Abstract
Objective
To develop a risk assessment algorithm that will increase the identification and treatment of women with cervical infection in rural Haiti.
Methods
Study participants were randomly selected from new patients who accessed services at a women's health clinic in rural Haiti between June 1999 and December 2002. This case‐control study included women who tested positive for chlamydia and/or gonorrhoea based on the Gen‐Probe PACE 2 laboratory test as cases. Controls were women who tested negative for both of these infections.
Results
Women from this area of rural Haiti had a limited level of education and lived in impoverished housing conditions. The sensitivity estimates of Haitian Ministry of Health and WHO algorithms for detecting chlamydia and/or gonorrhoea were generally low (ranging from 16.1% to 68.1%) in this population. Risk scores based on logistic regression models of local risk factors for chlamydia and gonorrhoea were developed and sensitivity estimates were higher for algorithms based on these risk scores (up to 98.8%); however, specificity was compromised.
Conclusions
A risk assessment algorithm to identify women with chlamydia and/or gonorrhoea is more sensitive and less specific than the syndromic management approach advocated by WHO and adapted by the Haitian Ministry of Health. Using a risk assessment tool with high sensitivity based on local risk factors of cervical infection will maximise access to care, improve outcomes, and decrease morbidity in women who have cervical infection in rural Haiti.
Keywords: chlamydia, gonorrhoea, Haiti
Sexually transmitted infections (STIs) exact a heavy burden worldwide. STIs increase the risk of HIV transmission1,2 and untreated cervical infections cause significant morbidity in women including pelvic inflammatory disease, tubo‐ovarian abscess, ectopic pregnancy, and infertility.3,4 In 1999, the World Health Organization (WHO) estimated that there were 340 million new cases of curable STIs worldwide. Nearly half of these infections are caused by chlamydia or gonorrhoea.4 Sadly, in the developing world, a significant percentage of women with cervical infection are not treated because of limited access to diagnostic testing.3
Given the long term health effects of untreated cervical infection and the increased risk of HIV transmission when STIs are present, it is imperative to identify strategies for improving case detection of chlamydia and gonorrhoea so that prompt treatment may be given. This is particularly critical for resource poor settings where the burden of STIs is high and access to care is generally low.5,6 The most widely implemented model for the management of STIs in resource poor settings has been the “syndromic approach,”7 in which a patient presents with symptoms associated with STIs and is treated empirically. Because of the limited ability to diagnose STIs in resource poor settings, the WHO has been advocating syndromic management of these infections since the 1970s8 and has recently updated these algorithms.7 The health ministries of many poor countries, such as Haiti, have adopted the syndromic management of STIs because such algorithms are easily used in areas with limited diagnostic capability. One key limitation of the syndromic approach is the low sensitivity and specificity for the detection of cervical infection.9 In particular, the low sensitivity of syndromic management of cervical infection is related to the fact that the majority of women with chlamydia and gonorrhoea are asymptomatic.3 To increase case detection of STIs in areas without access to diagnostic tests, some authors have advocated the use of epidemiological risk assessment rather than a syndromic approach. Such risk assessment tools are typically more sensitive in their ability to detect cervical infection compared with syndromic management algorithms.9,10
The purpose of the research described in this paper is to develop a risk assessment algorithm based on local risk factors of chlamydia and gonorrhoea that will increase the identification and treatment of women with cervical infection in rural Haiti. In addition, we will evaluate the performance of previously developed algorithms by the WHO and the Haitian Ministry of Health (MSPP) for use in this context.7,11 The accuracy of these algorithms (sensitivity and specificity) will be compared with a newly developed risk assessment algorithm based on data from rural Haiti that include laboratory diagnosis of chlamydia and gonorrhoea. From these comparisons, an algorithm will be identified that will maximise the likelihood that women with cervical infection in rural Haiti will receive appropriate and timely treatment.
Methods
Setting
This research study was performed in a deeply impoverished area of Haiti's central plateau. A rural area that is primarily comprised of subsistence farmers, Cange and the surrounding environs are largely inhospitable for cultivation. Zanmi Lasante was founded in the early 1980s to provide healthcare and related services for this population. Zanmi Lasante has a free standing women's health clinic, Proje Sante Fanm, that serves 12 000–15 000 women annually and provides comprehensive women's health services. All services and medications are provided free of charge given the high level of poverty in the region.
Study design and sample selection
The design was a case‐control study in which cases were women with chlamydia and/or gonorrhoea as confirmed through the Gen‐Probe PACE 2 laboratory test. Controls were women who did not have either of these infections and also presented for care at Proje Sante Fanm. New patients who presented for healthcare services at Proje Sante Fanm between June 1999 and December 2002 were selected randomly for participation. A more detailed description of the selection process of study participants and other methods has been provided elsewhere.12
Laboratory methods
Presence of chlamydia and/or gonorrhoea was determined using the Gen‐Probe PACE 2 test. The PACE 2 is a non‐amplified DNA probe assay for the detection of chlamydia and gonorrhoea in endocervical specimens (Gen‐Probe, San Diego, CA, USA). Using nucleic acid amplification as a gold standard, the sensitivity and specificity of the PACE 2 system in detecting chlamydia were 79.3% and 100%, respectively.13 For gonorrhoea, the overall sensitivity and specificity of endocervical specimens analysed using the Gen‐Probe PACE 2 test were 92.1% and 99.7%, respectively, using culture as the criterion.14
Statistical analysis
The prevalences of chlamydia, gonorrhoea, and of both infections were calculated based on the entire population of women tested for these infections (n = 3956) during the enrolment period. The sensitivity and specificity for different algorithms as indicated in table 1 were calculated using the laboratory diagnosis of chlamydia and/or gonorrhoea (Gen‐Probe PACE 2 test) as the “gold standard” from the case‐control study data (n = 944). Predictive value positive and negative were calculated based on the sensitivity, specificity, and prevalence of chlamydia and/or gonorrhoea using Bayes's theorem.
Table 1 Accuracy of algorithms for detection of cervical infection in rural Haiti.
| Algorithm | SN | SP | PVP | PVN | Percentage positive |
|---|---|---|---|---|---|
| Haiti MSPP algorithm for vaginal discharge (n = 944) | 16.1% | 93.8% | 17.2% | 93.3% | 9.1 |
| Haiti MSPP algorithm for vaginal discharge or lower abdominal pain (n = 944) | 16.9% | 93.7% | 17.7% | 93.4% | 9.4 |
| WHO algorithm for vaginal discharge (n = 939) | 35.0% | 63.6% | 7.1% | 92.4% | 36.0 |
| WHO algorithm for vaginal discharge (speculum and bimanual exam) (n = 939) | 38.6% | 58.6% | 6.9% | 92.3% | 40.6 |
| WHO algorithm for vaginal discharge (speculum and microscope) (n = 944) | 68.1% | 31.0% | 7.3% | 92.4% | 68.8 |
| Risk assessment algorithm developed in rural Haiti (n = 924) | 96.7% | 15.3% | 8.4% | 98.3% | 88.2 |
SN, sensitivity; SP, specificity; PVP, predictive value positive; PVN, predictive value negative.
Multiple logistic regression was used to identify risk factors that demonstrated the strongest prediction of chlamydia and/or gonorrhoea in this population. A “training” dataset (subset of the case‐control data) was constructed to run these initial analyses and included the first 548 women enrolled. The outcome variable for these models was the presence of chlamydia and/or gonorrhoea based on results from the laboratory test. Predictors were included in these models if they demonstrated an association with chlamydia and/or gonorrhoea at the 0.20 significance level or lower in univariate analyses. Using the backward elimination technique, four logistic regression models were considered based on statistical significance level and inclusion of STI related clinical signs and symptoms as predictor variables.
Based on these models, a risk score was calculated using the odds ratio (OR) estimates provided in these final models. If the factor was present, then the score for that given item was equal to the OR estimate produced in the final model. For example, for model 3, a woman who was less than 30 years of age and who reported difficulty in transport to the clinic would have a risk score of 6.3 (3.9+2.4) (see table 2). Based on the distributions of these risk scores, three cut‐off points were selected and sensitivity and specificity were calculated for each of these cut‐off points for the training dataset. In order to validate these findings, sensitivity and specificity were recalculated for a “testing” dataset that included the final 396 women enrolled in the case‐control study (see table 3).
Table 2 Multiple logistic regression analysis for the development of a new empirical algorithm to predict cervical infection.
| Odds ratio | 95% CI* | |
|---|---|---|
| Model 1 (n = 413)† | ||
| Age ⩽30 years | 3.8 | 1.7 to 8.3 |
| Occupation—Market vendor (no) | 2.6 | 1.3 to 5.3 |
| Occupation—Domestic servant (yes) | 6.4 | 1.5 to 27.1 |
| Partner's occupation—Mechanic (yes) | 6.3 | 1.0 to 38.7 |
| Partner's occupation—Construction (yes) | 3.0 | 1.1 to 8.3 |
| More than one lifetime sexual partner | 2.3 | 1.2 to 4.3 |
| Difficulty in transport to clinic | 2.7 | 1.4 to 5.1 |
| Cervical mucopus | 3.4 | 1.2 to 9.3 |
| Model 2 (n = 417)‡ | ||
| Age ⩽30 years | 3.5 | 1.7 to 7.4 |
| Occupation—Market vendor (no) | 2.4 | 1.2 to 4.7 |
| Occupation—Domestic servant (yes) | 4.6 | 1.2 to 17.8 |
| Partner's occupation—Construction (yes) | 2.8 | 1.0 to 7.6 |
| More than one lifetime sexual partner | 2.0 | 1.1 to 3.7 |
| Malodorous vaginal discharge (self report) | 2.5 | 1.1 to 5.7 |
| Difficulty in transport to clinic | 2.6 | 1.4 to 4.9 |
| Model 3 (n = 412)¶ | ||
| Age ⩽30 years | 3.9 | 1.8 to 8.3 |
| More than one lifetime sexual partner | 2.3 | 1.2 to 4.1 |
| Difficulty in transport to clinic | 2.4 | 1.3 to 4.4 |
| Model 4 (n = 417)§ | ||
| Age ⩽30 years | 3.6 | 1.8 to 7.5 |
| Difficulty in transport to clinic | 2.4 | 1.3 to 4.3 |
*CI, confidence interval.
†Significance level for retaining variables in model was 0.05. Clinical parameters significant at 0.20 level or less were included in this model.
‡Significance level for retaining variables in the model was 0.05. No clinical parameters were considered for this model.
¶Significance level for retaining variables in model was 0.01. Clinical parameters significant at 0.20 level or less were included in this model.
§Significance level for retaining variables in the model was 0.01. No clinical parameters were considered for this model.
Table 3 Accuracy of new empirical algorithms based on data from rural Haiti to detect cervical infection.
| Algorithm | Sensitivity | Specificity |
|---|---|---|
| Model 1, cut off >9.7 | ||
| Training dataset (n = 517) | 98.7% | 10.1% |
| Model 1, cut off >9.7 | ||
| Testing dataset (n = 351) | 96.4% | 11.5% |
| Model 1, cut off >12 | ||
| Training dataset (n = 517) | 93.3% | 18.1% |
| Model 1, cut off >12 | ||
| Testing dataset (n = 351) | 89.9% | 17.0% |
| Model 1, cut off >14.6 | ||
| Training dataset (n = 517) | 90.7% | 46.4% |
| Model 1, cut off >14.6 | ||
| Testing dataset (n = 351) | 80.5% | 31.9% |
| Model 2, cut off >2 | ||
| Training dataset (n = 511) | 97.4% | 9.0% |
| Model 2, cut off >2 | ||
| Testing dataset (n = 353) | 95.9% | 8.7% |
| Model 2, cut off >2.8 | ||
| Training dataset (n = 511) | 97.4% | 20.5% |
| Model 2, cut off >2.8 | ||
| Testing dataset (n = 353) | 94.7% | 15.8% |
| Model 2, cut off >5.4 | ||
| Training dataset (n = 511) | 85.5% | 43.0% |
| Model 2, cut off >5.4 | ||
| Testing dataset (n = 353) | 84.7% | 24.6% |
| Model 3, cut off > = 1 | ||
| Training dataset (n = 541) | 98.8% | 16.6% |
| Model 3, cut off > = 1 | ||
| Testing dataset (n = 383) | 95.7% | 12.2% |
| Model 3, cut off >2.3 | ||
| Training dataset (n = 541) | 94.0% | 27.3% |
| Model 3, cut off >2.3 | ||
| Testing dataset (n = 383) | 89.8% | 20.9% |
| Model 3, cut off >3.9 | ||
| Training dataset (n = 541) | 67.5% | 60.5% |
| Model 3, cut off >3.9 | ||
| Testing dataset (n = 383) | 62.6% | 54.1% |
| Model 4, cut off > = 1 | ||
| Training dataset (n = 544) | 94.0% | 27.3% |
| Model 4, cut off > = 1 | ||
| Testing dataset (n = 385) | 89.9% | 21.3% |
| Model 4, cut off >2.4 | ||
| Training dataset (n = 544) | 85.5% | 40.6% |
| Model 4, cut off >2.4 | ||
| Testing dataset (n = 385) | 79.8% | 35.0% |
| Model 4, cut off >3.6 | ||
| Training dataset (n = 544) | 39.8% | 84.4% |
| Model 4, cut off >3.6 | ||
| Testing dataset (n = 385) | 34.6% | 72.1% |
Results
Among new patients who presented for services at Proje Sante Fanm, the prevalence estimates of cervical infection were as follows: 6.2% for chlamydia; 1.7% for gonorrhoea; and 7.4% for chlamydia and/or gonorrhoea. In addition, 63.1% of the study population were pregnant at the time of enrolment. In this highly impoverished area of rural Haiti only 51.2% of women had ever attended school and 19.6% indicated that they had completed more than 6 years of schooling; 61.9% lived in a thatched or mud house and only 32.4% had a latrine. Over 50% earned $20 per month or less indicating a highly vulnerable situation for many of the women. Factors associated with cervical infection have been examined and published previously.12
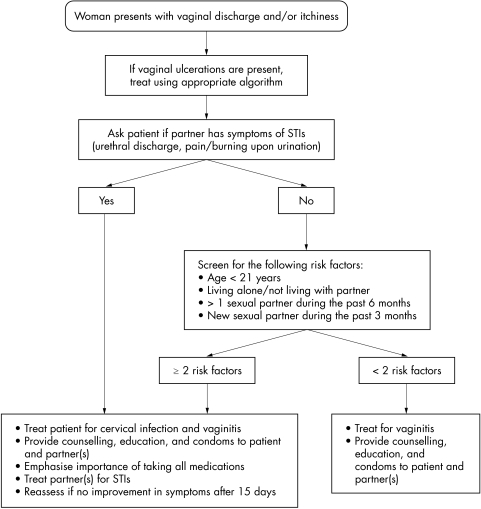
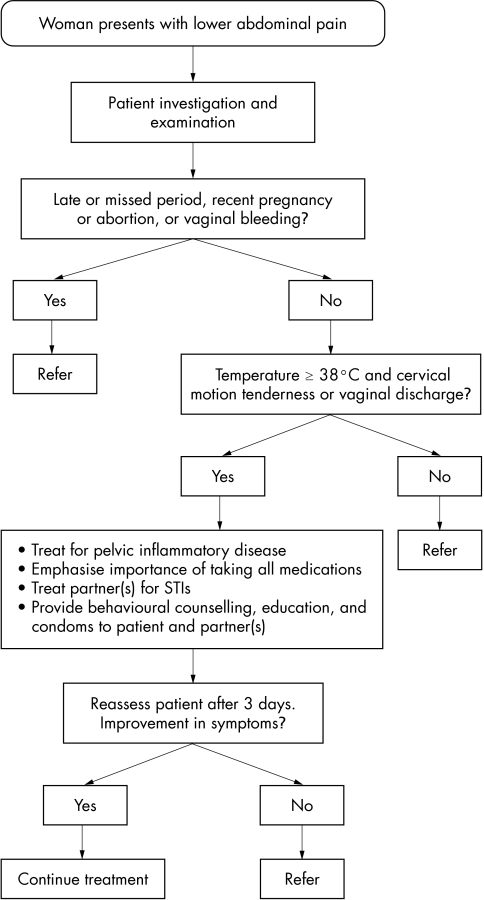
The accuracy of different algorithms for the detection of cervical infection is presented in table 1. The Haitian Ministry of Health algorithm for vaginal discharge was only 16.1% sensitive for detecting cervical infection. Although one might anticipate an improvement in sensitivity when including the lower abdominal pain (LAP) algorithm, sensitivity increased only 0.8% when adding the LAP algorithm to the vaginal discharge algorithm. Specificity for the Haitian MSPP algorithms was reasonable (93.8% and 93.7%). See figures 1 and 2 for the Haitian MSPP protocols for vaginal discharge and lower abdominal pain.
Figure 1 Protocol for treatment of vaginal discharge and/or itchiness among women in Haiti, Ministry of Health.
Figure 2 Protocol for treatment of pelvic inflammatory disease (PID) among women in Haiti, Ministry of Health.
The WHO algorithms, which also rely on the local risk assessment scores used in the MSPP algorithms reflected a modest degree of accuracy. For the WHO algorithm, which is based on vaginal discharge without more extensive clinical examination or laboratory assessment,15 the sensitivity was 35.0% and specificity 63.6%. Sensitivity improved slightly when adding a speculum and bimanual examination (38.6%); however, specificity was slightly lower (58.6%).16 Interestingly, the algorithm that included the speculum examination and laboratory assessment had a much higher sensitivity although still modest (68.1%).17 The difference between the first two WHO algorithms and the third is that the first two require that “abnormal discharge” is present according to a clinical examination, whereas the third does not. Without this criterion, sensitivity increased to 68.1% in this setting. At the same time, specificity decreased to 31.0% (see table 1).
The risk assessment algorithm developed in this study for use among women in rural Haiti demonstrated a higher sensitivity (96.7%) and a lower specificity (15.3%) than the MSPP and WHO algorithms. The percentage that would test “positive” for each of these algorithms is also presented in table 1 and ranges from 9.1% for the first MSPP algorithm to 88.2% for the risk assessment algorithm. Given the relatively low prevalence of cervical infection in the study population (7.4%) the “percentage positive” in this rural population is largely driven by the specificity of each algorithm in table 1.
Multiple logistic regression analysis was used to develop the risk assessment algorithm that is indicated in table 1. Results from the multivariate analysis are presented in table 2. As described in the methods section, four models were constructed using the “training” dataset. Model 1 included a sociodemographic variable (for example, age), economic variables (occupation, partner's occupation, and difficulty in transport to clinic), a sexual history factor (more than one lifetime sexual partner), and a clinical predictor (cervical mucopus). The factor that demonstrated the strongest association with chlamydia and/or gonorrhoea for model 1 was occupation as a domestic servant (OR = 6.4; 95% confidence interval (CI): 1.5 to 27.1). Model 2 considered the same set of factors at the 0.05 level except for the clinical predictor variables and resulted in a slightly different model, with the inclusion of self report of malodorous vaginal discharge (OR = 2.5; 95% CI: 1.1 to 5.7). In addition, partner's occupation as mechanic was not included in model 2. Model 3 included the same set of variables as model 1; however, the significance level was 0.01. Only three variables were retained in this model: age less than or equal to 30, more than one lifetime sexual partner, and difficulty in transport to clinic. Model 4 excluded the clinical predictor variables and retained variables significant at the 0.01 level. Compared with model 3, the “more than one lifetime sexual partner” was omitted from this final model (model 4).
Sensitivity and specificity estimates were compared for the “training” and “testing” datasets for models 1 to 4 at varying cut‐off points (see table 3). The highest sensitivity (98.8%) observed for the training dataset was for model 3 at a cut‐off score of ⩾1. Given the need to maximise sensitivity in order to prioritise identifying “true positives” this model was selected as the basis of our local risk assessment algorithm to increase access to care for cervical infection among women in rural Haiti. Since sensitivity was maximised, however, specificity was low for this algorithm (16.6%). Estimates of sensitivity and specificity for model 3 at the same cut‐off score (⩾1) were attenuated (95.7% and 12.2%) for the testing dataset, but were generally consistent with the training dataset results. Results presented in table 1 are combined estimates for the entire dataset of sensitivity and specificity for this risk assessment algorithm.
Discussion
The purpose of this research was to develop a risk assessment algorithm that would increase the identification of chlamydia and gonorrhoea to improve access to treatment for cervical infection in rural Haiti where diagnostic testing is not widely available. Based on our results, it was clear that the Haitian Ministry of Health algorithm currently in use, while having a reasonable specificity (93.7%), demonstrated a low sensitivity (16.9%). While improvements in sensitivity were observed for WHO algorithms for vaginal discharge,7 the highest sensitivity observed was only 68.1% for those women having a follow up speculum examination and laboratory evaluation in addition to a risk score assessment. The relatively low sensitivity of these algorithms may be related to the fact that they rely on the presentation of women arriving with complaints of vaginal discharge or lower abdominal pain. It is well known that a significant percentage of women with cervical infection are asymptomatic.18 To address this issue we developed a risk assessment algorithm that did not require initial presentation with STI symptoms. This strategy was similar to the “non‐hierarchical” approach employed by Vuylsteke and colleagues,9 who observed a significant improvement in sensitivity when they did not require these symptoms to be part of the risk assessment scale that they developed.
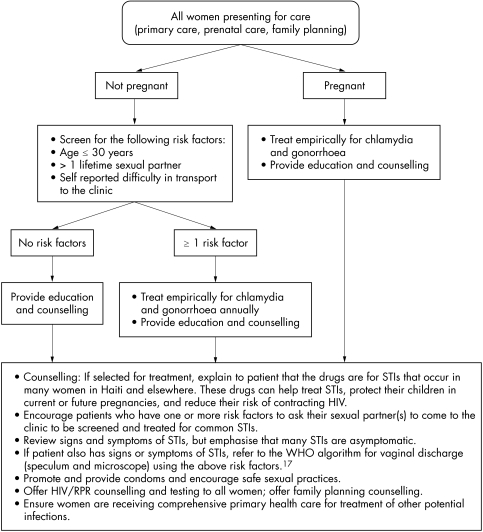
Based on the four models described in the results (see table 2), we developed risk scores and evaluated the sensitivity and specificity at different cut‐off points for these scores. We found that model 3 demonstrated the highest sensitivity for the training dataset (98.8%) at a cut‐off score of greater than or equal to “1.” Therefore, we would recommend the use of this model to serve as the basis for an algorithm for empirical treatment of chlamydia and gonorrhoea. This algorithm indicates that women who have one or more of the following characteristics would be treated empirically for chlamydia and gonorrhoea: age less than or equal to 30 years; more than one lifetime sexual partner; and self reported difficulties in transport to clinic. In addition to using the above risk assessment algorithm, all pregnant women will be empirically treated for chlamydia and gonorrhoea, given the negative health outcomes observed among infants exposed to chlamydia and gonorrhoea prenatally, including low birth weight, pneumonia, and blindness,3,4 and the reduced risk of premature rupture of membranes, preterm delivery, and low birth weight among pregnant women receiving prophylactic antibiotics19 (see fig 3 for recommended algorithm for rural Haiti).
Figure 3 Recommended protocol for treatment of chlamydia and gonorrhoea among women in rural Haiti.
Previously published algorithms for the identification of chlamydia and gonorrhoea have demonstrated a wide range of estimates of sensitivity and specificity. For example, Behets et al20 evaluated the use of different algorithms in Jamaica and found that a risk factor based algorithm was more sensitive (85%) compared with a clinical algorithm based on signs and symptoms (73%). Among women attending antenatal clinics in Tanzania, Mayaud et al21 compared sensitivity and specificity for different algorithms. These authors observed a fairly low sensitivity (43%) and specificity (58%) for detecting chlamydia and/or gonorrhoea for an algorithm based on vaginal discharge or genital itching. When considering a risk score algorithm based on sociodemographic characteristics and symptoms, sensitivity increased to 69% and specificity decreased to 54%.21 In Brazil, a clinical algorithm based on vaginal discharge had a low sensitivity (16%) and a high specificity (98%) for chlamydia and/or gonorrhoea. However, based on a national flow chart that included regional risk factors, sensitivity improved (68%) but specificity was much lower (47%).22 Similar to these results, we observed a low sensitivity for algorithms that relied on symptoms of vaginal discharge and/or lower abdominal pain, such as the WHO and Haitian Ministry of Health algorithms. Our findings as well as the above referenced studies indicate the important role of understanding the local risk factors for cervical infection in developing a risk assessment algorithm.
There are a number of limitations in the present study, including the use of the Gen‐Probe PACE 2 as the “gold standard” assessment. This study was initiated when amplification tests were prohibitive in rural Haiti because of cost and limitations in technical capacity. While sensitivity for gonorrhoea is acceptable (92.1%),14 the sensitivity of the Gen‐Probe PACE 2 to detect chlamydia is lower (79.3%) when compared with diagnosis through nucleic acid amplification.13 While this may result in potentially not detecting “true positive” cases of chlamydia, the objective of the study was to develop an algorithm that would approximate this specific laboratory test that is currently used in rural Haiti. No other laboratory tests for chlamydia and gonorrhoea are locally available. Secondly, the specificity of the proposed risk assessment algorithm is low (15.3%). However, the sensitivity for the proposed algorithm is high (96.7%). Given the priority of detecting women who may have infection, the utility of the proposed algorithm is reflected in this sensitivity estimate, ensuring that a greater number of women who have chlamydia and/or gonorrhoea in the target area will receive treatment. However, this low specificity will result in overtreatment of “false positives.” The primary concern for overtreatment involves the issue of potential drug resistance. This will not be a problem for women without any infection. However, for women who have other infections requiring antibiotics (for example, pneumonia) resistance to the prescribed drugs may develop. Women who access services at Zanmi Lasante receive comprehensive primary health care; therefore, it is anticipated that if women present with these other infections they will receive appropriate and timely treatment, significantly minimising the possibility of drug resistance. Given the morbidity of untreated cervical infection in women, such as the risk of tubo‐ovarian abscess and ectopic pregnancy in settings where obstetric care is not widely available, this strategy of “overtreatment” will save lives.
The widespread use of the proposed algorithm, based on figure 3, throughout rural Haiti would dramatically increase access to treatment for cervical infection for thousands of women. We have initiated use of this algorithm at Zanmi Lasante in rural Haiti and have found that women respond positively to this approach. While they understand that a positive result on the risk assessment algorithm does not indicate that they necessarily have chlamydia and/or gonorrhoea, they know that they are at an elevated risk and assuming treatment for these STIs may prevent them from acquiring HIV infection. An alternative to the risk assessment approach is the use of rapid point of care diagnostic tests. However, these tests have demonstrated low sensitivity (25–49%) for chlamydial infection in low prevalence populations.23,24,25 Therefore, the risk assessment algorithm described in this paper to identify and treat women for chlamydia and gonorrhoea in rural Haiti may prove to be a useful strategy until more sensitive rapid diagnostics for these infections are made widely available throughout the developing world.
Acknowledgements
This study was supported by a grant provided by the Fogarty International Center (D43‐TW00018; principal investigator: Dr Warren Johnson, Cornell University Medical School; principal investigator of Harvard substudy: Dr Paul Farmer, Department of Social Medicine, Harvard Medical School). J Singler was supported in part by a University of California San Francisco School of Medicine Student Research Committee Summer Fellowship as the 2001 Jane Shohl Colburn Research Fellow. The work at Zanmi Lasante and Proje Sante Fanm is made possible through the generosity of private foundations and donors, especially Thomas J White.
The authors would like to acknowledge the women who participated in the study and staff at Zanmi Lasante and Harvard Medical School who contributed significantly to this study. Special thanks are extended to Ledgy Astrel, Donna Barry, Fred Boehm, Augustin Bosquet, Friga François, Jean François, Kelourde Gracia, Yolène Gracia, René Guerrier, Kathryn Kempton, Lenéus Joseph, Tania Louis Jean, Julie Mann, Ludie Marcellus, Deogratias Niyizonkiza, Cynthia Orélus, Ulric Pierre, Sherly Pierre Louis, Sophonie Predestin, Lana Sloutsky, Cheri Verrilie, Loune Viaud, and Alice Yang.
Contributors
MCSF is affiliated with Harvard Medical School and Zanmi Lasante and was involved in all aspects of the study, including design, implementation, analysis, and manuscript preparation; WL, FL, PN, DB, MSC, JB, ML, LJ, and JGF are affiliated with Zanmi Lasante and were involved with study design and implementation; JS is affiliated with University of California, San Francisco Medical School and was involved with study design and implementation; JSM is affiliated with the Brigham and Women's Hospital, Boston, and Zanmi Lasante and was involved with manuscript preparation; EFC is affiliated with the Harvard School of Public Health and was involved with study analysis and manuscript preparation; JJS is affiliated with Harvard Medical School and was involved in study analysis and manuscript preparation; PF is affiliated with Harvard Medical School and Zanmi Lasante and was involved with study design, implementation, and manuscript preparation.
Abbreviations
LAP - lower abdominal pain
PID - pelvic inflammatory disease
MSPP - Ministère de la Santé Publique et de la Population
PIDSC - Program in Infectious Disease and Social Change
STI - sexually transmitted infection
Footnotes
There is no conflict of interest for any authors involved with this study.
References
- 1.Kapiga S H, Lyamuya E F, Lwihula G K.et al The incidence of HIV infection among women using family planning methods in Dar es Salaam, Tanzania. AIDS 19981275–84. [DOI] [PubMed] [Google Scholar]
- 2.Kilmarx P, Limpakarnjanarat K, Mastro T.et al HIV‐1 seroconversion in a prospective study of female sex workers in northern Thailand: continued high incidence among brothel‐based women. AIDS 199812188998. [DOI] [PubMed] [Google Scholar]
- 3.Rowley J, Berkley S. Sexually transmitted diseases. In: Murray CJL, Lopez AD, eds. Global burden of disease and injury series Volume III. Health dimensions of sex and reproduction: the global burden of sexually transmitted diseases, HIV, maternal conditions, perinatal disorders, and congenital anomalies. United States: Library of Congress, 199820–110.
- 4.World Health Organization Global prevalence and incidence of selected curable sexually transmitted infections overview and estimates. 2001. WHO/HIV_AIDS/2001. 02, WHO/CDS/CSR/EDC/2001. 10. Geneva, Switzerland ( www.who.int/docstore/hiv/GRSTI/who_hiv_aids_2001.02.pdf )
- 5.Moodley P, Pillay C, Goga R.et al Evolution in the trends of antimicrobial resistance in Neisseria gonorrhoeae isolated in Durban over a 5 year period: impact of the introduction of syndromic management. J Antimicrob Chemother 200148853–859. [DOI] [PubMed] [Google Scholar]
- 6.Cates W, Dallabetta G. The staying power of sexually transmitted diseases. Lancet. 1999;354 Suppl SIV62. [DOI] [PubMed]
- 7.World Health Organization Guidelines for the management of sexually transmitted diseases. WHO/RHR/01. 10–79 pages, Geneva, Switzerland ( www.who.int/docstore/hiv/STIManagemntguidelines/who_hiv_aids_2001.01 ) 2001
- 8.Djajakusumah T, Sudigdoadi S, Keersmaekers K.et al Evaluation of syndromic patient management algorithm for urethral discharge. Sex Transm Infect 199874(Suppl 1)S29–S33. [PubMed] [Google Scholar]
- 9.Vuylsteke B, Laga M, Alary M.et al Clinical algorithms for the screening of women for gonococcal and chlamydial infection: evaluation of pregnant women and prostitutes in Zaire. Clin Infect Dis 19931782–88. [DOI] [PubMed] [Google Scholar]
- 10.Kapiga S H, Vuylsteke B, Lyamuya E F.et al Evaluation of sexually transmitted diseases diagnostic algorithms among family planning clients in Dar es Salaam, Tanzania. Sex Transm Infect 199874(Suppl 1)S132–S138. [PubMed] [Google Scholar]
- 11.Ministère de la Santé Publique et de la Population (MSPP) Les Centres GHESKIO. OPS/OMS, Avec la participation des Centres pour le Développement et la Santé, Save the Children et la Coalition des ONG du Plateau Central Prise en charge des Maladies Sexuellment Transmissibles: Protocoles pour les soins de santé primaires 1998
- 12.Smith Fawzi M C, Lambert W, Singler J M.et al Prevalence and risk factors of STDs in rural Haiti: implications for policy and programming in resource‐poor settings. Int J STD AIDS 200314845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wylie J L, Moses S, Babcock R.et al Comparative evaluation of chlamydiazyme, PACE 2, and AMP‐CT assays for detection of Chlamydia trachomatis in endocervical specimens. J Clin Microbiol 1998363488–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koumans E H, Johnson R E, Knapp J S.et al Laboratory testing for Neisseria gonorrhoeae by recently introduced nonculture tests: a performance review with clinical and public health considerations. Clin Infect Dis 1998271171–1180. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Guidelines for the management of sexually transmitted diseases. WHO/RHR/01. 10–79 pages, Geneva, Switzerland ( www.who.int/docstore/hiv/STIManagemntguidelines/who_hiv_aids_2001.01 ) (p 23, Figure 6) 2001
- 16.World Health Organization Guidelines for the management of sexually transmitted diseases. WHO/RHR/01. 10–79 pages, Geneva, Switzerland ( www.who.int/docstore/hiv/STIManagemntguidelines/who_hiv_aids_2001.01 ) (p 24, Figure 7) 2001
- 17.World Health Organization Guidelines for the management of sexually transmitted diseases. WHO/RHR/01. 10–79 pages, Geneva, Switzerland ( www.who.int/docstore/hiv/STIManagemntguidelines/who_hiv_aids_2001.01 ) (p 25, Figure 8) 2001
- 18.Korenromp E L, Sudaryo M K, de Vlas S J.et al What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? Int J STD AIDS 20021391–101. [DOI] [PubMed] [Google Scholar]
- 19.Thinkhamrop J, Hofmeyr G J, Adetoro O.et al Prophylactic antibiotic administration in pregnancy to prevent infectious morbidity and mortality. Cochrane Review. Cochrane Library, Issue 4. 2004 [DOI] [PubMed]
- 20.Behets F, Williams Y, Brathwaite A.et al Management of vaginal discharge in women treated at a Jamaican sexually transmitted disease clinic: use of diagnostic algorithms versus laboratory testing. Clin Infect Dis 1995211450–1455. [DOI] [PubMed] [Google Scholar]
- 21.Mayaud P, Grosskurth H, Changalucha J.et al Risk assessment and other screening options for gonorrhoea and chlamydial infections in women attending rural Tanzanian antenatal clinics. Bull World Health Organ 199573621–630. [PMC free article] [PubMed] [Google Scholar]
- 22.Moherdaui F, Vuylsteke B, Siqueira Goes L F.et al Validation of national algorithms for the diagnosis of sexually transmitted diseases in Brazil: results from a multicentre study. Sex Transm Infect 199874(Suppl 1)S38–S43. [PubMed] [Google Scholar]
- 23.Vickerman P, Watts C, Alary M.et al Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex Transm Infect 200379363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hook E W, 3rd, Spitters C, Reichart C A.et al se of cell culture and a rapid diagnostic assay for Chlamydia trachomatis screening. JAMA 1994272867–870. [PubMed] [Google Scholar]
- 25.Rani R, Corbitt G, Killough R.et al Is there any role for rapid tests for Chlamydia trachomatis? Int J STD AIDS 20021322–24. [DOI] [PubMed] [Google Scholar]