Abstract
Background
Studies demonstrating previous herpes simplex virus (HSV) type 2 infection as a risk factor for HIV transmission, and the development of a HSV vaccine candidate, have emphasised the need for worldwide population based studies of HSV seroprevalence. The only nationwide seroprevalence studies have been conducted in the United States.
Methods
An Australia‐wide, population based study of HSV‐1 and HSV‐2 seroprevalence was conducted, using serum and sociodemographic data collected between 1999–2000, for a representative study of risk factors for diabetes in over 11 000 adults. A stratified random sample of 4000 was tested for HSV‐2 and 1000 for HSV‐1, with sampling and weighting for various demographic factors.
Results
Seroprevalence of HSV‐2 in Australian adults was 12%. Prevalence in women (16%) was twice that in men (8%). Rural populations had a lower prevalence (9%) than metropolitan (13%), and Indigenous had a higher prevalence (18%) than the non‐Indigenous populations (12%). The seroprevalence of HSV‐1 was 76% with significant differences by age group, sex and Indigenous status.
Conclusion
These are the first nationwide data to compare with US studies. HSV‐2 infection is less common in Australia than the United States, and this will allow planning for combating HIV transmission in high prevalence populations in northern Australia. In addition, the high HSV‐1 seroprevalence will be important for future deployment of genital herpes vaccines.
Keywords: herpes simplex virus, Australia, nationwide population based survey, seroprevalence
Infections caused by herpes simplex viruses (HSV) types 1 and 2 are common throughout the world, with considerable variation from country to country and within population groups.1 Studies in selected populations, considered to be at high risk for the acquisition of sexually transmitted infections (STIs), have shown that HSV‐2 occurs in 13–75% of patients attending STI clinics,2,3,4,5,6 24–87% of men who have sex with men,2,6,7,8,9 and 74–98% of female sex workers.10,11 In populations considered to be at lower risk for the acquisition of STIs, the prevalence of HSV‐2 is generally lower. In women attending antenatal clinics, reported prevalence ranges from 5–55%,4,5,12,13,14,15,16,17 in women attending family planning clinics 11–38%,12,18 and in blood donors 2–25%.2,5,19 Demographic risk factors and behaviours associated with HSV‐2 antibodies include increasing age, female gender, black race, previous STIs, young age at first intercourse, lower educational level, and lower social class.2,3,6,8,18,19,20,21,22 In the few studies of HSV‐1 seroprevalence that have been conducted, HSV‐1 infection occurs in 30–100% of adults aged 20 or above.5,9,13,14,15,16,17,19,20 Many of these seroprevalence studies have been small or conducted in highly selected or convenience samples and some have used suboptimal serological assays.
The only representative, nationwide studies, using HSV validated type specific serological assays to establish the prevalence of HSV in the general population, have been conducted in the United States. These studies used serum derived from the National Health and Nutrition Survey (NHANES), a multistage, stratified, cluster sample of the non‐institutionalised civilian population aged between 6 months and 74 years. In the first study (1976 and 1980), a random subsample was tested for antibodies to HSV‐2 and 16.4% of the population aged 15–74 were HSV‐2 positive.21 A second survey (1988 to 1994) showed that the seroprevalence of HSV‐2 in individuals aged 12 years or older was 21.9% and compared with the earlier study, the age adjusted seroprevalence of HSV‐2 rose by 30%. Independent predictors of HSV‐2 seropositivity were female gender, black race or Mexican‐American ethnic background, older age, less education, poverty, cocaine use, and a greater lifetime number of sexual partners.22 There have been a number of other population based studies. However, these have been in regions or individual cities and cannot be considered to be national.
With the potential availability of a vaccine to reduce HSV‐2 infection and HIV transmission,23 accurate determination of nationwide HSV prevalence and its demographic variability is essential.
Methods
The study used serum and sociodemographic data already collected for AusDiab, a nationwide, representative study designed to investigate diabetes and related risk factors in the Australian population (age ⩾25 years), derived from subjects residing in 42 randomly selected urban and non‐urban areas, based on 1996 census collection districts in each state and territory.24 The study was conducted between 1999 and 2000 and included over 11 000 participants (0.05% of the Australian population). The survey involved a short household interview, followed by a biomedical examination at a local survey centre. Householders not available at the initial interview were approached again, on up to four occasions. Household questionnaires were completed in 67% of the households (n = 11 479) that could be contacted and contained at least one eligible person. A total of 20 347 eligible individuals were interviewed in these 11 479 households. The final survey sample (those attending the biomedical examination) included 11 247 adults (5049 men and 6198 women), representing 55% of those completing the household interview; 44.9% were male, with the mean age being 51.5 years. This compares with 49.0% male in the 1998 Australian population, and a mean age in the 1996 census over 25 years of 48.1 years.25 Ninety five per cent of the people were of white European origin.
From the AusDiab survey, a stratified random sample was obtained, that involved oversampling some demographic groups in order to ensure sufficient numbers to address the analytic hypotheses of the study. Sample size calculations used 95% significance and 90% power for finding hypothesised differences in HSV‐2 prevalence, based on previous studies. As a consequence, all Indigenous subjects were included, and subjects resident in metropolitan regions other than capital cities, were oversampled; this provided more than sufficient numbers for precision of overall estimates and age group and sex comparisons. Although 3300 were indicated by sample size calculations, 4000 sera were randomly selected, yielding a sampling fraction of 40% for HSV‐2 determinations. In seven instances where sera were not available, additional subjects were randomly selected. For HSV‐1 analysis, 1000 serum samples were randomly selected from the 4000 sera used for HSV‐2 determination. This is a 10% sampling fraction of the total survey.
Ethical approval for the study was obtained from the International Diabetes Institute ethics committee.
Analysis
In AusDiab, the proportion of participants living in state capitals, other metropolitan, and non‐metropolitan areas were 65%, 7%, and 28% respectively, whereas in this study, the proportions were 54%, 20%, and 26% and the Indigenous proportion was 1%; in comparison, in this study, it is 3%. In addition, the age/sex distribution of the sample was found to differ marginally from the 1996 census because of differential response rates by age and sex.
For production of prevalence rates of HSV‐1 and HSV‐2, weights were calculated to adjust for: (a) purposeful oversampling of Indigenous and “other metropolitan” groups, and (b) the skewed age/sex distribution in AusDiab (compared to the census) from differential response by age and sex.24 Weight factors were calculated as the ratio of the proportion of a stratum in the 2001 Australian census to the proportion in the same stratum in the study population of sera. For the non‐Indigenous population, the strata were age group (6), sex (2), and geographic area (3). For the Indigenous population, because of the small numbers, the strata were age group (6) and sex (2). Strata specific weights were applied to cases before tabulations of weighted prevalences.
Analysis commenced by cross tabulation of HSV‐2 and HSV‐1 seroprevalence with age group, sex, geography of residence, and Indigenous status. Confidence intervals (95%) were calculated using the normal approximation of the binomial, except for instances where numerators were small (<30) and the exact binomial was used. The χ2 test was used to assess heterogeneity between categories of variables. p Values are provided, and p<0.05 used to establish statistical significance. Prevalences weighted to the 2001 census are given for comparison. In this instance, 95% confidence intervals were calculated using the weighted proportions, but the actual number in each stratum (given in the tables) was given to estimate the standard error.
To examine the effects of possible confounding, Poisson regression was undertaken with each variable separately, then together in a model. Poisson regression, rather than logistic regression, was used because prevalences were considerable and the odds ratio (OR) from logistic regression only approximates the relative risk (RR) when proportions are small. In this analysis, the RR derives from the ratio of prevalences.26 RRs are given with 95% confidence intervals. Statistical analyses were carried out in SPSS, SAS, and Stata.
HSV antibody testing
All sera were tested for antibody to HSV‐2 using an indirect enzyme linked immunosorbent assay (ELISA), specifically the HerpeSelect 2 ELISA IgG (Focus Technologies). The manufacturer recommends that an index value of >1.10 is presumptive for the presence of antibody to HSV‐2. However, we have found that using this cut‐off value yields a high rate of false positive results (unpublished). To overcome this problem, a cut‐off value of 3.5 was used to determine HSV‐2 seropositive sera. Sera that gave an equivocal result in the HerpeSelect 2 ELISA (index values 0.9–3.5) were resolved using western blot.27
A subset of the sera (see above) was tested for antibodies to HSV‐1. Depending on the HSV‐2 serostatus of these sera, they were tested by one of two strategies as previously described:14 If HSV‐2 antibody was negative, serum was tested using the Enzygnost anti‐HSV IgG ELISA (Behring) for detection of total antibody to HSV. If HSV‐2 antibody was positive, serum was tested using HSV‐1 western blot. This strategy was used owing to the lack of a low cost, sufficiently sensitive ELISA for antibody to HSV‐1.
Results
HSV‐2 seroprevalence
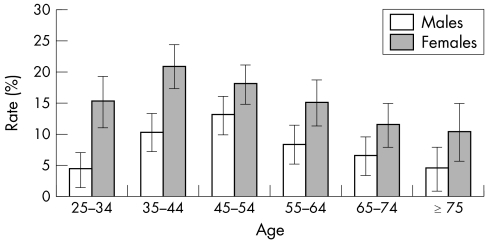
The prevalence of HSV‐2 in this large Australian population was 12%, and there were statistically significant differences by age group, sex, geography, and Indigenous status (table 1). Prevalence of HSV‐2 was highest in the 35–44 year age range (14–19%) and in comparison with the youngest age group (25–34 years), the RR of HSV‐2 seropositivity was 1.5 (p<0.001), adjusted for sex, geography, and Indigenous status (table 1). Women (16%) had a significantly higher prevalence of HSV‐2 than men (8%) (adjusted RR 1.8). The age specific pattern of HSV‐2 prevalence was similar for women and men, although the excess prevalence in women was greatest in the youngest age group (fig 1). Rural and remote populations had lower HSV‐2 prevalence (9%) than capital city or other metropolitan populations (adjusted RR 0.6 compared to the capital city referent group). The Indigenous population had a significantly higher prevalence of HSV‐2 (18%) than the non‐Indigenous (12%) (the RR was 1.5 adjusted for age, sex, and geography).
Table 1 Demographic variation in HSV‐2 seroprevalence in Australia.
| HSV‐2 | No | Rate (%) and 95% CI | Univariate RR | Multivariate RR* | |
|---|---|---|---|---|---|
| Weighted† | |||||
| Age (years) | χ2(5) = 33.6 p<0.001 | χ2 (5) = 34.0 p<0.001 | |||
| 25–34 | 496 | 10.7 (8.0 to 13.4) | 10.2 (12.8 to 7.5) | 1.00 | 1.00 |
| 35–44 | 935 | 16.4 (14.0 to 18.7) | 15.5 (13.1 to 7.8) | 1.53 (1.00 to 2.35) | 1.59 (1.20 to 2.12) |
| 45–54 | 1020 | 15.8 (13.6 to 18.0) | 14.4 (12.3 to 6.6) | 1.48 (0.96 to 2.26) | 1.53 (1.16 to 2.03) |
| 55–64 | 678 | 11.9 (9.5 to 14.4) | 10.8 (13.1 to 8.4) | 1.12 (0.69 to 1.80) | 1.18 (0.86 to 1.61) |
| 65–74 | 571 | 9.3 (6.9 to 11.7) | 8.3 (10.5 to 6.0) | 0.87 (0.51 to 1.47) | 0.94 (0.66 to 1.32) |
| ⩾75 | 300 | 7.7 (4.7 to 10.7) | 8.7 (11.8 to 5.5) | 0.72 (0.37 to 1.41) | 0.79 (0.51 to 1.24) |
| Sex | χ2(1) = 47.4 p<0.001 | χ2(1) = 49.5 p<0.001 | |||
| Male | 1739 | 8.9 (7.6 to 10.3) | 8.4 (9.7 to 7.1) | 1.00 | 1.00 |
| Female | 2261 | 16.3 (14.8 to 17.8) | 15.6 (14.1 to 7.1) | 1.83 (1.45 to 2.32) | 1.81 (1.52 to 2.14) |
| Geography | χ2(2) = 24.1 p<0.001 | χ2(2) = 26.4 p<0.001 | |||
| Capital city | 2162 | 14.4 (12.9 to 15.9) | 13.7 (12.2 to 5.1) | 1.00 | 1.00 |
| Other metropolitan | 804 | 15.3 (12.8 to 17.8) | 13.3 (10.9 to 5.6) | 1.06 (0.80 to 1.42) | 1.05 (0.87 to 1.27) |
| Rural and remote | 1034 | 8.7 (7.0 to 10.4) | 8.6 (10.4 to 6.9) | 0.61 (0.44 to 0.84) | 0.61 (0.49 to 0.75) |
| Indigenous status | χ2(1) = 7.8 p = 0.005 | χ2(1) = 4.3 p = 0.038 | |||
| Non‐Indigenous | 3885 | 12.8 (11.8 to 13.9) | 12.0 (11.0 to 3.0) | 1.00 | 1.00 |
| Indigenous | 115 | 21.7 (14.2 to 29.3) | 17.7 (10.7 to 4.6) | 1.69 (0.94 to 3.05) | 1.51 (1.05 to 2.18) |
| Australia | 4000 | 13.1 (12.1 to 14.2) | 12.1 (11.1 to 3.1) | ||
95% confidence interval (CI) of rates calculated by the normal approximation of the binomial; CIs for weighted rates are based on weighted proportions but actual n (given in the table). Relative risk (RR) calculated from a Poisson regression analysis. 95% CIs of RR calculated from the Wald method using SAS.
*Age group, sex, geography, Indigenous status.
†Weighted to the age, sex, geographic, and Indigenous structure of the 2001 Australian census population.
χ2(x), chi square at x degrees of freedom.
Figure 1 Seroprevalence of HSV‐2 in women and men in the Australian population according to age.
HSV‐1 seroprevalence
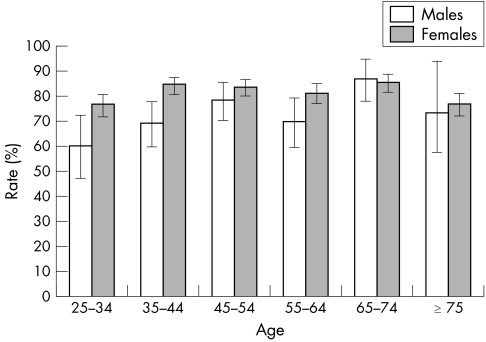
The prevalence of HSV‐1 was 76%, and there were statistically significant differences by age group, sex, and Indigenous status (table 2). Prevalence of HSV‐1 was highest in the 65–74 year age range (85%) and in comparison with the youngest age group (25–34 years), the RR of HSV‐1 seropositivity was 1.25, adjusted for sex, geography, and Indigenous status. Women (80%) had significantly higher prevalence of HSV‐1 than men (71%) (adjusted RR 1.12). The age specific pattern of HSV‐1 prevalence was broadly similar for women and men (fig 2). There were no statistically significant differences in HSV‐1 prevalence by geography. However, the Indigenous population had a higher prevalence of HSV‐1 (close to 100%) than the non‐Indigenous population (75%) (RR 1.24 adjusted for age, sex and geography) (table 2).
Table 2 Demographic variation in HSV‐1 seroprevalence in Australia.
| HSV‐1 | No | Rate (%) and 95% CI | Univariate RR | Multivariate RR* | |
|---|---|---|---|---|---|
| Weighted† | |||||
| Age | χ2(5) = 14.8 p = 0.011 | χ2(5) = 16.0 p = 0.007 | |||
| 25–34 | 124 | 68.5 (60.3 to 76.7) | 67.0 (58.7 to 75.3) | 1.00 | 1.00 |
| 35–44 | 233 | 77.3 (71.9 to 82.7) | 75.1 (69.6 to 80.7) | 1.13 (0.98 to 1.29) | 1.12 (1.00 to 1.26) |
| 45–54 | 232 | 81.0 (76.0 to 86.1) | 80.1 (75.0 to 85.2) | 1.18 (1.03 to 1.35) | 1.19 (1.06 to 1.34) |
| 55–64 | 176 | 75.6 (69.3 to 82.0) | 75.5 (69.2 to 81.9) | 1.10 (0.95 to 1.27) | 1.11 (0.98 to 1.25) |
| 65–74 | 170 | 85.9 (80.7 to 91.1) | 85.2 (79.9 to 90.5) | 1.25 (1.09 to 1.44) | 1.25 (1.11 to 1.41) |
| ⩾75 | 65 | 75.4 (64.9 to 85.9) | 76.6 (66.3 to 86.9) | 1.10 (1.10 to 1.33) | 1.09 (0.93 to 1.28) |
| Sex | χ2(1) = 11.2 p = 0.001 | χ2(1) = 11.4 p = 0.001 | |||
| Male | 516 | 71.3 (67.4 to 75.2) | 71.3 (67.4 to 75.2) | 1.00 | 1.00 |
| Female | 480 | 80.4 (76.9 to 84.0) | 80.4 (76.9 to 84.0) | 1.13 (1.05 to 1.21) | 1.12 (1.05 to 1.19) |
| Geography | χ2(2) = 4.2 p = 0.122 | χ2(2) = 3.9 p = 0.141 | |||
| Capital city | 536 | 75.7 (72.1 to 79.3) | 73.6 (69.9 to 77.3) | 1.00 | 1.00 |
| Other metropolitan | 193 | 82.4 (77.0 to 87.8) | 78.9 (73.1 to 84.7) | 1.09 (0.98 to 1.20) | 1.08 (1.00 to 1.18) |
| Rural and remote | 271 | 79.7 (74.9 to 84.5) | 78.6 (73.7 to 83.5) | 1.05 (0.96 to 1.15) | 1.04 (0.97 to 1.12) |
| Indigenous status | χ2(1) = 5.7 p = 0.017 | χ2(1) = 5.4 p = 0.020 | |||
| Non‐Indigenous | 972 | 77.6 (75.0 to 80.2) | 75.4 (72.7 to 78.1) | 1.00 | 1.00 |
| Indigenous | 28 | 96.4 (81.7 to 99.9)¶ | 100.0 (87.7 to 100.0)‡¶ | 1.24 (1.01 to 1.53) | 1.24 (1.04 to 1.47) |
| Australia | 1000 | 78.1 (75.5 to 80.1) | 75.7 (73.0 to 78.4) | ||
95% confidence interval (CI) of rates calculated by the normal approximation of the binomial except for those indicated. CIs for weighted rates are based on weighted proportions but actual n (given in the table). 95% CI of rates calculated by the normal approximation of the binomial. Relative risk (RR) calculated from a Poisson regression analysis. 95% CIs of RR calculated from the Wald method using SAS.
*Age group, sex, geography, Indigenous status.
†Weighted to the age, sex, geographic, and Indigenous structure of the 2001 Australian census population.
‡One sided test. ¶Exact binomial.
χ2(x), chi square at x degrees of freedom.
Figure 2 Seroprevalence of HSV‐1 in women and men in the Australian population according to age.
Discussion
This is the first representative, nationwide, population based survey of HSV‐1 and HSV‐2 seroprevalence undertaken outside the United States and showed that the prevalence of HSV‐1 and HIV‐2 antibodies in Australia were 76% and 12%, (at a mean age of 45–50). Analysis of HSV‐1 and HSV‐2 seroprevalence for available demographic factors; age, geography, sex, and Indigenous status revealed marked and significant differences for HSV‐2, but much smaller differences for HSV‐1 seroprevalence. Thus, the HSV‐2 seroprevalence increased to a peak of 16% at ages between 35 and 44, and declined thereafter to ∼8% in those over 65 years. Females had a significantly higher seroprevalence than males, again peaking at 35–44 and subjects in metropolitan areas, especially capital cities, had a significantly higher seroprevalence compared with those in rural areas. There was also a significantly higher HSV‐1 (100% v 75%) and HSV‐2 seroprevalence (18% v 12%), comparing Indigenous and non‐Indigenous Australians.
The higher prevalence of HSV‐2 antibody in Indigenous populations is consistent with a relatively high incidence of sexually transmitted infections in some of these populations.28 The relatively small number of Indigenous people in our survey did not allow further distinction between rural and metropolitan communities. Further studies should focus specifically on Indigenous people, particularly in census districts where they predominate.
This study and our previous studies in pregnant women in western Sydney confirm that Australia has a relatively low HSV‐2 seroprevalence compared with most other countries.1,5,29,30 Conversely, HSV‐1 seroprevalence in Australia is higher than in the United States and many parts of Europe.1 In some developed countries, there has been a reduction in overall HSV‐1 seroprevalence over recent decades as a result of decreased acquisition in infancy.31 In contrast, worldwide, there is a trend towards increasing sexual acquisition of HSV‐1 in adolescence and early adulthood,32,33 and this trend is also apparent in Australia.34 Therefore, HSV‐2 seroprevalence is now less accurate as a predictor of the overall burden of genital herpes. This is offset by the fact that genital HSV‐1 recurs much less frequently than HSV‐2.35 HSV‐1 seroprevalence and early infant acquisition is much higher in many developing countries, where adult seroprevalence nears 100%.1 This is similar to the HSV‐1 seroprevalence documented in the Indigenous people in our survey. Such high levels of HSV‐1 acquisition probably reflect extensive infant salivary spread in poorer hygienic conditions. Nevertheless, the reasons for higher overall HSV‐1 seroprevalence in Australia, compared with the United States, require further studies of acquisition in infancy and in adolescence.
Unfortunately, this study lacked data from people aged 25 or younger. Information from NHANES II and III and other studies in high risk individuals, or selected populations around the world, have shown that HSV‐2 seroprevalence increases rapidly from the mid‐teens to the mid‐twenties and then continues to slowly increase in the older age groups.1,2,3,5,6,12,15,16,18,21,22 The few studies that have looked at HSV‐2 seroprevalence in younger adolescents and children, have shown that this infection is uncommon in people below the age of 15. In Australia, further studies will be required to determine HSV‐2 seroprevalence in these younger age groups.
Many studies7 and a meta‐analysis of them,36 have indicated that previous genital HSV‐2 infection is a risk factor for spread of HIV. The contribution to spread (attributable risk) is proportional to seroprevalence. Thus, in some African countries, where HSV‐2 seroprevalence is more than 80%, the attributable risk may rise to 40%. This provides a rationale for the current trials of suppression of recurrences of genital HSV‐2 with aciclovir in an attempt to reduce HIV transmission. Knowledge of variations of HSV‐2 seroprevalence and hence susceptibility to HIV between populations or countries, should allow closer monitoring and early detection of HIV in these populations. Spread of HIV from Papua New Guinea to populations in northern Australia could be facilitated by previous HSV‐2 infection among high risk (Indigenous) Australians.
As previous HSV‐1 infection reduces the severity of disease and may or may not protect against HSV‐2 infection, differences in seroprevalence between countries may alter the patterns of genital HSV‐2 disease or even infection. Differences in HSV‐1 seroprevalence are likely to influence the efficacy of the HSV‐2 glycoprotein D2‐dMPL vaccine manufactured by GSK (Simplirix), as efficacy has only been demonstrated in women with no previous antibodies to HSV‐1 or HSV‐2.23 Therefore, in preparing for potential deployment of the vaccine in each country, it is important that there is an accurate estimate of HSV‐1 seroprevalence in adolescent and adult populations. In some countries, or even some populations in other countries, very high HSV‐1 seroprevalence might make deployment very difficult.
Acknowledgments
We thank Dr P Zimmet for assistance facilitating the study and invaluable advice about access to the AusDiab database and to the serum bank.
Contributors
ALC, RT, and AM, concept and design; ALC, AM, RT,and JT, mauscript writing; JS, epidemiological data collection; JT, HIV serological testing; RT, CM, data analysis; all authors, manuscript review.
Abbreviations
ELISA - enzyme linked immunosorbent assay
HSV - herpes simplex virus
NHANES - National Health and Nutrition Survey
OR - odds ratio
RR - relative risk
STI - sexually transmitted infections
Footnotes
Financial support: This study was funded by an unconditional research grant from GlaxoSmithKline Australia.
Conflict of interest: none.
References
- 1.Smith J S, Robinson N J. Age‐specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 2002186(Suppl 1)S3–28. [DOI] [PubMed] [Google Scholar]
- 2.Cowan F M, Johnson A M, Ashley R.et al Antibody to herpes simplex virus type 2 as serological marker of sexual lifestyle in populations. BMJ 19943091325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langeland N, Haarr L, Mhalu F. Prevalence of HSV‐2 antibodies among STD clinic patients in Tanzania. Int J STD AIDS 19989104–107. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham A L, Lee F K, Ho D W.et al Herpes simplex virus type 2 antibody in patients attending antenatal or STD clinics. Med J Aust 1993158525–528. [DOI] [PubMed] [Google Scholar]
- 5.Hashido M, Lee F K, Nahmias A J.et al An epidemiologic study of herpes simplex virus type 1 and 2 infection in Japan based on type‐specific serological assays. Epidemiol Infect 1998120179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van de Laar M J, Termorshuizen F, Slomka M J.et al Prevalence and correlates of herpes simplex virus type 2 infection: evaluation of behavioural risk factors. Int J Epidemiol 199827127–134. [DOI] [PubMed] [Google Scholar]
- 7.Stamm W E, Handsfield H H, Rompalo A M.et al The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA 19882601429–1433. [PubMed] [Google Scholar]
- 8.Dukers N H, Bruisten S M, van den Hoek J A.et al Strong decline in herpes simplex virus antibodies over time among young homosexual men is associated with changing sexual behavior. Am J Epidemiol 2000152666–673. [DOI] [PubMed] [Google Scholar]
- 9.Russell D B, Tabrizi S N, Russell J M.et al Seroprevalence of herpes simplex virus types 1 and 2 in HIV‐infected and uninfected homosexual men in a primary care setting. J Clin Virol 200122305–313. [DOI] [PubMed] [Google Scholar]
- 10.Nzila N, Laga M, Thiam M A.et al HIV and other sexually transmitted diseases among female prostitutes in Kinshasa. AIDS 19915715–721. [DOI] [PubMed] [Google Scholar]
- 11.Limpakarnjanarat K, Mastro T D, Saisorn S.et al HIV‐1 and other sexually transmitted infections in a cohort of female sex workers in Chiang Rai, Thailand. Sex Transm Infect 19997530–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogaerts J, Ahmed J, Akhter N.et al Sexually transmitted infections among married women in Dhaka, Bangladesh: unexpected high prevalence of herpes simplex type 2 infection. Sex Transm Infect 200177114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown Z A, Selke S, Zeh J.et al The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997337509–515. [DOI] [PubMed] [Google Scholar]
- 14.Mindel A, Taylor J, Tideman R L.et al Neonatal herpes prevention: a minor public health problem in some communities. Sex Transm Infect 200076287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ades A E, Peckham C S, Dale G E.et al Prevalence of antibodies to herpes simplex virus types 1 and 2 in pregnant women, and estimated rates of infection. J Epidemiol Commun Health 19894353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsgren M, Skoog E, Jeansson S.et al Prevalence of antibodies to herpes simplex virus in pregnant women in Stockholm in 1969, 1983 and 1989: implications for STD epidemiology. Int J STD AIDS 19945113–116. [DOI] [PubMed] [Google Scholar]
- 17.Arvaja M, Lehtinen M, Koskela P.et al Serological evaluation of herpes simplex virus type 1 and type 2 infections in pregnancy. Sex Transm Infect 199975168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breinig M K, Kingsley L A, Armstrong J A.et al Epidemiology of genital herpes in Pittsburgh: serologic, sexual, and racial correlates of apparent and inapparent herpes simplex infections. J Infect Dis 1990162299–305. [DOI] [PubMed] [Google Scholar]
- 19.Wutzler P, Doerr H W, Farber I.et al Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations‐relevance for the incidence of genital herpes. J Med Virol 200061201–207. [DOI] [PubMed] [Google Scholar]
- 20.Vyse A J, Gay N J, Slomka M J.et al The burden of infection with HSV‐1 and HSV‐2 in England and Wales: implications for the changing epidemiology of genital herpes. Sex Transm Infect 200076183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson R E, Nahmias A J, Magder L S.et al A seroepidemiologic survey of the prevalence of herpes simplex virus type 2 infection in the United States. N Engl J Med 19893217–12. [DOI] [PubMed] [Google Scholar]
- 22.Fleming D T, McQuillan G M, Johnson R E.et al Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med 19973371105–1111. [DOI] [PubMed] [Google Scholar]
- 23.Stanberry L R, Spruance S L, Cunningham A L.et al Glycoprotein‐D‐adjuvant vaccine to prevent genital herpes. N Engl J Med 20023471652–1661. [DOI] [PubMed] [Google Scholar]
- 24.Dunstan D, Zimmet P, Welborn T, Australian Diabetes, Obesity and Lifestyle Study (AusDiab) et al The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)—methods and response rates. Diabetes Res Clin Pract 200257119–129. [DOI] [PubMed] [Google Scholar]
- 25.Australian Government Canberra Census of Population and Housing. CDATA96, Australia Catalog No 2019.0.30.001. Canberra: Australian Bureau of Statistics, 1997
- 26.Walter S D. Choice of effect measure for epidemiological data. J Clin Epidemiol 200053931–939. [DOI] [PubMed] [Google Scholar]
- 27.Ho D W, Field P R, Irving W L.et al Detection of immunoglobulin M antibodies to glycoprotein G‐2 by western blot (immunoblot) for diagnosis of initial herpes simplex virus type 2 genital infections. J Clin Microbiol 1993313157–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden F J, Paterson B A, Mein J.et al Estimating the prevalence of Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and human papillomavirus infection in indigenous women in northern Australia. Sex Transm Infect 199975431–434. [PMC free article] [PubMed] [Google Scholar]
- 29.Lo J Y, Lim W W, Ho D W.et al Difference in seroprevalence of herpes simplex virus type 2 infection among antenatal women in Hong Kong and southern China. Sex Transm Infect 199975123. [PubMed] [Google Scholar]
- 30.Dobbins J G, Mastro T D, Nopkesorn T.et al Herpes in the time of AIDS: a comparison of the epidemiology of HIV‐1 and HSV‐2 in young men in northern Thailand. Sex Transm Dis 19992667–74. [DOI] [PubMed] [Google Scholar]
- 31.Tunback P, Bergstrom T, Andersson A S.et al Prevalence of herpes simplex virus antibodies in childhood and adolescence: a cross‐sectional study. Scand J Infect Dis 200335498–502. [DOI] [PubMed] [Google Scholar]
- 32.Stanberry L R, Rosenthal S L, Mills L.et al Longitudinal risk of herpes simplex virus (HSV) type 1, HSV type 2, and cytomegalovirus infections among young adolescent girls. Clin Infect Dis 2004391433–1438. [DOI] [PubMed] [Google Scholar]
- 33.Cowan F M, Copas A, Johnson A M.et al Herpes simplex virus type 1 infection: a sexually transmitted infection of adolescence? Sex Transm Infect 200278346–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran T, Druce J D, Catton M C.et al Changing epidemiology of genital herpes simplex virus infection in Melbourne, Australia, between 1980 and 2003. Sex Transm Infect 200480277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corey L. First‐episode, recurrent, and asymptomatic herpes simplex infections. J Am Acad Dermatol 198818(1 Pt 2)169–172. [DOI] [PubMed] [Google Scholar]
- 36.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2‐seropositive persons: a meta‐analysis. J Infect Dis 200218545–52. [DOI] [PubMed] [Google Scholar]