Abstract
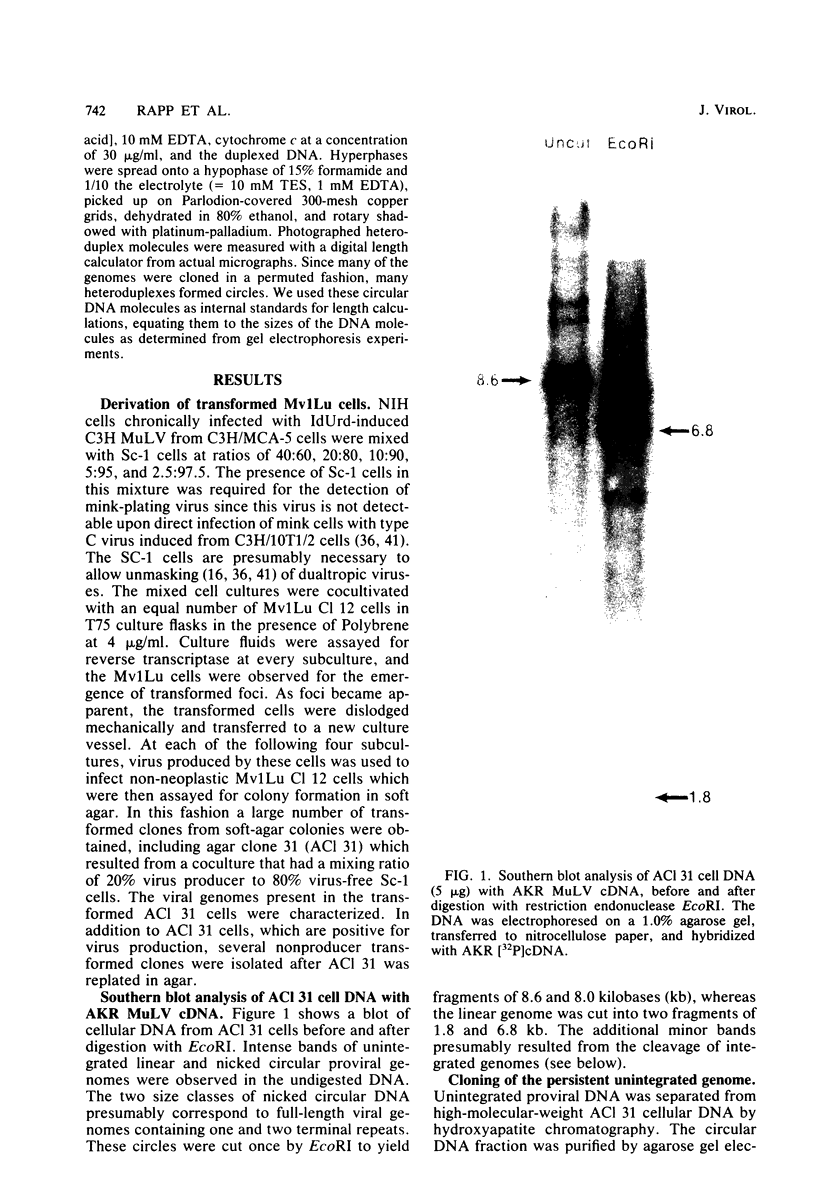
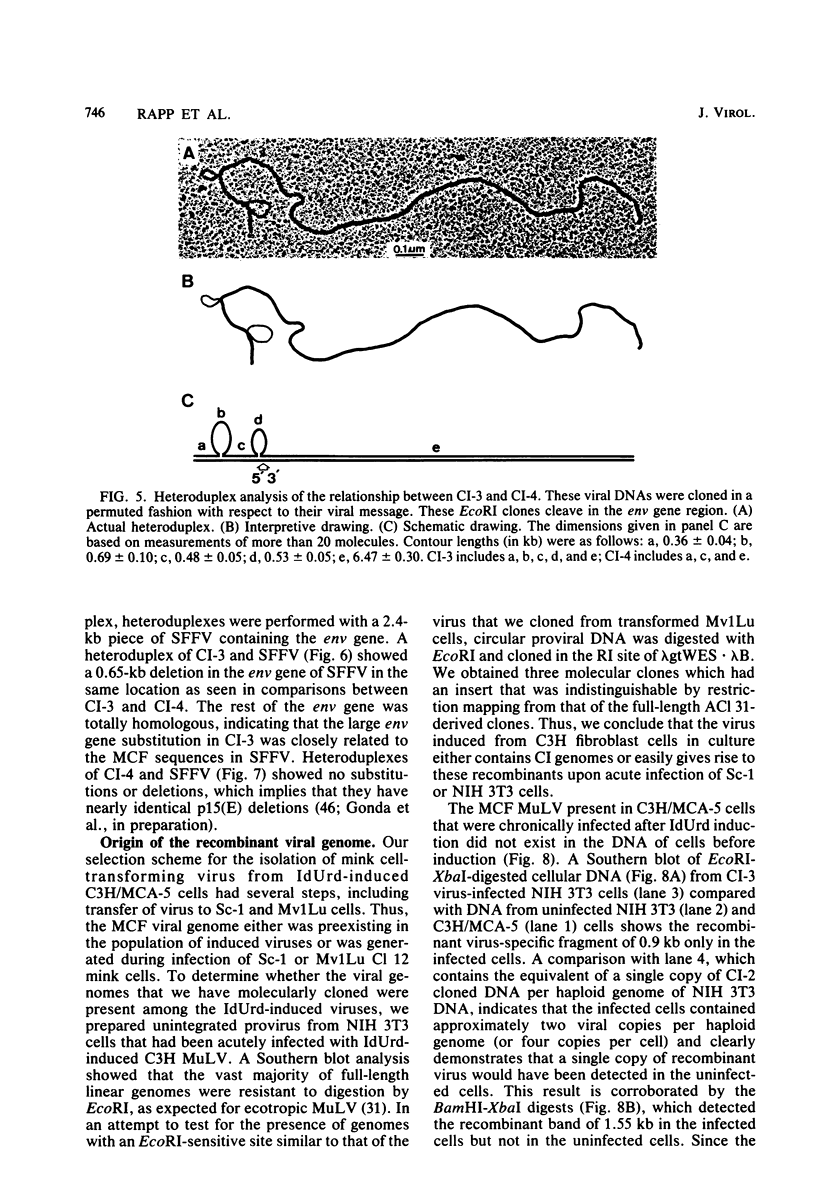
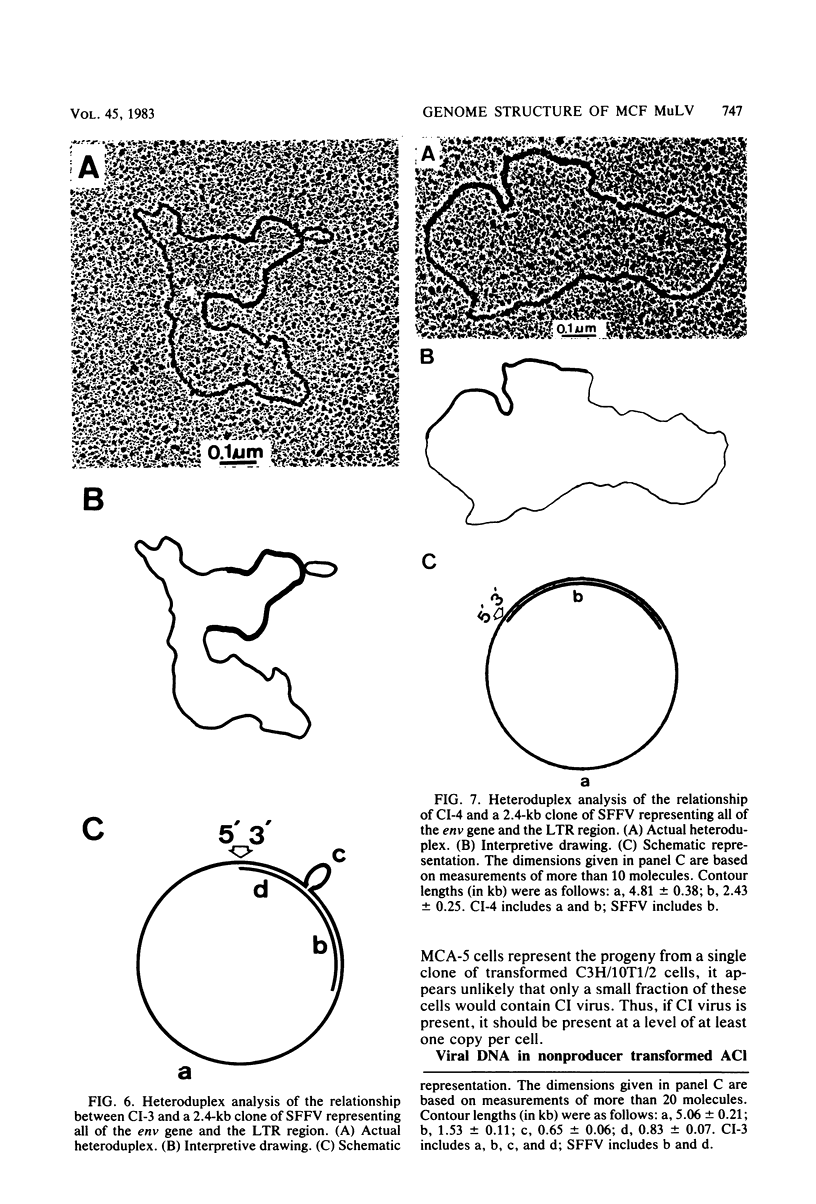
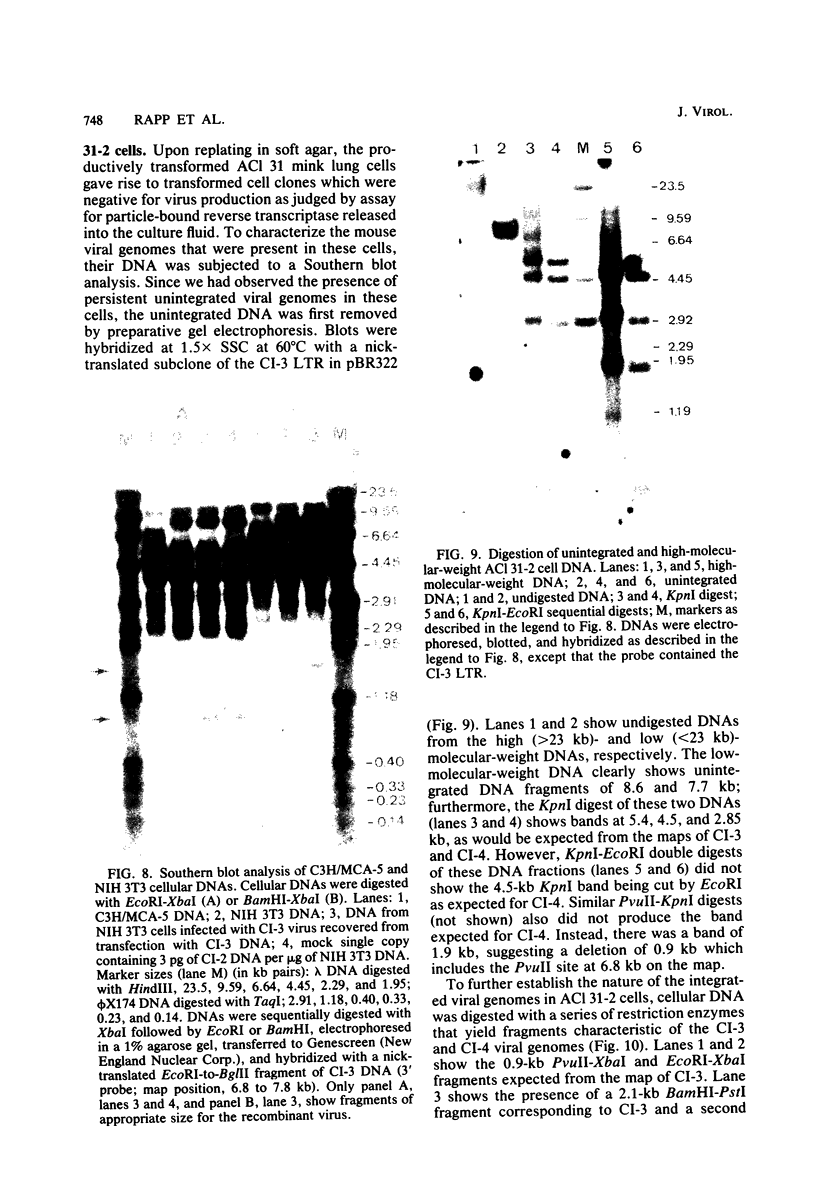
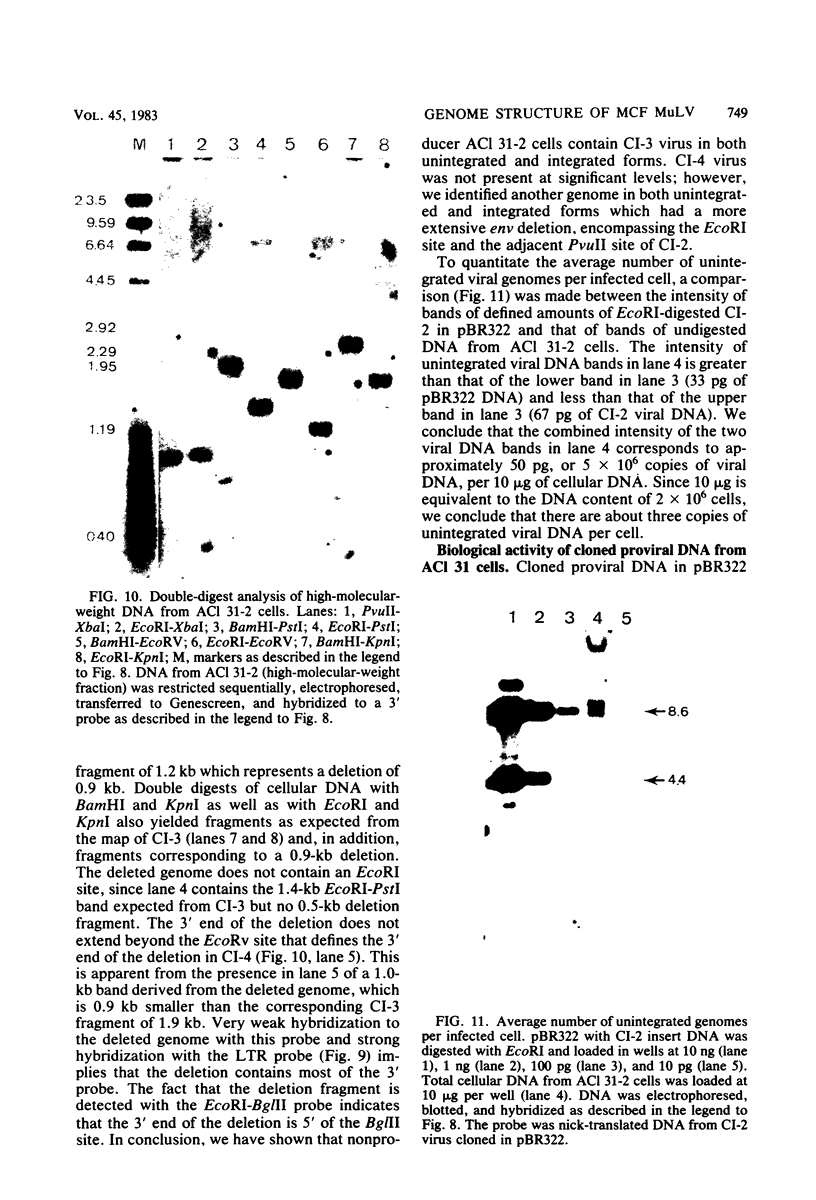
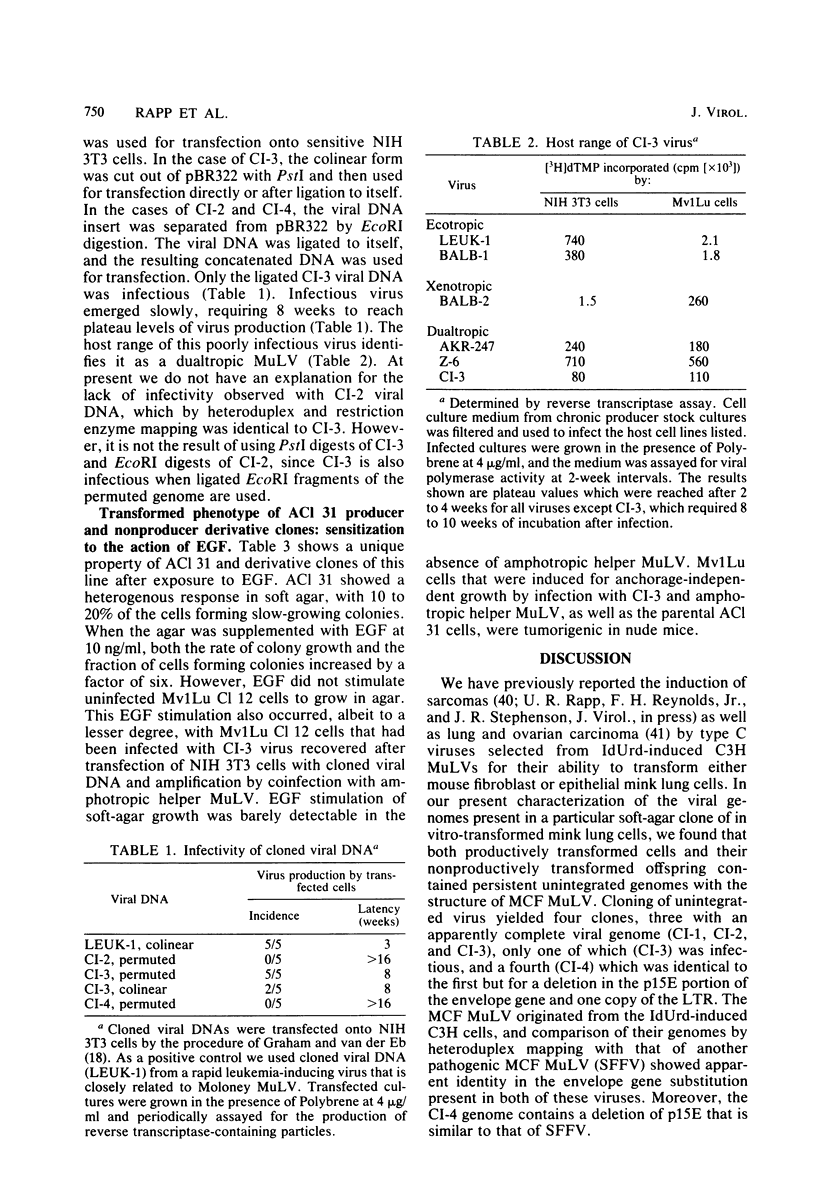
We characterized mink cell focus-forming murine leukemia viruses that were isolated from C3H/MCA-5 cells after induction with 5-iododeoxyuridine in culture. Mink lung epithelial cells malignantly transformed in vitro by induced virus were the source of four molecular clones of mink cell focus-forming virus. CI-1, CI-2, CI-3, and CI-4. Three clones, CI-1, CI-2, and CI-3, had full-length mink cell focus-forming viral genomes, one of which (CI-3) was infectious. In addition, we obtained a defective viral genome (CI-4) which had a deletion in the envelope gene. A comparison between the envelope genes of CI-4 and those of spleen focus-forming virus by heteroduplex mapping showed close homology in the substitution region and defined the deletion as being identical to the p15E deletion of spleen focus-forming virus. The recombinant mink cell focus-forming genomes are not endogenous in C3H/MCA-5 cells and therefore must have been formed in culture after induction by 5-iododeoxyuridine. CI-3, the infectious clone of mink cell focus-forming murine leukemia virus, was dualtropic, and mink cells infected with CI-3 were altered in their response to epidermal growth factor. In the presence of epidermal growth factor at 10 ng/ml, uninfected mink cells retained their epithelial morphology in monolayer culture and did not form colonies in soft agar. In contrast, CI-3 virus-infected mink cells grew with fibroblastic morphology in monolayer culture and showed an increased growth rate in soft agar in the presence of epidermal growth factor.
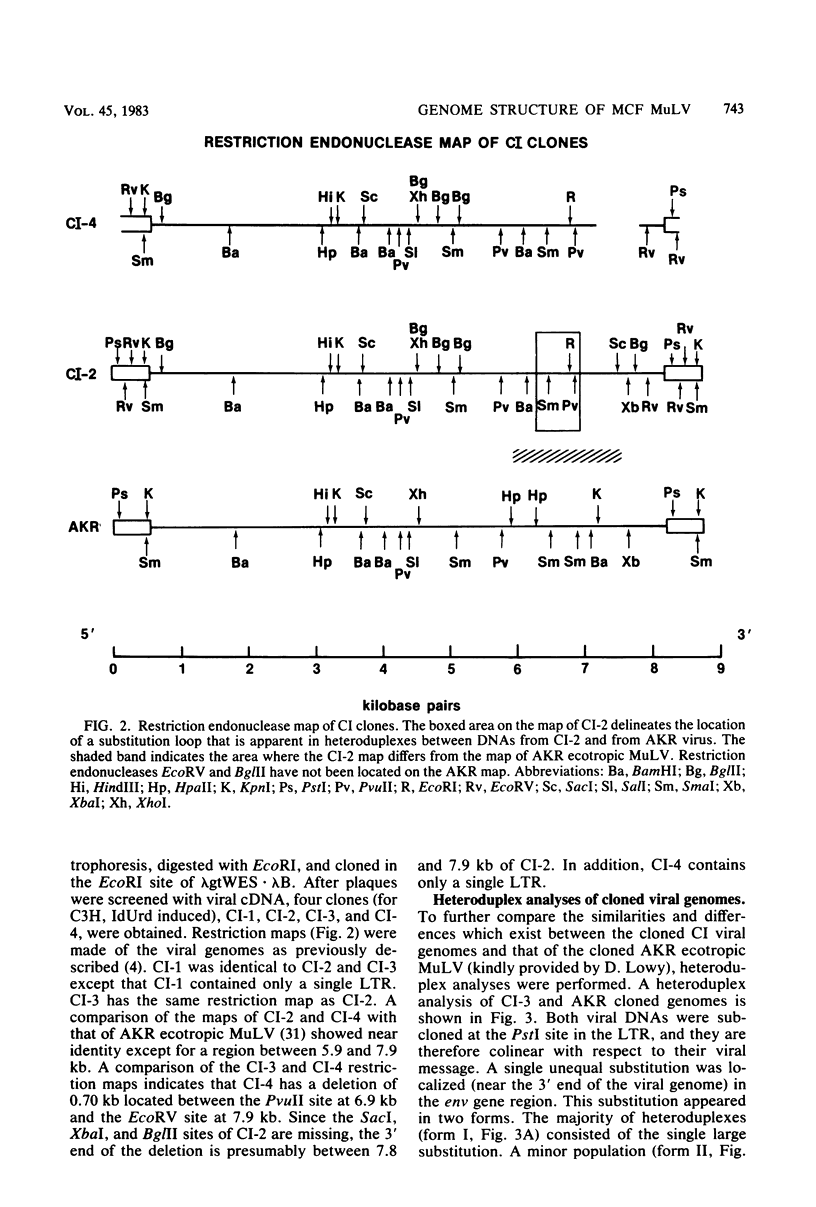
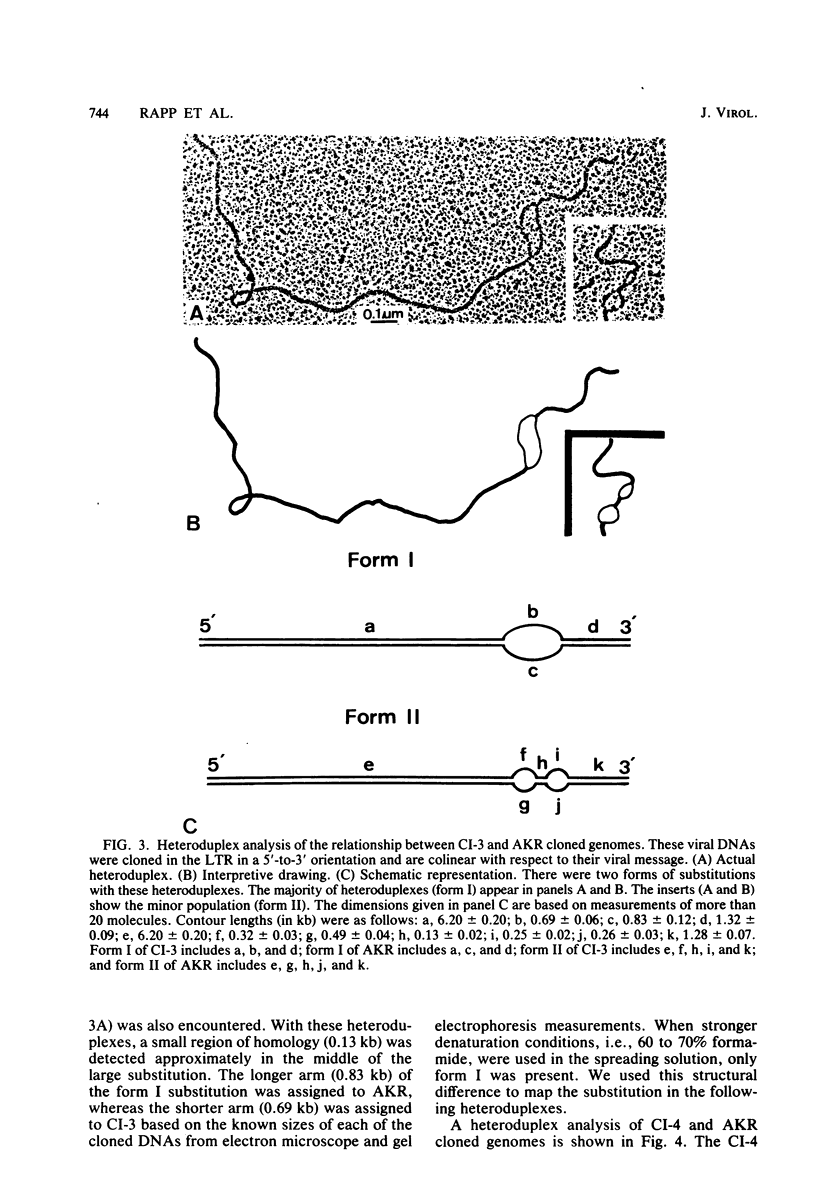
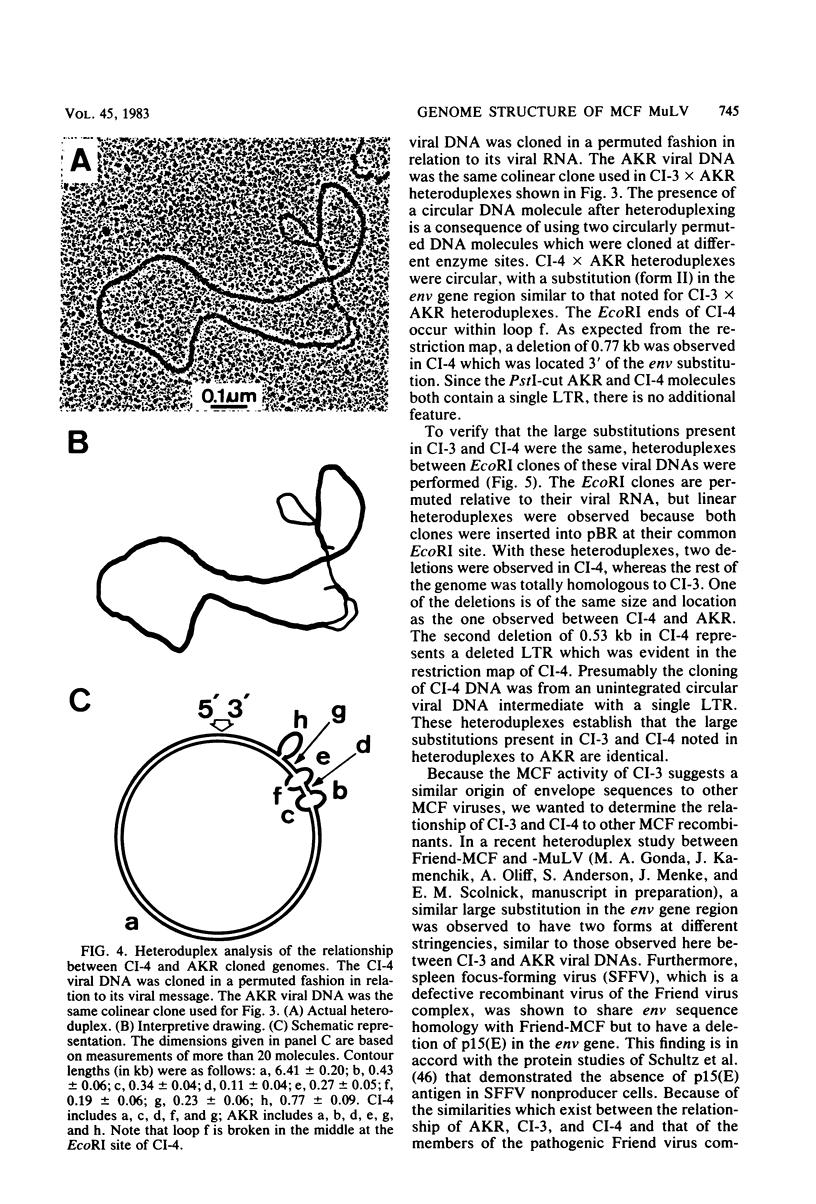
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battula N., Todaro G. J. Physical map of infectious baboon type C viral DNA and sites of integration in infected cells. J Virol. 1980 Dec;36(3):709–718. doi: 10.1128/jvi.36.3.709-718.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berns A., Jaenisch R. Increase of AKR-specific sequences in tumor tissues of leukemic AKR mice. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2448–2452. doi: 10.1073/pnas.73.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Bonner T. I., Reynolds K., Searfoss G. H., Todaro G. J. Colobus type C virus: molecular cloning of unintegrated viral DNA and characterization of the endogenous viral genomes of Colobus. J Virol. 1982 Mar;41(3):842–854. doi: 10.1128/jvi.41.3.842-854.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Verma I. M., Shih T. Y., Scolnick E. M., Davidson N. Heteroduplex analysis of the sequence relations between the RNAs of mink cell focus-inducing and murine leukemia viruses. J Virol. 1978 Oct;28(1):352–360. doi: 10.1128/jvi.28.1.352-360.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Rapp U. R., Todaro G. J., Stephenson J. R. Acquisition of oncogenicity by endogenous mouse type C viruses: effects of variations in env and gag genes. J Virol. 1978 Nov;28(2):457–465. doi: 10.1128/jvi.28.2.457-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Famulari N. G., Jelalian K. Cell surface expression of the env gene polyprotein of dual-tropic mink cell focus-forming murine leukemia virus. J Virol. 1979 Jun;30(3):720–728. doi: 10.1128/jvi.30.3.720-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G., Tung J. S., O'Donnell P. V., Fleissner E. Murine leukemia virus env-gene expression in preleukemic thymocytes and leukemia cells of AKR strain mice. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1281–1287. doi: 10.1101/sqb.1980.044.01.140. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Dunlop N. M. Genomic masking of nondefective recombinant murine leukemia virus in Moloney virus stocks. Science. 1978 Aug 4;201(4354):457–459. doi: 10.1126/science.663667. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hager G. L., Chang E. H., Chan H. W., Garon C. F., Israel M. A., Martin M. A., Scolnick E. M., Lowy D. R. Molecular cloning of the Harvey sarcoma virus closed circular DNA intermediates: initial structural and biological characterization. J Virol. 1979 Sep;31(3):795–809. doi: 10.1128/jvi.31.3.795-809.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J., Auersperg N. Epidermal growth factors enhances viral transformation of granulosa cells. Science. 1981 Jul 10;213(4504):218–219. doi: 10.1126/science.6264597. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Fischinger P., Bolognesi D., Elder J., Gautsch J. W. B-MuX: a unique murine C-type virus containing the "env" gene of xenotropic viruses and the "gag" gene of the ecotropic virus. Virology. 1978 Oct 15;90(2):255–264. doi: 10.1016/0042-6822(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Moloney leukemia virus gene expression and gene amplification in preleukemic and leukemic BALB/Mo mice. Virology. 1979 Feb;93(1):80–90. doi: 10.1016/0042-6822(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Jaenisch R. Conformation of free and of integrated Moloney leukemia virus proviral DNA in preleukemic and leukemic BALB/Mo mice. Virology. 1980 Feb;101(1):111–123. doi: 10.1016/0042-6822(80)90488-2. [DOI] [PubMed] [Google Scholar]
- Linemeyer D. L., Ruscetti S. K., Scolnick E. M., Evans L. H., Duesberg P. H. Biological activity of the spleen focus-forming virus is encoded by a molecularly cloned subgenomic fragment of spleen focus-forming virus DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1401–1405. doi: 10.1073/pnas.78.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F. Oncogenicity of AKR endogenous leukemia viruses. J Virol. 1978 Jul;27(1):13–18. doi: 10.1128/jvi.27.1.13-18.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E., Obata Y., Old L. J. Leukemogenic properties of AKR dualtropic (MCF) viruses: amplification of murine leukemia virus-related antigens on thymocytes and acceleration of leukemia development in AKR mice. Virology. 1981 Jul 30;112(2):548–563. doi: 10.1016/0042-6822(81)90301-9. [DOI] [PubMed] [Google Scholar]
- Rapp U. R. Kinetics of expression of infectious ecotropic, xenotropic, and mink cell focus-forming murine leukemia virus after 5-iododeoxyuridine induction of cells from high- and low-leukemia mouse strains. J Virol. 1983 Feb;45(2):755–765. doi: 10.1128/jvi.45.2.755-765.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Marshall T. H. Cell surface receptors for endogenous mouse type C viral glycoproteins and epidermal growth factor: tissue distribution in vivo and possible participation in specific cell-cell interaction. J Supramol Struct. 1980;14(3):343–352. doi: 10.1002/jss.400140308. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Todaro C. Generation of new mouse sarcoma viruses in cell culture. Science. 1978 Sep 1;201(4358):821–824. doi: 10.1126/science.210501. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Todaro G. J. Generation of oncogenic mouse type C viruses: in vitro selection of carcinoma-inducing variants. Proc Natl Acad Sci U S A. 1980 Jan;77(1):624–628. doi: 10.1073/pnas.77.1.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Todaro G. J. Generation of oncogenic type C viruses: rapidly leukemogenic viruses derived from C3H mouse cells in vivo and in vitro. Proc Natl Acad Sci U S A. 1978 May;75(5):2468–2472. doi: 10.1073/pnas.75.5.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Cloyd M. W., Hartley J. W. Status of the association of mink cell focus-forming viruses with leukemogenesis. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1265–1268. doi: 10.1101/sqb.1980.044.01.137. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Feild J. A., Scolnick E. M. Polycythaemia- and anaemia-inducing strains of spleen focus-forming virus differ in post-translational processing of envelope-related glycoproteins. Nature. 1981 Dec 17;294(5842):663–665. doi: 10.1038/294663a0. [DOI] [PubMed] [Google Scholar]
- Ruscetti S., Linemeyer D., Field J., Troxler D., Scolnick E. Type-specific radioimmunoassays for the gp70s of mink cell focus-inducing murine leukemia viruses: expression of a cross-reacting antigen in cells infected with the Friend strain of the spleen focus-forming virus. J Exp Med. 1978 Sep 1;148(3):654–663. doi: 10.1084/jem.148.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Ruscetti S. K., Scolnick E. M., Oroszlan S. The env-gene of the spleen focus-forming virus lacks expression of p15(E) determinants. Virology. 1980 Dec;107(2):537–542. doi: 10.1016/0042-6822(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staal S. P., Hartley J. W., Rowe W. P. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Keshet E., Weller S. K. Correlation of transient accumulation of linear unintegrated viral DNA and transient cell killing by avian leukosis and reticuloendotheliosis viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):773–778. doi: 10.1101/sqb.1980.044.01.083. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Scolnick E. M. Rapid leukemia induced by cloned friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology. 1978 Mar;85(1):17–27. doi: 10.1016/0042-6822(78)90408-7. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Yuan E., Linemeyer D., Ruscetti S., Scolnick E. M. Helper-independent mink cell focus-inducing strains of Friend murine type-C virus: potential relationship to the origin of replication-defective spleen focus-forming virus. J Exp Med. 1978 Sep 1;148(3):639–653. doi: 10.1084/jem.148.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M. Properties of "mink cell focus-inducing" (MCF) virus isolated from spontaneous lymphoma lines of BALB/c mice carrying Moloney leukemia virus as an endogenous virus. Virology. 1979 Feb;93(1):226–236. doi: 10.1016/0042-6822(79)90290-3. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]
- Young H. A., Gonda M. A., De Feo D., Ellis R. W., Nagashima K., Scolnick E. M. Heteroduplex analysis of cloned rat endogenous replication-defective (30 S) retrovirus and Harvey murine sarcoma virus. Virology. 1980 Nov;107(1):89–99. doi: 10.1016/0042-6822(80)90275-5. [DOI] [PubMed] [Google Scholar]