Abstract
Objective
To examine the type specific seroprevalence of herpes simplex virus (HSV) types 1 and 2 infections, stratified by age and gender, and associated risk factors for HSV‐2 seropositivity in Poland.
Methods
2257 serum samples of individuals from 15–65 years were randomly selected from serum banks in four different geographical regions of Poland, including the Zachodnio‐pomorskie, Warmińsko‐mazurskie, Lubelskie, and Mazowieckie districts. Type specific serum antibodies to HSV‐1 and HSV‐2 were detected using HerpeSelect IgG ELISA tests.
Results
Overall prevalences of type specific HSV‐1 and HSV‐2 serum antibodies were 90.4% and 9.3%, respectively. Age standardised HSV‐2 seroprevalence was higher in women (9.7%) than men (8.8%) (p = 0.06), and increased notably with age from 4% in 15–24 year olds to 12% in those aged 50–65 years. HSV‐1 seroprevalence was consistently higher than HSV‐2 seroprevalence in each specific age group, ranging from 74.5% in 15–24 year olds to 98.8% in 50–65 year olds. HSV‐2 seroprevalence varied significantly by geographical region, with the highest prevalence in the Zachodnio‐pomorskie district (12%). Significant multivariate risk factors for HSV‐2 seropositivity included older age, female gender, and geographical place of residence.
Conclusion
This large survey found a notably high seroprevalence of HSV‐1, even among young female adolescents 15–19 years of age (80%). HSV‐2 seropositivity was under 12% in all age groups surveyed in Poland, tending to be among the lowest overall HSV‐2 seropositivity rates reported thus far in Europe.
Keywords: herpes simplex virus, seropositivity, Poland
Herpes simplex virus (HSV) infections are among the most common infections worldwide and cause recurrent infections throughout life.1 HSV infects humans of all ages, and causes a spectrum of diseases, ranging from asymptomatic infections to neonatal death.2
HSV‐1 and HSV‐2 type viruses share approximately 50% nucleotide sequence homology. HSV‐1 is usually transmitted via non‐sexual contact and is generally clinically associated with oro‐labial infection, whereas HSV‐2 is typically transmitted sexually and infects ano‐genital sites.3,4,5 HSV‐1 and HSV‐2 are both capable of infecting ano‐genital and oro‐labial sites, and produce clinically indistinguishable lesions. HSV‐1 is an important cause of genital herpes in some countries.6 Individuals with genital HSV‐2 generally have more frequent recurrences7,8 and viral shedding in the absence of symptoms than those with genital HSV‐1.9
HSV‐1 seroprevalence in adults is generally 60% or more in different areas worldwide and increases with age, reaching a plateau at around 30 years. HSV‐1 is more prevalent than HSV‐2 at any given age group in non‐high risk populations.10,11 HSV‐2 seroprevalence varies notably across geographical regions. In similar aged adults, HSV‐2 prevalence is generally higher in the United States than Europe, and is higher in women than men.10 In Europe, HSV‐2 seropositivity appears to be higher in northern than southern Europe, and to be the lowest in Spain and Italy.11
Data from central Europe are sparse. In Poland, no data on type specific HSV seroprevalence are available. This study examines HSV‐1 and HSV‐2 seroprevalence, stratified by age and gender, and associated risk factors for HSV‐2 seropositivity in Poland.
Materials and methods
Samples
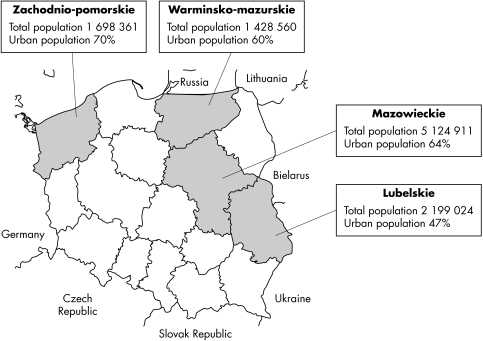
Serum samples were selected from a serum bank in Poland established in 2000, with a catchment area of the following four geographical regions (fig 1): Zachodnio‐pomorskie, Warmińsko‐mazurskie, Lubelskie, and Mazowieckie. These four regions comprise 27.3% of total Polish population.12 The bank contains residual sera from public health laboratories that offer comparable services, including diagnostic screening before surgery (∼75%), health screening for new employees (∼15%), diagnostics for infectious diseases other than sexually transmitted infections and other services, including visas for international travel (∼10%).
Figure 1 Map of Poland indicating four districts included in HSV seroprevalence survey.12
A sample of 5 ml of peripheral venous blood was collected, allowed to clot, and centrifuged at 1000 g for 10 minutes. Sera were transferred to microtubes of 0.5–1 ml and sent to the National Institute of Hygiene (NIH) in Warsaw for storage at −70°C. All personal identifiers were removed. The development of this serum bank was approved by the institutional review board of the NIH in Warsaw, Poland.
Sampling
A total of 2263 serum samples collected in 2002 were selected using a stratified sampling design. Samples were randomly selected in each age and gender group from the Mazowieckie district (the largest population of all four regions) to obtain about 100 people per age and gender group, and then from the other three regions to obtain about 200 people overall per age and gender group; age distributions were similar for each region. Additionally, all available samples in 2000 from Mazowieckie district (n = 222) were tested, and HSV prevalence results were compared to 838 samples collected from the same district in 2002.
Laboratory tests
Type specific serum antibodies to HSV‐1 and HSV‐2 were detected using HerpeSelect‐1 and HerpeSelect‐2 IgG ELISAs (Focus Diagnostics). These tests use glycoprotein G antigens: gG1 from HSV‐1 and gG2 from HSV‐2 to elicit type specific antibody responses. Results are reported according to manufacturer's instructions. Equivocal samples were retested using the same test kit. In total, 49 samples were equivocal and, after retesting, six remained equivocal (three for HSV‐1 and three for HSV‐2) and were excluded from analyses.
Quality control
The same 170 samples (6.8% of 2485) were blindly retested with HerpeSelect HSV‐1 and HSV‐2 ELISA. For both HSV‐1 and HSV‐2, 165 samples (97.1%) had concordant results. Initial laboratory results were used for final analyses.
Statistical analysis
The multiple logistic regression model was used to evaluate risk factors for HSV‐2 seropositivity after controlling for age, gender, HSV‐1 serostatus, and region. This regression model was fitted by starting with a model containing all first and second order terms and proceeding by backward selection procedure using SAS statistical software (version 8.2). A Mantel‐Haenszel χ2 test was used to determine differences in HSV‐1 and HSV‐2 seroprevalence between samples from 2000 and 2002 in the Mazowieckie district.
Results
Type specific serological responses were available in 2002 from sera of 2257 Polish individuals (1218 female, 1039 male) with an overall median age of 34 years. HSV‐1 and HSV‐2 seropositivity were 89.8% and 9.1%, respectively. Antibodies for both HSV‐1 and HSV‐2 were present in 186 samples (8.2%).
HSV‐2 seroprevalence was slightly higher in women (9.6%) than men (8.7%) (p = 0.06), and varied significantly by geographical region. The highest HSV‐2 prevalence was in Zachodnio‐pomorskie (12%) and the lowest in regions of Lubelskie (6.5%) and Warmińsko‐mazurskie (7%). Overall, HSV‐1 seroprevalence in women (91.5%) was higher than men (87.9%) (p<0.0001) (table 1). HSV‐1 seropositivity was high across all regions, from ∼87% in Mazowieckie and Zachodnio‐pomorskie to 92% in Lubelskie and 94% in Warminsko‐mazurskie.
Table 1 Type specific seroprevalence of herpes simplex virus types 1 (HSV‐1) and 2 (HSV‐2) in Polish women and men, stratified by geographical region.
| Region | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample size | Median age (years) | HSV‐1 (%) | HSV‐2 (%) | Sample size | Median age (years) | HSV‐1 (%) | HSV‐2 (%) | |
| Lubelskie | 154 | 39 | 91.6 | 7.7 | 155 | 42 | 92.3 | 5.2 |
| Mazowieckie | 554 | 33 | 90.3 | 10.2 | 281 | 38 | 85.4 | 6.8 |
| Warmińsko‐mazurskie | 314 | 35.5 | 94 | 8.6 | 144 | 46.5 | 95.1 | 3.5 |
| Zachodnio‐pomorskie | 196 | 28 | 90.8 | 10.7 | 459 | 28 | 85.7 | 12.6 |
| Poland | 1218 | 33 | 91.5 | 9.6 | 1039 | 35 | 87.9 | 8.7 |
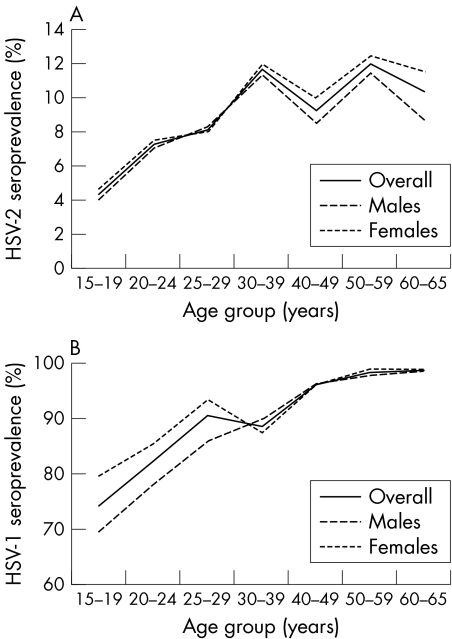
HSV‐2 seroprevalence increased notably with age in both genders (fig 2A). HSV‐2 seropositivity was the lowest in 15–19 years olds (4.3%), rose steadily to 11.7% in 30–39 year olds, and reached a plateau over 40 years (varying from 9.3% to 12.0%). Women had higher HSV‐2 seroprevalence than men at any given age, except 25–29 year olds where prevalence was comparable at ∼8%. HSV‐1 was consistently higher in women than men and lower in younger age groups, ranging from 74.5% at 15–24 years to 98.8% by 50–65 years (fig 2B).
Figure 2 HSV‐2 (A) and HSV‐1 (B) seroprevalence in Poland by age in 2002, stratified by gender.
Older age was the most significant risk factor for HSV‐2 seropositivity (OR = 2.6 for 50–65 year olds versus 15–24 year olds). Gender was of borderline significance (OR = 1.4 for females versus males; 95% CI: 0.99 to 1.8). HSV‐1 sero status was not associated with HSV‐2 (OR = 0.79). HSV‐2 seropositivity was higher in Zachodnio‐pomorskie than Mazowieckie region (OR = 1.7, 95% CI: 1.2 to 2.5) (table 2).
Table 2 Selected risk factors for the HSV‐2 infection and adjusted HSV‐2 seroprevalence rates in Poland.
| No | HSV‐2 seroprevalence (%) | OR (95% CI) | ||
|---|---|---|---|---|
| Age group (years) | 15–24 | 630 | 6.0* | 1¶ |
| 25–49 | 1081 | 9.8* | 1.9 (1.3 to 2.7) | |
| 50–65 | 546 | 11.5* | 2.6 (1.7 to 4.1) | |
| Overall | 2257 | 9.3‡ | p for trend = 0.004 | |
| Sex | Male | 1039 | 9.8† | 1¶ |
| Female | 1218 | 9.7† | 1.4 (0.99 to 1.8) | |
| HSV‐1 serostatus | Negative | 227 | 9.8‡ | 1¶ |
| Positive | 2030 | 10.7‡ | 0.79 (0.48 to 1.3) | |
| Region in Poland | Mazowieckie | 835 | 8.3‡ | 1¶ |
| Lubelskie | 309 | 6.6‡ | 0.69 (0.41 to 1.2) | |
| Warminsko‐mazurskie | 458 | 5.9‡ | 0.72 (0.47 to 1.1) | |
| Zachodnio‐pomorskie | 655 | 13.4‡ | 1.7 (1.2 to 2.5) |
OR, multivariate odds ratio adjusted for age, gender, HSV‐1 serostatus, and region.
*Gender adjusted HSV‐2 seroprevalence; †age group adjusted HSV‐2 seroprevalence; ‡age group and gender adjusted HSV‐2 seroprevalence; CI: confidence interval, 1¶, reference.
Age adjusted HSV‐2 seroprevalence, using the Polish census population in 2002 as the reference standard (www.stat.gov.pl), was 9.3% overall (8.8% in men; 9.7% in women), similar to crude results (9.1% overall; 9.7% in women, 8.7% in men). After adjusting for age and gender, HSV‐2 seropositivity was significantly higher in Zachodnio‐pomorskie (13.4%) than other regions (5.9%–8.4%) (table 2). Age adjusted HSV‐1 seroprevalence (90.4%) was slightly higher among women (92.1%) then men (88.5%) (data not shown).
From 2000 to 2002 in the Mazowieckie region, overall HSV‐1 seropositivity increased slightly from 81.5% to 89.8% (table 3). In contrast, HSV‐2 seropositivity was slightly higher in 2000 (11.3%) than 2002 (9.5%). Age and gender adjusted HSV‐1 and HSV‐2 seroprevalences did not differ significantly between 2000 and 2002. HSV‐1 and HSV‐2 rate ratios between 2000 and 2002 were 1.18 (95% CI: 0.88 to 1.55) and 0.77 (95% CI: 0.53 to 1.11), respectively.
Table 3 Differences in HSV‐1 and HSV‐2 seroprevalence in the Mazowieckie region between 2000 and 2002.
| 2000 | 2002 | |||||
|---|---|---|---|---|---|---|
| Sample size | HSV‐1 seroprevalence (%) | HSV‐2 seroprevalence (%) | Sample size | HSV‐1 seroprevalence (%) | HSV‐2 seroprevalence (%) | |
| Females overall | 140 | 83.6 | 12.9 | 557 | 90.3 | 10.2 |
| Males overall | 82 | 78.1 | 8.5 | 281 | 85.4 | 6.8 |
| Total | ||||||
| 15–24 | 99 | 67.7 | 11.1 | 208 | 78.8 | 6 |
| 25–49 | 97 | 90.7 | 10.3 | 409 | 91.9 | 9.8 |
| 50+ | 26 | 100 | 15.4 | 201 | 98.5 | 11.5 |
| Overall | 222 | 81.5 | 11.3 | 838 | 89.8 | 9.5 |
Discussion
This is the first study of HSV‐2 and HSV‐1 seroprevalence among women and men in Poland using type specific serological methods. Age standardised HSV‐1 and HSV‐2 seroprevalence were 90.4% and 9.3%, respectively, in 2257 samples from serum banks. Age standardised HSV‐2 seropositivity was higher in women (9.7%) than men (8.8%), and increased notably with age from 4% in 15–19 year olds to 12% in those 40 years or older. HSV‐1 seropositivity was higher than HSV‐2 in each age group surveyed and was not associated with HSV‐2 serostatus. Significant multivariate risk factors for HSV‐2 included older age, female gender, and geographical region of residence.
Reliability of these results is based on a large sample size and standardised laboratory procedures for type specific HSV tests. This is the largest study of HSV‐2 in Poland to date (n = >2000 sera), including both genders from four regions. ELISAs for type specific HSV‐1 and HSV‐2 serum antibody detection in order to determine cumulative lifetime exposure to infection. Previous validation studies show high sensitivity and specificity for HSV‐1 and HSV‐2 Focus Diagnostics ELISAs when compared to the gold standard HSV western blot.13,14 Further, a high percentage agreement (97%) for reproducibility of HSV serological results was found. One limitation, however, is that determination of HSV‐2 seroprevalence may underestimate the total burden of genital herpes in areas where a notable proportion of genital herpes is caused by HSV‐1.5
This study is based on a large serum bank from Polish regional public health laboratories that form a countrywide network offering similar services in all regions. Catchment areas are large, including a wide reference population referred for surgery and other diagnostic testing. Given that a notable proportion of individuals are seen for routine pre‐surgery screening, it is plausible that participants are not likely to differ significantly from a population based sample of similarly aged women and men in these four regions. However, serum bank donors in Poland may differ from the general Polish population, and thus study results may not be representative of the Polish population at large.
HSV‐2 seroprevalence in this Polish population (median age of 34) (9.1%) is comparable to similarly aged women from population based surveys in Barcelona, Spain (11%)15 and England (12.3%),16 and slightly higher than in population based samples from Spain (2.7–∼5%),17,18 Czech Republic (6%),19 and Central Italy (4.8%).20 HSV‐2 seropositivity overall in Poland was lower, however, than in women from northern and eastern Europe,10,19 such as a population based sample in Germany (14%);19 two studies of women in Norway(25–28%)21,22; Swedish studies of pregnant women (∼34%)23,24; and population based studies19 in Finland (13%), the Netherlands (9%), Bulgaria (24%), and Belgium (11%). Comparisons of age specific HSV‐2 seroprevalence between studies, however, require caution given differences in type specific assays and in populations surveyed.
Few surveys have included both genders to compare age specific HSV‐2 seroprevalence using identical type specific HSV‐1 and HSV‐2 laboratory methods. HSV seroepidemiological studies in Poland have generally been conducted using non‐specific serological tests that do not effectively distinguish HSV‐1 and HSV‐2 antibody responses, by using a complement fixation test25 or laboratory specific, non‐standardised HSV‐1 ELISA.26 In our study, Polish women had a higher risk of HSV‐2 seropositivity than men (OR = 1.4). These results are similar to US and European studies27,28,29 and provide consistent evidence that women are probably at a higher risk of HSV‐2 acquisition than men.
A higher HSV‐2 seroprevalence was found among women and men from Zachodnio‐pomorskie than the other three Polish regions, and the reason for this is unclear. Differences in HSV‐2 seropositivity may be the result of regional differences in sexual norms or population based seroprevalence. Higher HSV‐2 seroprevalence in Zachodnio‐pomorskie is consistent with a higher incidence of non‐gonococcal urethritis in this district than the three other regions (www.pzh.gov.pl/epimeld). However, reported rates of other sexually transmitted infections (STIs) in 2002 have been higher in the other regions than in Zachodnio‐pomorskie (that is, a higher prevalence of syphilis was reported in Warminsko‐mazurskie; a higher prevalence gonorrhoea in Mazowieckie). Differences in reported STI rates across regions might represent true differences in STI prevalence or regional differences in diagnosis or reporting patterns. HSV‐2 seropositivity may be a useful indicator of future HIV population risk30 or be an actual risk factor for HIV transmission.31 Thus, these data indicate that future public health efforts may need to consider regional approaches to STI/HIV prevention in Poland.
Consistent with previous studies, HSV‐1 seropositivity level in four areas in Poland (∼89%) was more prevalent than HSV‐2. HSV‐1 burden in Poland is similar to that of similarly aged men and women (∼34 years) in Germany (82% in blood donors),32 Bulgaria (84% in a population based study),19 Spain (84% in a population based study),17 the Czech Republic (81% in a population based study),19 and central Italy (93% in 30 year old health personnel).33 HSV‐1 seropositivity in Poland is slightly lower than a similarly aged national sample from England and Wales (∼69%) and than population based studies19 in the Netherlands (57%), Belgium (67%), and Finland (52%).
In this study, HSV‐1 seropositivity was not associated HSV‐2 serostatus. It has been hypothesised that HSV‐1 seropositive individuals may have a lower acquisition of HSV‐2 infection because of cross protection of HSV‐1 against HSV‐2, yet results from two HSV‐2 prophylactic clinical trials have inconsistent results.34,35 Our ability to address this issue is limited, however, because of this study's cross sectional design and lack of information on other potential sexual behavioural confounders.
Data on HSV‐1 seroprevalence in female adolescents/young women (aged 15–24 years) have important implications for the future introduction of an HSV‐2 prophylactic vaccine because this vaccine will probably only be effective in dually HSV‐1/HSV‐2 seronegative women.34 HSV‐1 seroprevalence in Polish females aged 15 and 24 was 83.4%, suggesting that vaccination earlier in life than adolescence could maximise potential vaccine benefits. These data are consistent with European studies that found a range of 60% to 90% of HSV‐1 in females aged ∼15–24 years from Germany (∼60%),32,19 Belgium(∼65%),19 Spain (72%),17 Finland (72%),36 the Czech Republic(∼75%),19 central Italy (85%),33 Bulgaria (∼85%),19 and Slovenia (85%),19 but are slightly higher than in similarly aged women in the Netherlands (∼40%),19 Wales and England (∼40–50%),19,37 and Finland (40%).19 In female adolescents aged 15 years in Poland, the prevalence of dual HSV‐1/HSV‐2 seronegativity was 18.9% (24/127)—13.8% in Zachodnio‐pomorskie, 13.9% in Warminsko‐mazurskie, 29% in Lubelskie, and 22% in Mazowieckie.
Our results indicate a relatively low age standardised prevalence of HSV‐2 seropositivity in serum bank specimens from women and men aged 15–65 years of age in four regions of Poland. Overall age standardised HSV‐2 seroprevalence (9.3%) in Poland appears to be similar to other “low” HSV‐2 prevalence areas within Europe, and was lower than many other European countries, particularly in northern Europe. Age standardised HSV‐1 seropositivity among serum samples in Poland (90.4%) was consistent with other European surveys. The HSV‐2 prophylactic vaccine currently in phase III trials is planned to target female adolescents 15 years of age. Given that ∼20% of young women in Poland would be HSV‐1/HSV‐2 seronegative and thus eligible for vaccination, future strategies may benefit from vaccinating females younger than 15 years of age, while considering the length of proved HSV‐2 vaccine efficacy.
Key messages
HSV‐2 seropositivity was under 12% in all age groups surveyed from blood banks in four regions of Poland, tending to be among the lowest reported thus far in Europe
HSV‐1 seroprevalence was high in Poland, even among young female adolescents aged 15–19 years (80%)
HSV‐2 serostatus was associated with older age, female gender, and geographical place of residence, but not with HSV‐1 serostatus
In Poland, approximately 20% of young women aged 15 years would be potentially eligible for vaccination with a prophylactic HSV vaccine targeted at HSV‐1/HSV‐2 dually negative young female adolescents
Acknowledgements
The authors thank Jane Markley of Focus Diagnostics for helping to initiate and progress this study; and Phillippa Humphreys, Jamie Robinson, and Lisa Lindsay for their helpful comments.
Contributors
JMP and BL initiated the project, and BL is the study guarantor; JS and AT were responsible for performed tests, internal quality control, data collection, and analysis; JSS and MR provided methodological and statistical part of the project and were responsible for epidemiological analysis of data; all contributors were involved in paper preparation.
Abbreviations
HSV - herpes simplex virus
NIH - National Institute of Hygiene
STI - sexually transmitted infections
Footnotes
Funding: GlaxoSmithKline Research & Development.
Competing interests: JMP is a full time employee of GSK. All other others have no competing interests to declare.
References
- 1.Whitley R, Kimberlin D W, Roizman B. Herpes simplex viruses. Clin Infect Dis 199826541–555. [DOI] [PubMed] [Google Scholar]
- 2.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields BN, Knipe DM, Howley PM, eds. Fields virology. Philadelphia: Lippincott‐Raven, 1996
- 3.Aurelian L. Herpes simplex viruses (Herpesviridae). General features. In, Granoff A, Webster RG, eds. Encyclopedia of virology London, Academic Press 1999
- 4.Ashley R L, Wald A. Genital herpes: review of the epidemic and potential use of type‐ specific serology. Clin Microbiol Rev 1999121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinghorn G R. Herpes simplex type 1 genital infections. Herpes 199964–7. [Google Scholar]
- 6.Brugha R, Keersmaekers K, Renton A.et al Genital herpes infection: a review. Int J Epidemiol 199726698–709. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first‐episode infection. Ann Intern Med 1994121847–854. [DOI] [PubMed] [Google Scholar]
- 8.Wald A, Corey L, Cone R.et al Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest 1997991092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wald A, Zeh J, Selke S.et al Virologic characteristics of subclinical and symptomatic genital herpes infection. N Engl J Med 1995333770–775. [DOI] [PubMed] [Google Scholar]
- 10.Smith J S, Robinson N J. Age‐specific prevalence of infection with herpes simplex virus type 2 and 1: a global review. J Infect Dis 2002186(Suppl 1)S3–S8. [DOI] [PubMed] [Google Scholar]
- 11.Stanberry L. The epidemiology of herpes simplex virus infections in adolescents. Herpes 1999612–15. [Google Scholar]
- 12.Central Statistical Office Statistical yearbook of the regions—Poland, 2003 (data for 2002). Warsaw,2003
- 13.Strick L, Wald A. Type‐specific testing for herpes simplex virus. Expert Rev Mol Diagn 20044443–453. [DOI] [PubMed] [Google Scholar]
- 14.Ashley‐Morrow R, Nollkamper J, Robinson N J.et al Performance of Focus ELISA tests for herpes simplex virus type 1 (HSV‐1) and HSV‐2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect 200410530–536. [DOI] [PubMed] [Google Scholar]
- 15.De Sanjosé S, Muñoz N, Bosch F X.et al Sexually transmitted agents and cervical neoplasia in Colombia and Spain. Int J Cancer 199456358–363. [DOI] [PubMed] [Google Scholar]
- 16.Morris‐Cunnington M, Brown D, Pimenta J.et al New estimates of herpes simplex virus type 2 seroprevalence in England: ‘high' but stable seroprevalence over the last decade. Sex Transm Dis 200431243–246. [DOI] [PubMed] [Google Scholar]
- 17.De Ory F, Pachon I, Echevarria J M.et al Seroepidemiological study of herpes simplex virus in the female population in the autonomous region of Madrid, Spain. Eur J Clin Microbiol Infect Dis 199918678–680. [DOI] [PubMed] [Google Scholar]
- 18.Garcia‐Corbeira P, Dal Re R, Aguilar L.et al Is sexual transmission an important pattern for herpes simplex type 2 virus seroconversion in the Spanish general population? J Med Virol 199959194–197. [DOI] [PubMed] [Google Scholar]
- 19.Pebody R G, Andrews N, Brown D.et al The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect 200480185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquini P, Mele A, Franco E.et al Prevalence of herpes simplex virus type 2 antibodies in selected population groups in Italy. Eur J Clin Microbiol Infect Dis 1988754–56. [DOI] [PubMed] [Google Scholar]
- 21.Eskild A, Jeansson S, Jenum P A. Herpes simplex‐virus type 2‐antistoffer hos gravide i Norge. Tidsskr Nor Loegeforen 19991192323–2326. [PubMed] [Google Scholar]
- 22.Olsen A O, Orstavik I, Dillner J.et al Herpes simplex virus and human papillomavirus in a population‐based case‐control study of cervical intraepithelial neoplasia grade II–III. APMIS 1998106417–424. [DOI] [PubMed] [Google Scholar]
- 23.Persson K, Mansson A, Jonsson E.et al Decline of herpes simplex virus type 2 and Chlamydia trachomatis infections from 1970 to 1993 indicated by a similar change in antibody pattern. Scand J Infect Dis 199527195–199. [DOI] [PubMed] [Google Scholar]
- 24.Forsgren M, Skoog E, Jeansson S.et al Prevalence of antibodies to herpes simplex virus in pregnant women in Stockholm in 1969, 1983 and 1989: implications for STD epidemiology. Int J STD AIDS 19945113–116. [DOI] [PubMed] [Google Scholar]
- 25.Imbs D, Rudnicka H. Seroepidemiological investigations for cytomegalovirus (CMV) and herpes simplex virus (HSV) infections in girls and women. Przeg Epid 198741286–294. [PubMed] [Google Scholar]
- 26.Laskus T, Radkowski G, Halama R.et al Cytomegalovirus and herpes simplex virus infection in alcoholics and drug addicts. Przeg Epid 199145263–266. [PubMed] [Google Scholar]
- 27.Fleming D T, McQuillan G M, Johnson R E.et al Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med 19973371105–1111. [DOI] [PubMed] [Google Scholar]
- 28.Eberhart‐Phillips J, Dickson N P, Paul C.et al Herpes simplex type 2 infection in a cohort aged 21 years. Sex Transm Infect 199874216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laubereau B, Zwahlen M, Neuenschwander B.et al Herpes simplex virus type 1 and 2 in Switzerland. Schweiz Med Wochenschr 2000130143–150. [PubMed] [Google Scholar]
- 30.Dobbins J G, Mastro T D, Nopkesorn T.et al Herpes in the time of AIDS: a comparison of the epidemiology of HIV‐1 and HSV‐2 in young men in northern Thailand. Sex Transm Dis 19992667–74. [DOI] [PubMed] [Google Scholar]
- 31.Celum C L. The interaction between herpes simplex virus and human immunodeficiency virus. Herpes 200411(Suppl 1)36A–45A. [PubMed] [Google Scholar]
- 32.Wutzler P, Doerr H W, Farber I.et al Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations‐relevance for the incidence of genital herpes. J Med Virol 200061201–207. [DOI] [PubMed] [Google Scholar]
- 33.Franco E, Caprilli F, Zaratti L.et al Prevalence of antibodies to herpes simplex virus type 1 in different population groups in Italy [letter]. Eur J Clin Microbiol 19876322. [DOI] [PubMed] [Google Scholar]
- 34.Stanberry L R, Spruance S L, Cunningham A L.et al Glycoprotein‐D‐adjuvant vaccine to prevent genital herpes. N Engl J Med 20023471652–1661. [DOI] [PubMed] [Google Scholar]
- 35.Langenberg A G, Corey L, Ashley R L.et al A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med 19993411432–1438. [DOI] [PubMed] [Google Scholar]
- 36.Arvaja M, Lehtinen M, Koskela P.et al Serological evaluation of herpes simplex virus type 1 and type 2 infections in pregnancy. Sex Transm Infect 199975168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vyse A J, Gay N J, Slomka M J.et al The burden of infection with HSV‐1 and HSV‐2 in England and Wales: implications for the changing epidemiology of genital herpes. Sex Transm Infect 200076183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]