is satisfactory in a group and school setting. The number of people reached by outreach work in groups is limited. School screening may have an impact on community prevalence of Ct infections
Outreach workers experienced the combination of prevention activities with a test offer as enriching. They reported more in‐depth discussions in the field, but it remains to be seen whether increased interest in sexual health has a positive effect on safe sex behaviour in the high risk group.
Currently, there is no Ct screening programme in the Netherlands, but a policy exists of offering active STI testing to high risk groups. The current study was performed to explore the application of such a policy targeting a hard to reach risk group. The street setting was least efficient, but as many of these young people might not access health care otherwise; this service may provide a safety net offered by the Municipal Health Service.29,31 In group and school settings the offering of Ct testing was most efficient in terms of people tested and infections found, compared to time investment (and thus costs). Our findings are in line with cost efficiency reports of school based screening,12 with recent reports of community tailored outreach testing projects,16,21,32 and the less favourable outcomes in street settings.15 However, a cost efficiency analysis including effects on population level cannot be performed at this micro‐level of activity.
Only a limited number of people can be contacted by outreach group activities. Our Municipal Health Service may reach about 1000 people per year. Assuming a Ct positivity of 10%, this means that 100 infections would otherwise probably remain undetected. Of course the individuals concerned may benefit from such a test offer, but this will not influence Ct prevalence in the population in any significant way. The settings described are individual testing facilities and cannot replace a systematic Ct screening programme. When looking at public health effects, it seems that targeting the vocational schools could reach large number of students (40 000), representing all ethnic groups of the Rotterdam population. If Ct screening were adopted in the Netherlands, schools might offer opportunities to increase the participation rate as an alternative testing facility for those who are hard to motivate by postal screening. This deserves further study.
Acknowledgements
A Luyendijk and EG Dullaart conducted the laboratory analyses. Without the commitment of the outreach workers and their coordinator, LWouters, and the dedication of the MHS nurses (B Nuradini and A van't Westeinde) in STI education and counselling this project could not have been executed. We are grateful to Dr J van Bergen from STI‐AIDS Netherlands for his advice and useful comments. Questionnaire and information material is adapted from the CT‐Pilot,9 and we thank the CT‐Pilot group for use of this material.
Contributors
HG and OdZ designed the study and HG was project leader; JR was scientific supervisor of the study; HG, IV, JO, and OdZ have contributed to the study protocol and collection of data; analysis was done by HG and IV; all authors contributed to the interpretation of data and critically reviewed the draft, which was written and finalised by HG.
Abbreviations
Ct - Chlamydia trachomatis
PID - pelvic inflammatory diseases
STI - sexually transmitted infections
Footnotes
This research has been financed by a grant from the Dutch Public Health Fund (fonds OGZ).
Conflict of interest: none declared.
References
- 1.Stamm W. Chlamydia trachomatis. In: Sexually transmitted diseases. New York: McGraw‐Hill, 1999
- 2.Bloomfield P J, Kent C, Campbell D.et al Community‐based chlamydia and gonorrhea screening through the United States mail, San Francisco. Sex Transm Dis 200229294–297. [DOI] [PubMed] [Google Scholar]
- 3.Klausner J D, McFarland W, Bolan G.et al Knock‐knock: a population‐based survey of risk behavior, health care access, and Chlamydia trachomatis infection among low‐income women in the San Francisco Bay area. J Infect Dis 20011831087–1092. [DOI] [PubMed] [Google Scholar]
- 4.Ku L, Sonenstein F L, Turner C F.et al The promise of integrated representative surveys about sexually transmitted diseases and behavior. Sex Transm Dis 199724299–309. [DOI] [PubMed] [Google Scholar]
- 5.Turner C F, Rogers S M, Miller H G.et al Untreated gonococcal and chlamydial infection in a probability sample of adults. JAMA 2002287726–733. [DOI] [PubMed] [Google Scholar]
- 6.Andersen B, Olesen F, Moller J K.et al Population‐based strategies for outreach screening of urogenital Chlamydia trachomatis infections: a randomized, controlled trial. J Infect Dis 2002185252–258. [DOI] [PubMed] [Google Scholar]
- 7.Fenton K A, Korovessis C, Johnson A M.et al Sexual behaviour in Britain: reported sexually transmitted infections and prevalent genital Chlamydia trachomatis infection. Lancet 20013581851–1854. [DOI] [PubMed] [Google Scholar]
- 8.Low N, McCarthy A, Macleod J.et al The chlamydia screening studies: rationale and design. Sex Transm Infect 200480342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Bergen J E A M, Gotz H M, Richardus J H.et al Prevalence of urogenital Chlamydia trachomatis increases significantly with level of urbanisation and suggests targeted screening approaches: results from the first national population based study in the Netherlands. Sex Transm Infect 20058117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Valkengoed I G, Boeke A J, Morre S A.et al Disappointing performance of literature‐derived selective screening criteria for asymptomatic Chlamydia trachomatis infection in an inner‐city population. Sex Transm Dis 200027504–507. [DOI] [PubMed] [Google Scholar]
- 11.Burstein G R, Waterfield G, Joffe A.et al Screening for gonorrhea and chlamydia by DNA amplification in adolescents attending middle school health centers. Opportunity for early intervention. Sex Transm Dis 199825395–402. [DOI] [PubMed] [Google Scholar]
- 12.Cohen D A, Nsuami M, Etame R B.et al A school‐based Chlamydia control program using DNA amplification technology. Pediatrics 1998101E1. [DOI] [PubMed] [Google Scholar]
- 13.Poulin C, Alary M, Bernier F.et al Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae among at‐risk women, young sex workers, and street youth attending community organizations in Quebec City, Canada. Sex Transm Dis 200128437–443. [DOI] [PubMed] [Google Scholar]
- 14.Debattista J, Clementson C, Mason D.et al Screening for Neisseria gonorrhoeae and Chlamydia trachomatis at entertainment venues among men who have sex with men. Sex Transm Dis 200229216–221. [DOI] [PubMed] [Google Scholar]
- 15.Gunn R A, Podschun G D, Fitzgerald S.et al Screening high‐risk adolescent males for Chlamydia trachomatis infection. Obtaining urine specimens in the field. Sex Transm Dis 19982549–52. [DOI] [PubMed] [Google Scholar]
- 16.Jones C A, Knaup R C, Hayes M.et al Urine screening for gonococcal and chlamydial infections at community‐based organizations in a high‐morbidity area. Sex Transm Dis 200027146–151. [DOI] [PubMed] [Google Scholar]
- 17.Van Leeuwen J M, Rietmeijer C A, LeRoux T.et al Reaching homeless youths for Chlamydia trachomatis and Neisseria gonorrhoeae screening in Denver, Colorado. Sex Transm Infect 200278357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rietmeijer C A, Yamaguchi K J, Ortiz C G.et al Feasibility and yield of screening urine for Chlamydia trachomatis by polymerase chain reaction among high‐risk male youth in field‐based and other nonclinic settings. A new strategy for sexually transmitted disease control. Sex Transm Dis 199724429–435. [DOI] [PubMed] [Google Scholar]
- 19.Bauer H M, Chartier M, Kessell E.et al Chlamydia screening of youth and young adults in non‐clinical settings throughout California. Sex Transm Dis 200431409–414. [DOI] [PubMed] [Google Scholar]
- 20.Richardson E, Sellors J W, Mackinnon S.et al Prevalence of Chlamydia trachomatis infections and specimen collection preference among women, using self‐collected vaginal swabs in community settings. Sex Transm Dis 200330880–885. [DOI] [PubMed] [Google Scholar]
- 21.Moss N J, Gallaread A, Siller J.et al “Street medicine”: collaborating with a faith‐based organization to screen at‐risk youths for sexually transmitted diseases. Am J Public Health 2004941081–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotz H M, van Bergen J E, Veldhuijzen I K.et al A prediction rule for selective screening of Chlamydia trachomatis infection. Sex Transm Infect 20058124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer S, Torgerson D J. Economic notes: definitions of efficiency. BMJ 19993181136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rours G I, Verkooyen R P, Willemse H F.et al Use of pooled urine samples and automated DNA isolation to achieve improved sensitivity and cost‐effectiveness of large‐scale testing for Chlamydia trachomatis in pregnant women. J Clin Microbiol 2005434684–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Valkengoed I G, Boeke A J, van den Brule A J.et al [Systematic home screening for Chlamydia trachomatis infections of asymptomatic men and women in family practice by means of mail‐in urine samples]. Ned Tijdschr Geneeskd 1999143672–676. [PubMed] [Google Scholar]
- 26.Cohen D A, Nsuami M, Martin D H.et al Repeated school‐based screening for sexually transmitted diseases: a feasible strategy for reaching adolescents. Pediatrics 19991041281–1285. [DOI] [PubMed] [Google Scholar]
- 27.Cohen D A, Nsuami M, Brooks B.et al School‐based screening for sexually‐transmitted diseases. J LA State Med Soc 1999151617–621. [PubMed] [Google Scholar]
- 28.Nsuami M, Cohen D A. Participation in a school‐based sexually transmitted disease screening program. Sex Transm Dis 200027473–479. [DOI] [PubMed] [Google Scholar]
- 29.Wiesenfeld H C, Lowry D L, Heine R P.et al Self‐collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis 200128321–325. [DOI] [PubMed] [Google Scholar]
- 30.Kahn R H, Moseley K E, Thilges J N.et al Community‐based screening and treatment for STDs: results from a mobile clinic initiative. Sex Transm Dis 200330654–658. [DOI] [PubMed] [Google Scholar]
- 31.Stephenson J, Carder C, Copas A.et al Home screening for chlamydial genital infection: is it acceptable to young men and women? Sex Transm Infect 20007625–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bull S S, Jones C A, Granberry‐Owens D.et al Acceptability and feasibility of urine screening for Chlamydia and gonorrhea in community organizations: perspectives from Denver and St Louis. Am J Public Health 200090285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]