Abstract
Unlike properly folded and assembled proteins, most misfolded and incompletely assembled proteins are retained in the endoplasmic reticulum of mammalian cells and degraded without transport to the Golgi complex. To analyze the mechanisms underlying this unique sorting process and its fidelity, the fate of C-terminally truncated fragments of influenza hemagglutinin was determined. An assortment of different fragments was generated by adding puromycin at low concentrations to influenza virus-infected tissue culture cells. Of the fragments generated, <2% was secreted, indicating that the system for detecting defects in newly synthesized proteins is quite stringent. The majority of secreted species corresponded to folding domains within the viral spike glycoprotein. The retained fragments acquired a partially folded structure with intrachain disulfide bonds and conformation-dependent antigenic epitopes. They associated with two lectin-like endoplasmic reticulum chaperones (calnexin and calreticulin) but not BiP/GRP78. Inhibition of the association with calnexin and calreticulin by the addition of castanospermine significantly increased fragment secretion. However, it also caused association with BiP/GRP78. These results indicated that the association with calnexin and calreticulin was involved in retaining the fragments. They also suggested that BiP/GRP78 could serve as a backup for calnexin and calreticulin in retaining the fragments. In summary, the results showed that the quality control system in the secretory pathway was efficient and sensitive to folding defects, and that it involved multiple interactions with endoplasmic reticulum chaperones.
INTRODUCTION
Transport of newly synthesized proteins from the endoplasmic reticulum (ER) to the Golgi complex and beyond is strictly regulated (Pfeffer and Rothman, 1987; Lodish, 1988; Rose and Doms, 1988; Hurtley and Helenius, 1989; Klausner, 1989). As a rule these proteins, soluble or membrane-bound, are transported only when they have acquired a fully folded, native conformation. Typically, misfolded proteins, folding intermediates, unassembled subunits, and incompletely assembled oligomers remain in the ER. If they fail to reach the proper conformation they eventually undergo degradation without reaching the Golgi complex. By separating native from nonnative proteins, this conformation-based sorting process guarantees the deployment of properly folded proteins and regulates protein expression post-translationally. It has been called quality control and architectural editing (Hurtley and Helenius, 1989; Klausner, 1989).
Results obtained after expression of recombinant and wild-type proteins in a variety of cell systems suggest that quality control can be quite stringent (see Hammond and Helenius, 1995). It is evident that relatively minor defects can lead to retention. Mere aggregation of misfolded proteins into large covalently or noncovalently cross-linked aggregates may confine misfolded products to the ER (Hurtley and Helenius, 1989; Tooze et al., 1989). In other cases, the persistence of chaperone binding may result in protein retention (Gething and Sambrook, 1992). Furthermore, it has been shown that exposure of free sulfhydryl groups may result in permanent ER confinement (Sitia et al., 1990; Guenzi et al., 1994). It is likely that no single molecular mechanism is responsible for discriminating between proteins of different conformation and preventing proteins from entering the secretory pathway.
In this study, we address the overall properties of quality control by following the fate of C-terminally truncated fragments of influenza hemagglutinin (HA), a viral spike glycoprotein. HA is a well-characterized, homotrimeric type I membrane protein (Wiley and Skehel, 1987). Each subunit (84 kDa) has six intrachain disulfide bonds and seven N-linked glycans. They fold and assemble in the ER or/and intermediate compartment, and each subunit is cleaved late in the secretory pathway into a N-terminal (HA1) and a C-terminal (HA2) fragment. The crystal structure of the ectodomain shows that each subunit consists of two domains: a globular top domain formed by HA1, and a stem domain formed by HA2 plus N- and C-terminal portions of HA1. This cleaved HA structure is metastable. It will undergo a significant rearrangement under acidic pH or mild denaturing conditions (Carr and Kim, 1993; Bullough et al., 1994). Without HA1, HA2 alone can fold into its acidic form (Chen et al., 1995a). When misfolded because of mutations, inhibition of glycosylation, or side reactions during normal folding, HA is retained in the ER of the cell and slowly degraded (Hurtley et al., 1989). During normal folding, it interacts with two lectin-like chaperones, calnexin (CNX) and calreticulin (CRT), which promote efficient folding and homotrimer formation (Hebert et al., 1996). Binding to BiP/GRP78, an abundant HSP70 homologue in the ER, is not observed unless misfolding occurs (Hurtley et al., 1989).
We generated a large panel of randomly truncated N-terminal fragments of HA in living cells using puromycin, a protein synthesis inhibitor. The fate of these fragments was determined and their folding status and interactions with molecular chaperones were analyzed. Only a small fraction of the fragments was secreted. The majority of the generated fragments was retained in the ER in association with CNX and CRT. The results revealed that quality control was stringent in retaining the fragments in the ER, that molecular chaperones played a central role, and that redundant mechanisms were involved.
MATERIALS AND METHODS
Reagents, Cell, Virus
Promix 35S-labeled cysteine and methionine mixture was purchased from Amersham Corp. (Arlington Heights, IL). CHAPS was purchased from Pierce (Rockford, IL). PNGase F was purchased from Boehringer-Mannheim (Indianapolis, IN). Other chemicals and enzymes were purchased from Sigma (St. Louis, MO). Chinese hamster ovary (CHO) cells were cultured in αMEM with 8% fetal calf serum, 1 U/ml penicillin, and 1 μg/ml streptomycin. The X31/A/Aichi/1968 strain of influenza virus was prepared as described previously (Doxsey et al., 1985).
The rabbit polyclonal anti-HA antiserum was raised against a peptide of the 12 N-terminal residues of HA (Chen et al., 1995b). Mouse monoclonal antibodies against various epitopes were harvested from hybridoma cell lines provided by Dr. J. Skehel (Medical Research Council, London, United Kingdom). The polyclonal rabbit anti-CNX antiserum were raised against the C-terminal 19 amino acids deduced from canine CNX sequence (Wada et al., 1991). Polyclonal rabbit anti-CRT antibodies was purchased from Affinity Bioreagents (Neshanic Station, NJ). The rabbit anti-BiP/GRP78 antibodies were gifts from Dr. H.-D. Söling (Georg-August Universität, Göttingen, Germany).
Preparation of Anti-Puromycin Antibodies
Immunogen used in generating antibodies against puromycin was produced by covalently attaching puromycin to keyhole limpet hemocyanin (KLH). Final reaction conditions were 5 mg/ml KLH (determined according to the method of Bradford, with bovine serum albumin used as a protein standard), 2–5 mM puromycin, 200 mM sodium phosphate (pH 7–7.5), 100 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), and 5 mM N-hydroxysulfosuccinimide. The reaction was initiated by the addition of EDC as a solid, and allowed to proceed at 22°C, with progress of the reaction monitored by the appearance of turbidity. After 15 min, before any visible turbidity, the volume of one-half the reaction was brought to 50 mM with Tris-HCl (pH 7.5), and the second half was treated identically after 35 min, when it was fully turbid. Both halves were exhaustively dialyzed against 10 mM potassium phosphate (pH 7.4), 144 mM NaCl. The dialysates, including a large amount of precipitated material, were mixed together and then emulsified with 1.2 volumes of Freund’s complete adjuvants by repeated passage between connected syringes. Rabbits were injected with a quantity of this emulsion containing approximately 1 mg of KLH. Boosting injections were given every 2–3 wk with a similar amount of the dialyzed reaction mixtures emulsified with Freund’s incomplete adjuvant.
The reaction conditions described above generated a peptide bond between the sole primary amino group of puromycin and carboxyl groups within KLH, and therefore produced a connection closely resembling that formed when translation reacts the same amino group with the carboxyl terminus of a nascent polypeptide. Because the molecular mass of KLH was 3000 to 7500 kDa, between 500 and 700 mol of puromycin were incorporated per mol of KLH, as assessed by disappearance of puromycin from a trichloroacetic acid-soluble form during the reaction. The antibodies recognized the purine ring structure of puromycin.
Viral Infection and Metabolic Labeling
Dishes (6 cm) of nearly confluent CHO cells were infected with influenza virus X31 as described (Braakman et al., 1991). Five to 6 hours after infection, cells were washed with phosphate-buffered saline and starved with Cys/Met-free medium for 30 min. For experiments using tunicamycin (TM) or castanospermine, the drugs were added during the starvation period. The cells were then pulsed for 1 h with 200 μCi/dish in Cys/Met-free medium in the presence of indicated puromycin concentrations. The chase was started by removing pulse medium and adding αMEM containing 500 μM cycloheximide, 10 μg/ml each of chymostatin, leupeptin, antipain and pepstatin (CLAP) to inhibit proteolysis, and an additional unlabeled 5 mM Cys/5 mM Met. During the pulse and chase period, dishes were rocked in a 37°C incubator. At the end of the chase, the medium was collected and mixed with N-ethylmaleimide (NEM) and phenylmethylsulfonyl fluoride (PMSF) to final concentrations of 20 mM and 1 mM, respectively.
Lysis, Immunoprecipitation, and SDS-PAGE
The cells were lysed with 0.5% Triton X-100 in MNT buffer (20 mM MES, 100 mM NaCl, and 30 mM Tris-HCl, pH 7.5) containing 10 μg/ml CLAP, 1 mM PMSF, 20 mM NEM, and 1 mM EDTA. For experiments involving coprecipitation with CNX, CRT, and BiP/GRP78, cells were lysed in 2% CHAPS in HBS buffer (10 mM HEPES, 40 mM NaCl, pH 7.6) containing 10 μg/ml CLAP, 1 mM PMSF, 20 mM NEM, and 50 U apyrase.
Postnuclear supernatants prepared by centrifugation at 16,000 × g for 5 min were incubated for 2 h with antibodies in the presence of protein A-Sepharose beads under continuous shaking. The immunoprecipitates containing mouse monoclonal antibodies were washed twice with wash buffer B (1 mM EDTA, 300 mM NaCl, 50 mM Tris-HCl, pH 8.0) for 10 min at room temperature. The immunoprecipitates containing antibodies against CNX, CRT, and BiP/GRP78 were washed twice with 0.2% CHAPS/HBS (40 mM NaCl, 10 mM HEPES) for 10 min at 4°C. For the second precipitation, the precipitates from the first precipitation were redissolved in 1% SDS/HBS and diluted with 20 volumes of 0.5% Triton X-100/MNT. The Sepharose beads were removed by centrifugation and the new antibodies and protein A-Sepharose were added and incubated overnight. The second precipitates were then resuspended in SDS-containing sample buffer, boiled for 5 min before loading on SDS-PAGE. One and two-dimensional SDS-PAGE was performed as described (Braakman et al., 1991; Chen et al., 1995b).
PNGase F and Endo H Digestion
For PNGase F digestion, immunoprecipitates were resuspended in 0.2% SDS in 50 mM NaH2PO4/Na2HPO4 and 25 mM EDTA, pH 6.8, and boiled for 5 min. The samples were then cooled on ice and an equal volume of 2% Triton X-100 in 50 mM NaH2PO4/Na2HPO4 and 25 mM EDTA, pH 6.8, was added. Samples were then incubated with 0.2 U of PNGase for 1 h at 37°C.
For Endo H digestion, immunocomplexes were resuspended in 0.2% SDS in 100 mM sodium acetate (pH 5.5) and boiled for 5 min. The equal volume of 100 mM sodium acetate (pH 5.5) was then added. Endo H (0.2 U) was added to each sample and incubated for 16 h at 37°C.
RESULTS
Generating C-Terminally Truncated HA Molecules
To produce C-terminally truncated HA molecules, we took advantage of puromycin, a protein synthesis inhibitor that causes premature chain termination. It mimics amino acyl-tRNA by attaching covalently to the C-terminal amino acid (Nathans, 1964). The nascent chains are dissociated from the ribosome. In the case of membrane-bound ribosomes in the ER, they are released into the lumen (Redman and Sabatini, 1966). The addition of puromycin thus allows interruption of elongation and the production of C-terminally truncated proteins translocated into the ER.
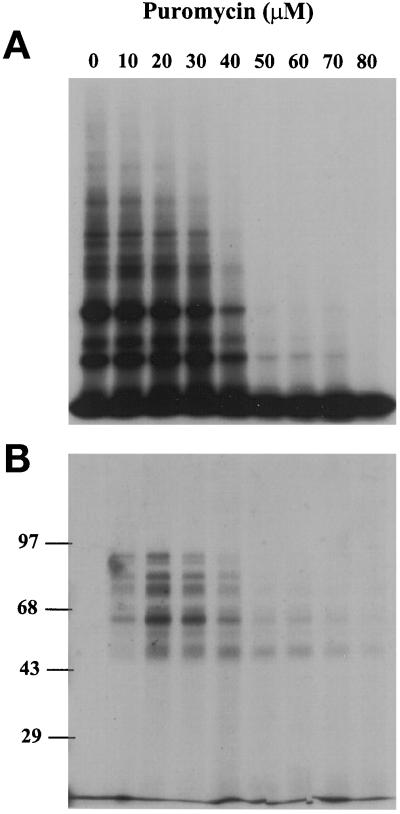
When CHO cells were labeled with 35S-Met/Cys in the presence of increasing concentrations of puromycin, protein synthesis was inhibited. At intermediate concentrations, the synthesis of larger proteins was clearly decreased (Figure 1A). When puromycin was added to influenza infected CHO cells, HA fragments were produced as indicated by double immunoprecipitation of the cell lysates using polyclonal antibodies against the N-terminal 12 amino acids of HA and antibodies against puromycin (Figure 1B). In this procedure, the cell lysates were precipitated first with antibodies against HA, the precipitate was then washed and redissolved in 1% SDS/HBS and diluted with 20 volumes of 0.5% Triton X-100/MNT. The diluted solutions were then precipitated with puromycin antibodies. Since the two antibodies recognize opposite ends of polypeptide fragments, the double precipitation protocol ensured that full-length HA and fragments generated by proteolysis were excluded. When no puromycin was added to the cell, no protein was precipitated (lane 1 of Figure 1B and Figure 2D).
Figure 1.
Puromycin caused protein synthesis inhibition (A) and the production of N-terminal fragments of HA (B). CHO cells were labeled for 1 h with 200 μCi 35S-Promix per 6-cm dish in the presence of different puromycin concentrations. The cells were lysed with 0.1% Triton X-100/MNT. The lysates were either directly subjected to reducing SDS-PAGE and fluorography (A) or double immunoprecipitated with antibodies against N-terminal peptide of HA and antibodies against puromycin before SDS-PAGE (B).
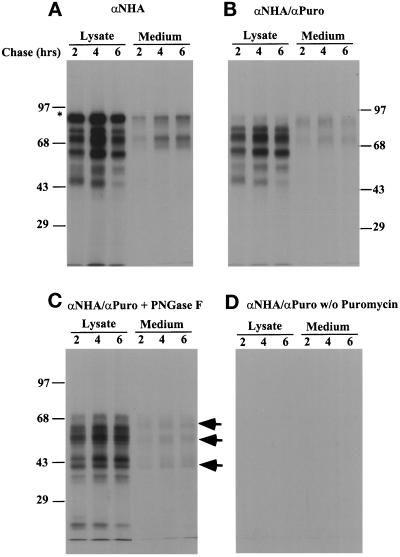
Figure 2.
Secretion and retention of N-terminal fragments of HA. CHO cells were labeled as in Figure 1 in the absence (D) or presence of 30 μM puromycin (A–C), and then chased for 2, 4, and 6 h in the presence of protease inhibitors. After the chase, cell lysates and the medium were collected and immunoprecipitated with HA antibodies (A) or HA antibodies followed by puromycin antibodies (B, C, and D). The precipitated proteins were digested with PNGase F at 37°C for 1 h to remove oligosaccharides (C). At each chase point, the lysate and medium were derived from the same cells.
The amount of C-terminally truncated HA fragments generated during 1-h labeling period peaked at concentrations of 20–40 μM puromycin (Figure 1B). The apparent molecular masses of the major fragments observed ranged from 45 kDa to nearly the full-length protein (84 kDa).
The truncated N-terminal HA fragments did not represent a collection of polypeptides continuously decreasing size. Instead, a series of bands was seen. This uneven size distribution was caused in part by gaps introduced by the cotranslational addition of N-linked oligosaccharides (Chen et al., 1995b). Since the uneven distribution was still observed after removal of oligosaccharides by treatment with PNGase F (Figure 2C), it was also likely to arise from uneven elongation rate of the nascent polypeptide chain. Because of specific secondary structure elements in the mRNA (Wolin and Walter, 1988) or rarely used codons (Varenne et al., 1984), ribosomes will pause and stack up at certain sites, which in turn provides enrichment of some nascent chain species. Alternatively, some fragments may have been particularly sensitive to degradation and therefore selectively eliminated. Nevertheless, a large collection of HA fragments of different sizes was generated and could be used to analyze the quality control system.
A Small Fraction of Truncated HA Fragments Is Secreted
HA is a type I membrane glycoprotein. It has a glycosylated N-terminal ectodomain (514 amino acid residues), a 26-amino acid transmembrane domain and a 10-residue cytosolic tail. When synthesized and translocated into the ER, the ectodomain begins to fold cotranslationally (Chen et al., 1995b). Folding continues after chain termination, and six intrachain disulfide bonds are formed (Braakman et al., 1991). Once the chains are fully oxidized they assemble into homotrimers that are transported via the Golgi complex to the cell surface (Braakman et al., 1991; Tatu et al., 1993; Tatu et al., 1995). The folding efficiency of wild-type HA is >90% and the majority of folded HA is efficiently transported to the cell surface (Copeland et al., 1986).
To determine whether any of the puromycin-generated fragments were secreted, infected cells were labeled for 1 h in the presence of 30 μM puromycin and chased for 2, 4, and 6 h. The chase medium and cell lysates were then analyzed for N-terminal HA fragments by immunoprecipitation with antibody against N-terminal HA peptide alone or by double immunoprecipitation as described above. The anti-HA antibody precipitations (Figure 2A) showed that some of the labeled full-length HA (marked with an asterisk in this and subsequent Figures) and labeled HA fragments were present in the cells as well as in the medium. No fragment was observed in the medium during the 1-h pulse period. Within the cells, they remained at a rather constant level during the 6-h chase period. The amount of fragments in the medium was low, indicating that the majority was not released by the cells.
When double precipitation with antibodies against the N-terminal peptide and C-terminally attached puromycin was performed, similar patterns of HA fragments were observed except that the full-length protein was not seen (Figure 2B). If the puromycin was omitted, no fragments were detected in the lysate or in the medium (Figure 2D). It was clear that some HA fragments produced in the ER by puromycin action indeed were secreted. When quantified by densitometry, the amount of N-terminal fragments in the medium was 0.5 to 2% of total. We concluded that the vast majority of N-terminal HA fragments was retained in the cell and they were stable for 6 h or more.
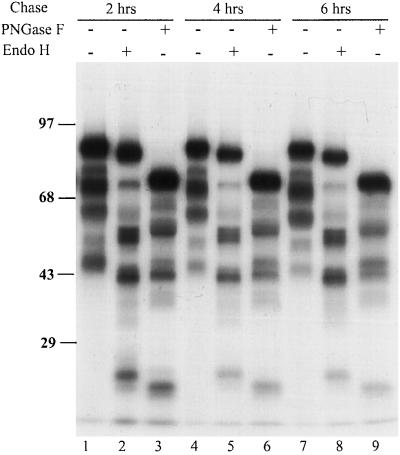
The N-terminal fragments in the cell and medium were derived from the ER as indicated by their sensitivity to PNGase F and Endo H, which remove N-linked oligosaccharides added in the ER. When digested with PNGase F, the molecular mass of all fragments decreased due to the removal of the oligosaccharides (Figure 2C, Figure 3). The apparent molecular mass of the smallest retained fragment observed was reduced from 45 to 22 kDa, consistent with the presence of multiple glycosylation sites in the extreme N-terminal part of HA. Furthermore, the fragments in the cell were all sensitive to Endo H as indicated by the increase in the mobility of the fragments (Figure 3). This indicated that the fragments were properly translocated into the ER and the majority of the cell-associated fragments was still in a pre-Golgi compartment. The secreted fragments were Endo H resistant (our unpublished results), indicating that they were secreted through the regular secretory pathway via the Golgi complex.
Figure 3.
Endo H and PNGase F digestion of HA fragments generated from addition of puromycin. Influenza-infected CHO cells were labeled as in Figure 1 in presence of 30 μM puromycin, and then chased for 2, 4, and 6 h in the presence of protease inhibitors. After the chase, cell lysates were collected and immunoprecipitated with HA antibodies. The precipitated proteins were digested with PNGase F at 37°C for 1 h to remove oligosaccharides or Endo H at 37°C overnight. The top band of each lane represents the full-length HA.
The low level of secretion of HA fragments was unlikely to arise from the attachment of puromycin. Puromycin, as previously shown on apoprotein B, does not affect the secretion of proteins or peptidyl puromycin (Siuta-Mangano and Lane, 1981). The low level of secretion observed here demonstrated that the fragments were efficiently retained in the cells by stringent quality control. The lack of terminal endoglycosidase H resistance indicated that the quality control occurred at the level of the ER.
A Subset of Fragments Are Secreted
Whereas many different bands were present inside cells, only three fuzzy bands could be discerned from samples of the medium fraction after deglycosylation, which eliminated the interference of oligosaccharide on the mobility of the protein fragments (Figure 2C). The largest had a mobility slightly shorter than full-length HA, approximating the size of the so-called “anchor-free HA,” a truncated molecule missing the transmembrane domain and cytosolic tail (∼60 kDa after deglycosylation). It appeared to be secreted efficiently. The smallest secreted fragment had a molecular mass of around 40 kDa after deglycosylation. Thus, it corresponded in size to the HA1 subunit of mature, proteolytically cleaved HA. The intermediate size fragment had a molecular mass of approximately 55 kDa after deglycosylation, indicating that it contained about 130 amino acids from HA2 in addition to HA1. Secretion of the two smallest fragments, however, was apparently not very efficient because most of them remained in the cells.
The selective secretion of the fragments suggested that quality control operates at the level of folding domains. The largest secreted fragment clearly contained all sequence necessary for generating the entire ectodomain. Its recombinant versions (anchor-free HA) have previously been shown to be secretion-competent when expressed in cells (Gething and Sambrook, 1982; Singh et al., 1990). The smallest fragment contained the sequence of the top domain of HA. The intermediate secreted fragment contained HA1 plus about 130 amino acids. It contained the majority of the sequence in the stem domain of HA2 including the long helix, but it lacked the small subdomain of HA2 present at the base of the spike. The secretion of these particular fragments, therefore, implied that the likelihood of a truncated molecule being secreted increases if truncation occurs between domains rather than within a domain.
HA Fragments Acquire Disulfide Bonds
Two-dimensional SDS-PAGE and immunoprecipitation with conformation specific anti-HA antibodies were used to analyze the oxidation and folding status of the various fragments.
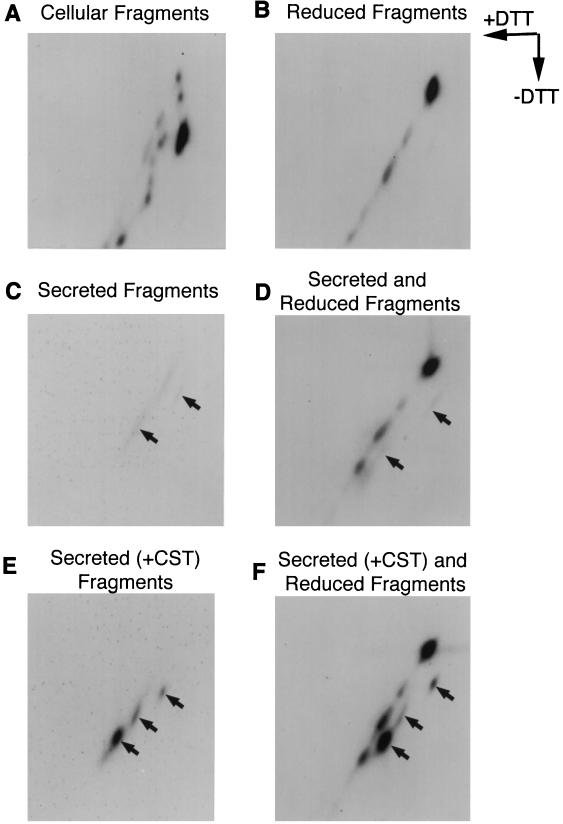
We have previously developed a two-dimensional SDS-PAGE system to analyze the formation of disulfide bonds in growing chains of HA (Chen et al., 1995b). Radioactively labeled immunoprecipitated samples are first subjected to SDS-PAGE separation without reduction. They are then reduced and separated in a second dimension according to molecular weight. Proteins without disulfide bonds will have the same mobility in the first and second dimensions and run on the diagonal. Proteins that participate in intermolecular disulfide bonding run above the diagonal due to lower mobility in the first dimension. In contrast, proteins with intramolecular disulfide bonds usually run below the diagonal due to higher mobility in the first dimension as the disulfide cross-links render the SDS-protein complexes more compact than their fully reduced counterparts.
As shown in Figure 4A, the majority of C-terminal HA fragments retained in the cells ran below the diagonal. When reduced before analysis, the fragments all were recovered on the diagonal as expected (Figure 4B). The results indicated that the HA fragments did acquire intrachain disulfide bonds. The lack of labeled material above the diagonal indicated the fragments were not disulfide cross-linked to each other or to other proteins in the ER.
Figure 4.
Two-dimensional SDS-PAGE of HA fragments. Influenza infected cells were labeled for 1 h in the presence of 30 μM puromycin with (A) or without (B) addition of 1 mM DTT. The cells were lysed in 0.5% Triton X-100/MNT and the HA fragments were precipitated with antibodies against the HA N-terminal peptide. After a 2-h chase in the absence (lane C) and presence (lane E) of 1 mM CST, the secreted fragments were also prepared by precipitating with the antibodies against the HA N-terminal peptide. The precipitates were resuspended in nonreducing sample buffer and then subjected to two-dimensional SDS-PAGE directly (lanes A–C, and E). In lanes D and F, the secreted fragments were mixed with the reduced samples used in lane B. This was done to mark the diagonal of the gel.
The fragments in the medium were also analyzed, although the amount of label was quite low. The fragments recovered from the medium 2 h after a 1-h pulse of 35S-Met/Cys are shown in Figure 4C. The three bands are separated into defined streaks. That they run below the diagonal is better seen in Figure 4D, where the sample was mixed with a completely reduced sample from Figure 4A to mark the position of the diagonal. To increase the signal, we repeated the experiment using castanospermine to increase the amount of fragment secretion (see below). The three fragments and their position below the diagonal is clearly visible in Figure 4E (medium fragments alone) and Figure 4F (medium fragments mixed with reduced intracellular fragments from Figure 4A).
We concluded from the two-dimensional analysis that the secreted fragments are oxidized with no traces of interchain disulfide bond and few unoxidized forms. Most of the fragments retained in the cells are also oxidized extensively, and they are indistinguishable from the secreted forms in their electrophoretic mobility.
Antigenic Properties of Retained HA Fragments
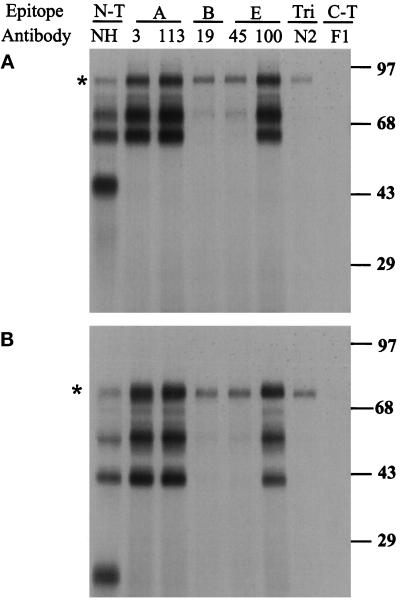
To determine whether the retained fragments shared conformational epitopes with known conformers of HA, the fragments were precipitated by conformation-specific HA monoclonal and polyclonal antibodies. Monoclonal H3 and HC113 are specific for epitope A, HC19 for epitope B, and HC45 and HC100 for epitope E, all in the top domain (Daniels et al., 1983; Daniels et al., 1984). N2 is a well-characterized epitope B antibody that only reacts with folded HA homotrimers in their neutral pH conformation (Copeland et al., 1986).
When the puromycin fragments of HA were produced in the presence of DTT to prevent formation of disulfide bonds (Braakman et al., 1992), they did not react with any of these conformation-specific antibodies (our unpublished results). When HA fragments were produced under conditions that allowed oxidation, some of the antibodies did precipitate fragments (Figure 5). The antibodies to the A epitope recognized all the fragments that were 40 kDa or larger after oligosaccharide removal. The fragment of 22 kDa after glycan removal, although oxidized (see Figure 5B), was not precipitated. HC100, a monoclonal antibody against the E epitope, appeared to recognize a set of fragments similar to the A epitope-specific antibodies, but the second E epitope-specific antibody HC45 only precipitated the full-length HA and one fragment, as did the B epitope-specific antibody HC19 (Figure 5). The trimer-specific N2 antibody precipitated the full-length HA only, indicating the fragments did not trimerize. None of the secreted fragments reacted with N2 antibody, consistent with previous studies that the anchor-free HA did not trimerize (Singh et al., 1990).
Figure 5.
Interaction of HA fragments with conformation-specific antibodies. The HA fragments produced after 1 h of labeling in the presence of 30 μM puromycin were precipitated with the specified antibodies, and subjected to SDS-PAGE before (A) or after (B) PNGase F digestion. The antibodies included NH and F1 (specific to the N and C terminus), HC3 and HC113 specific for epitope A, HC19 specific to epitope B, HC45 and HC100 specific to epitope E, and N2 specific for native trimers. Asterisk marks position of full-length HA.
In contrast, a monoclonal antibody (F1) against an epitope in the stem domain transiently expressed during HA folding (Braakman et al., 1991; Chen et al., 1995b), did not precipitate any of the fragments. This indicated that although the fragments displayed some of the normal conformational epitopes their conformations did not correspond to major intermediates during HA maturation. Since none of the fragments was precipitated with trimer-specific antibody, they did not form correctly assembled trimers.
Taken together, the results indicated that the retained HA fragments were extensively oxidized, and exhibited some antigenic properties in common with intact properly folded HA. They were, however, trapped in off-pathway conformations, which may explain their retention.
CNX and CRT Are Involved in the Retention of HA Fragments
The failure to detect any intermolecular disulfide bonds between HA fragments or between HA and other ER proteins, indicated that the formation of transient intermolecular disulfides, observed for Ig (Fra et al., 1993; Guenzi et al., 1994), was not involved in the retention. To determine whether molecular chaperones were involved, we performed coimmunoprecipitation studies with antibodies to CNX, CRT, and BiP/GRP78. CNX and CRT are lectin-like chaperones that interact with HA during cotranslational and posttranslational folding (Hammond et al., 1994; Chen et al., 1995b; Peterson et al., 1995; Hebert et al., 1996). BiP/GRP78 interacts with nonglycosylated, misfolded forms of HA (Hurtley et al., 1989).
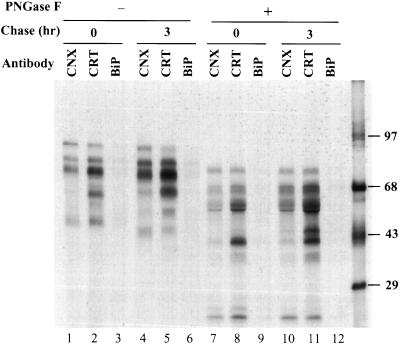
The 35S-labeled fragments produced in the presence of puromycin were precipitated immediately after a pulse or after a 3-h chase, with antibodies against CNX, CRT, and BiP/GRP78 followed by a sequential precipitation with HA N-terminal antibodies. The precipitates were subjected to SDS-PAGE. As shown in Figure 6, BiP/GRP78 did not display any binding to HA fragments. However, both CNX and CRT did associate, and the association persisted for 3 h of chase. A larger fraction of the fragments associated with CRT than with CNX. Although fragments of all different sizes coprecipitated with CRT, CNX showed a preference for the larger ones. A fragment with a molecular mass of around 37 kDa, corresponding to the size that contained most of the HA1 subunit, did not associate with CNX but bound efficiently to CRT, whereas fragments as small as 22 kDa (Figure 6, lanes 7 and 8, or 10 and 11) bound well to both chaperones. We estimated that 40% or more of the total HA fragment was associated with either CNX or CRT (Figure 7A, lanes 1–3).
Figure 6.
Association of HA fragments with CNX and CRT. The HA fragments in the cell immediately after the pulse or after a 3-h chase were first precipitated with antibodies against CNX, CRT, or BiP and then with HA antibodies. Half of the precipitated proteins were treated with PNGase F before SDS-PAGE.
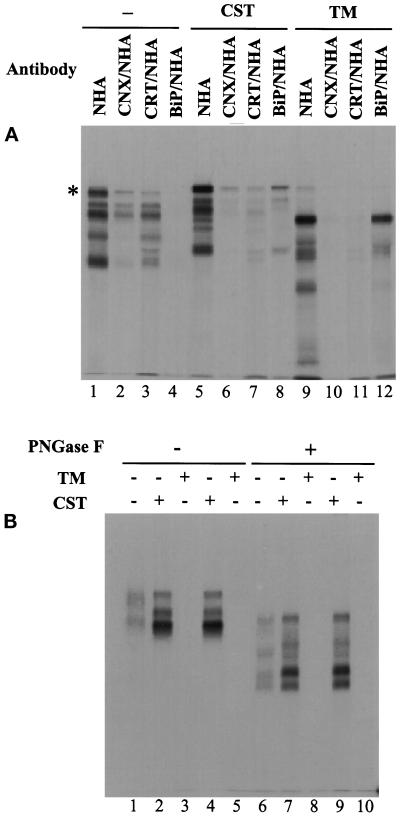
Figure 7.
Effect of CST and TM on association of HA fragments with CNX, CRT and BiP/GRP78 (A); CST increase in secretion of HA fragments (B). The HA fragments were generated as described in previous Figures but in the presence of 1 mM CST or 5 μg/ml TM. (A) The fragments were precipitated with HA antibodies immediately after a 1-h pulse. (B) The secreted fragments were precipitated from the 2-h chase medium after 1 h pulse in the absence (lanes 1 and 6) or presence (lanes 2, 4, 7 and 9) of CST or TM (lanes 3, 5, 8 and 10). When CST or TM was added during the pulse, the chase medium contained either CST (lanes 4 and 9) or TM (lanes 5 and 10) or neither (the other lanes).
Castanospermine Causes Increased Fragment Secretion
CNX and CRT have been shown previously to participate in the retention of misfolded glycoproteins, folding intermediates and unassembled oligomeric proteins in the ER (Ou et al., 1993; Hammond and Helenius, 1994b; Rajagopalan et al., 1994). To block binding of substrate glycoproteins to the chaperones, castanospermine (CST), an α-glucosidase inhibitor can be used (Hammond et al., 1994; Hebert et al., 1995; Peterson et al., 1995). It blocks trimming of the core oligosaccharides, thus preventing the formation of the monoglucosylated N-linked oligosaccharides needed for attachment to the two lectins. It does not, however, completely impair HA folding (Hammond et al., 1994; Chen et al., 1995b; Hebert et al., 1995).
When CST was present during the pulse labeling period, association of puromycin-generated fragments with CNX and CRT was considerably decreased (Figure 7A, lanes 2, 3, 6, and 7), and the secretion of fragments was significantly increased (Figure 7B). Compared with the control experiments in which secretion was about 2%, about 10% of the generated fragments was released to the medium. The same result was seen whether CST was present during the pulse and chase (lane 4), or only during the pulse (Figure 7B, lane 2). In contrast, addition of an N-glycosylation inhibitor, TM, inhibited secretion completely (lanes 3 and 5).
Taken together, the results indicated that binding of glycosylated fragments to CNX and CRT was involved in retention of fragments in the cell. CNX and CRT apparently serve as retention factors for at least some of the N-terminal HA fragments.
BiP/GRP78 as a Backup in Fragment Retention
Since the majority of fragments still failed to be secreted in CST-treated cells, it seemed likely that other factors prevented them from leaving the cell. We tested whether BiP/GRP78, another ER chaperone, might be involved. BiP/GRP78 is known to associate with many proteins retained in the ER including VSV G protein during folding (Gething and Sambrook, 1992; Hammond and Helenius, 1995). Although we have no indication that BiP/GRP78 binds to HA during normal folding or to puromycin-induced fragments in the absence of CST (Figure 7A, lane 4), it was possible that it could bind when the proteins were not associated with CNX and CRT.
When double precipitations were performed using anti-BiP/GRP78 and anti-HA N-terminal antibodies using lysates obtained from cells treated with CST and puromycin, evidence of BiP/GRP78 binding could be observed (Figure 7A, lane 8). Some of the fragments and the full-length HA molecules were precipitated with anti-BiP/GRP78 under conditions of ATP depletion by adding apyrase to cell lysates.
This showed that when CNX and CRT did not interact with full-length HA or with N-terminal HA fragments, BiP/GRP78 could bind. The access of BiP/GRP78 to suitable peptide moieties may normally be blocked by the binding of the lectin-like chaperones. Evidently, the binding of BiP/GRP78 contributed to retention of nontrimmed fragments in the ER. In essence, it seemed to serve as back-up retention system for HA fragments. However, since the amount of BiP/GRP78-bound fragments was not high enough to explain the entire retention observed, other mechanisms may be involved.
DISCUSSION
Sorting of newly synthesized proteins is an important and ubiquitous activity in the living cell. It is necessary for the establishment and maintenance of compartments and membranes with different composition and function, and for the selective secretion of proteins. Typically, molecular sorting relies on signals displayed by the proteins themselves. These are usually sequence motifs that serve as signals for targeting to the ER, to mitochondria, to chloroplasts, and to the nucleus (see Gorlich and Mattaj, 1996; Schatz and Dobberstein, 1996). Mannose-6-phosphate groups serve as signals for protein targeting to lysosomes (Griffiths et al., 1988), and KDEL and KKxK signals are involved in the retrieval of resident ER proteins from the Golgi complex (Nilsson et al., 1989; Pelham, 1989). Each type of signal is recognized by receptors that are, in turn, coupled to the machinery needed for relocation or selective retention.
The quality control process, which oversees the transport of proteins from the ER, is different in that signal sequences are not likely to be involved. Thousands of soluble and membrane-bound proteins undergo quality control without identifiable signal peptides or primary sequence homologies. Instead, the system senses general structural differences between native and nonnative proteins. In some cases, minor defects detected by the ER quality control system actually lead to human diseases. The mutant ΔF508 of cystic fibrosis transmembrane regulator CFTR expressed in a majority of cystic fibrosis patients, and the PiZ α1-antitrypsin expressed in some patients with α1-antitrypsin deficiency are examples (Cheng et al., 1990; Sifers et al., 1992). Although these mutant proteins reach a functional conformation, they remain trapped in the ER and bound to chaperones. Exposed hydrophobic peptides and free sulfhydryl groups have also been implicated in the retention of proteins (Sitia et al., 1990; Gething and Sambrook, 1992). The molecular criteria for sorting, however, remain poorly understood.
By analyzing random C-terminal truncations of HA, we were able to address some of the general properties of quality control. The results demonstrated that retention can be very stringent. No more than 2% of truncated HA fragments were secreted. Transport of full-length HA is, in contrast, close to 90% efficient (Copeland et al., 1986). The vast majority of truncated HA molecules was apparently unable to acquire a conformation that fulfilled the structural requirements for export to the extracellular medium. These fragments accumulated in the ER.
Analysis of the three fragments that were secreted indicated that they were extensively folded and contained intrachain disulfide bonds. The molecular weights after removal of the N-linked carbohydrates revealed that they corresponded to molecules truncated roughly in the interface between known folding domains. The largest fragments corresponded to the HA ectodomain devoid of transmembrane and cytoplasmic tail sequences. Their secretion competency could be anticipated from studies using recombinant anchor-free HA (Gething et al., 1986; Singh et al., 1990). Although they fail to trimerize, anchor-free HA ectodomains are known to fold correctly and to be efficiently secreted. The efficient secretion of the puromycin-attached ectodomain observed here, demonstrated that the covalently associated puromycin molecule at the C terminus was tolerated by the quality control system, as observed for apoprotein B (Siuta-Mangano and Lane, 1981). This means that the low efficiency of fragment secretion was not simply due to the presence of puromycin at the C terminus.
Fragments equivalent to the HA1 subunit of intact mature proteolytically activated HA were also transported. The top of the HA monomer comprises an independently folded domain that includes about two-thirds of HA1 (Wiley and Skehel, 1987). HA1 can be released in folded form from mature HA by reduction of a disulfide bond (Graves et al., 1983). The third secreted fragment appeared to contain the sequences for the HA domain and the portion of HA2 that forms the actual stem domain. It lacked a terminal globular region of the stem domain located close to the membrane. This globular structure could be viewed as a separate domain. The selective secretion of these fragments supported the notion that fragments truncated in interfaces between folding domains stand a better chance of reaching transport-competent conformations than molecules truncated in the middle of a domain. From the point of view of protein structure, this is understandable because folding domains have many of the structural features and compact folding properties of native proteins (Richards, 1977).
Apparently, the quality control machinery distinguishes between fragments that correspond to folded domains and fragments that do not. If this turns out to apply generally, cells may be used to investigate the domain structure of proteins for which structural information is not available. Random fragments can be generated using puromycin or by other means, and the secreted fragments identified. The same strategy may also be used to define secretion-competent fragments of membrane and secretory proteins. Such fragments are often needed for X-ray crystallography and biochemical analysis.
Most of the fragments that were retained in the ER were extensively folded, judging by the presence of disulfide bonds and antigenic epitopes. Unlike misfolded proteins generated by TM treatment and amino acid analogs, they were not aggregated nor did they undergo rapid degradation. There was nothing obviously wrong with their overall properties to distinguish them from the secretion competent fragments. The reason for the lack of transport was not that they were unable to trimerize, because in contrast to full-length HA, trimerization is not a prerequisite for transport of anchor-free HA to the extracellular space (Singh et al., 1990).
Many of the retained HA fragments were associated with two lectin-like molecular chaperones, CNX and CRT. These are homologues that bind to folding intermediates and misfolded glycoproteins that carry monoglucosylated N-linked oligosaccharides (for reviews, see Hammond and Helenius, 1993; Bergeron et al., 1994; Helenius et al., 1997). The monoglucosylated sugar moieties are generated from the core oligosaccharides by the action of two trimming enzymes, glucosidase I and II. They are also formed by reglucosylation of glucose-free high mannose glycans by an enzyme called UDP-glucose: glycoprotein glucosyltransferase (Trombetta and Parodi, 1992). The latter enzyme only uses incompletely folded glycoproteins as a substrate and is therefore thought to serve as a folding sensor in the CNX/CRT cycle (Sousa et al., 1992).
Like other molecular chaperones, CNX and CRT are found to bind to nonnative proteins, but they do so in a very different way. Recent studies with RNase B suggest that they bind to any protein, whether folded or not, that carries monoglucosylated glycans (Rodan et al., 1996; Zapun et al., 1997). This means that UDP-glucose:glycoprotein glucosyl transferase is a factor that determines to which proteins the chaperones bind. Attachment to the chaperones prevents irreversible aggregation and helps keep the proteins in the proper folding pathway. That they have the added responsibility of participating in quality control has been shown previously for VSV G protein and MHC class I (Hammond and Helenius, 1994a; Vassilakos et al., 1996). Apparently, they serve as a retention trap. They were clearly involved in retaining HA fragments in the ER because when binding to CNX and CRT was inhibited by CST, a fivefold increase in secretion was observed.
The CST-induced collapse in quality control was only partial. Of the fragments, 90% remained in the cells after CST addition. Many were found to associate with BiP/GRP78 instead, a member of the HSP70 family. BiP/GRP78 is known to interact with many newly synthesized proteins in the ER (Gething and Sambrook, 1992). It has a binding site for hydrophobic peptides exposed on incompletely folded proteins. It does not normally form stable complexes with folding intermediates of HA, but apparently it can bind when CNX and CRT binding is inhibited. When glycosylation was prevented by TM, BiP/GRP78 was the main chaperone associating with the fragments. BiP/GRP78 binding to nonglycosylated, misfolded full-length HA has been previously described (Hurtley et al., 1989). Evidently, HA has peptide binding sites that can support BiP/GRP78 binding but they are not accessible when CNX and CRT are associated.
Our results showed that quality control and folding are intimately linked to the action of molecular chaperones. While the chaperones are responsible for assisting the folding process, they also serve to retain folding intermediates and misfolded proteins that they bind to in the ER. The system of chaperones does not distinguish between folding intermediates and irreversibly misfolded proteins: both are retained by association with chaperones. The structural criteria underlying sorting depend on the binding specificities of the various chaperones. In other words, it is probably correct to predict that when full understanding is acquired about the binding specificities of chaperones to their substrates, one will also understand the structural criteria for quality control. When a protein reaches a conformation that does not offer binding sites for chaperones, it is allowed to exit the ER. For BiP/GRP78 this may involve the loss of hydrophobic surface peptides. For UDP-glucose glycoprotein glucosyltransferase, the principles of selectivity are more complex and still incompletely understood.
ACKNOWLEDGMENTS
We thank Dr. Jiang Yu for contributions during initiation of the project, members of the Helenius-Mellman group for discussions, and B. Sodeik, D. Hebert, J. Simons, S. Trombetta, K. Cannon and Mr. J. Peterson for helpful comments on the manuscript. We also thank Dr. P. Walter in whose laboratory the puromycin antibodies were produced. The work was supported by National Institutes of Health grants to A. Helenius and a National Institutes of Health postdoctoral fellowship to J.-X.Z., and a grant from the Royal Netherlands Academy of Art and Science to I.B.
Footnotes
Abbreviations used: CNX, calnexin; CRT, calreticulin; CST, castanospermine; HA, hemagglutinin; NEM, N-ethylmaleimide; TM, tunicamycin.
REFERENCES
- Bergeron JJ, Brenner MB, Thomas DY, Williams DB. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Braakman I, Helenius J, Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J, 1992;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, Hoover-Litty H, Wagner KR, Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991;114:401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Carr CM, Kim PS. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Chen J, Wharton SA, Weissenhorn W, Calder LJ, Hughson FM, Skehel JJ, Wiley DC. A soluble domain of the membrane-anchoring chain of influenza virus hemagglutinin (HA2) folds in Escherichia coli into the low-pH-induced conformation. Proc Natl Acad Sci USA. 1995a;92:12205–12209. doi: 10.1073/pnas.92.26.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Helenius J, Braakman I, Helenius A. Cotranslational folding and calnexin binding of influenza hemagglutinin in the endoplasmic reticulum. Proc Natl Acad Sci USA. 1995b;92:6229–6233. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Gregory RJ, Marshall J, Palu S, Souza DW, White GA, O’Riordian CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Copeland CS, Doms RW, Bolzau EM, Webster RG, Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986;103:1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RS, Douglas AR, Skehel JJ, Wiley DC. Analyses of antigenicity of influenza hemagglutinin at the pH optimum for virus-mediated membrane fusion. J Gen Virol. 1983;64:1657–1662. doi: 10.1099/0022-1317-64-8-1657. [DOI] [PubMed] [Google Scholar]
- Daniels RS, Douglas AR, Skehel JJ, Wiley DC, Naeve CW, Webster RG, Rogers GN, Paulson JC. Antigenic analysis of Influenza virus haemagglutinins with different receptor-binding specificities. Virology. 1984;138:174–177. doi: 10.1016/0042-6822(84)90158-2. [DOI] [PubMed] [Google Scholar]
- Doxsey SJ, Sambrook J, Helenius A, White J. An efficient method for introducing macromolecules into living cells. J Cell Biol. 1985;101:19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra AM, Fagioli C, Finazzi D, Sitia R, Alberini CM. Quality control of ER synthesized proteins: an exposed thiol group as a three-way switch mediating assembly, retention and degradation. EMBO J. 1993;12:4755–4761. doi: 10.1002/j.1460-2075.1993.tb06164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M-J, McCammon K, Sambrook J. Expression of wild-type and mutant forms of Influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986;46:939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gething M-J, Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982;300:598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Gething M-J, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gorlich D, Mattaj IW. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- Graves PN, Schulman L, Young JF, Palese P. Preparation of influenza subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive determinants. Virology. 1983;126:106–119. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Guenzi S, Fra A, Sparvoli A, Rocco M, Sitia R. The efficiency of cystein-mediated intracellular retention determines the differential fate of secretory IgA and IgM in B and plasma cells. Eur J Immunol. 1994;24:2477–2482. doi: 10.1002/eji.1830241033. [DOI] [PubMed] [Google Scholar]
- Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharides, glucose trimming and calnexin during glycoprotein folding in the endoplasmic reticulum. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Helenius A. A chaperone with a sweet tooth. Curr Biol. 1993;3:884–885. doi: 10.1016/0960-9822(93)90226-e. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994a;266:456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment and Golgi apparatus. J Cell Biol. 1994b;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determines glycoprotein association with calnexin. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J. 1996;15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- Helenius, A., Trombetta, E.S., Hebert, D.N., and Simons, J.F. (1997). Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol.(in press). [DOI] [PubMed]
- Hurtley SM, Bole DG, Hoover-Litty H, Helenius A, Copeland CS. Interactions of misfolded Influenza hemagglutinin with binding protein (BiP) J Cell Biol. 1989;108:2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Klausner RD. Architectural editing: determining the fate of newly synthesized membrane proteins. New Biol. 1989;1:3–8. [PubMed] [Google Scholar]
- Lodish HF. Transport of secretory and membrane glycoproteins from the rough endoplasmic reticulum to Golgi. J Biol Chem. 1988;263:2107–2110. [PubMed] [Google Scholar]
- Nathans D. Puromycin inhibition of protein synthesis: incorporation of puromycin into peptide chains. Proc Natl Acad Sci USA. 1964;51:585–592. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Jackson M, Peterson PA. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989;58:707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Ou W-J, Cameron PH, Thomas DY, Bergeron JJM. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Pelham HRB. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Ora A, Nguyen Van’ P, Helenius A. Transient, lectin-like association of calreticulin with folding intermediates of cellular viral glycoproteins. Mol Biol Cell. 1995;6:1173–1184. doi: 10.1091/mbc.6.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR, Rothman JE. Biosynthetic protein transport and sorting by endoplasmic reticulum and the Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Xu Y, Brenner MB. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994;263:387–390. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- Redman CM, Sabatini DD. Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc Natl Acad Sci USA. 1966;56:608–615. doi: 10.1073/pnas.56.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards FM. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Rodan AR, Simons JF, Trombetta ES, Helenius A. N-linked oligosaccharides are necessary and sufficient for association of RNase B with calnexin and calreticulin. EMBO J. 1996;15:6921–6930. [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Doms RW. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Sifers RN, Finegold MJ, Woo SLC. Molecular biology and genetics of alpha-1-antitrypsin deficiency. Semin Liver Dis. 1992;12:301–310. doi: 10.1055/s-2008-1040399. [DOI] [PubMed] [Google Scholar]
- Singh I, Doms RW, Wagner KR, Helenius A. Intracellular transport of soluble and membrane-bound glycoproteins: folding, assembly and secretion of anchor-free influenza hemagglutinin. EMBO J. 1990;9:631–639. doi: 10.1002/j.1460-2075.1990.tb08155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990;60:781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Siuta-Mangano P, Lane MD. Very low density lipoprotein synthesis and secretion. Extrusion of apoprotein B nascent chains through the membrane of the endoplasmic reticulum without protein synthesis. J Biol Chem. 1981;256:2094–2097. [PubMed] [Google Scholar]
- Sousa MC, Ferrero-Garcia MA, Parodi AJ. Recognition of the oligosaccharide and protein moities of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry. 1992;31:97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- Tatu U, Braakman I, Helenius A. Membrane glycoprotein folding, oligomerization and intracellular transport: effects of dithiothreitol in living cells. EMBO J. 1993;12:2151–2157. doi: 10.1002/j.1460-2075.1993.tb05863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatu U, Hammond C, Helenius A. Folding and oligomerization of influenza hemagglutinin in the ER and the intermediate compartment. EMBO J. 1995;14:1340–1348. doi: 10.1002/j.1460-2075.1995.tb07120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Kern HF, Fuller SD, Howell KE. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J Cell Biol. 1989;109:35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta SE, Parodi AJ. Purification to apparent homogeneity and partial characterization of rat liver UDP-glucose:glycoprotein glucosyltransferase. J Biol Chem. 1992;267:9236–9240. [PubMed] [Google Scholar]
- Varenne S, Buc J, Lloubes R, Lazdunski C. Translation is a non-uniform process. Effect of tRNA availability on the rate of elongation of nascent polypeptide chains. J Mol Biol. 1984;180:549–576. doi: 10.1016/0022-2836(84)90027-5. [DOI] [PubMed] [Google Scholar]
- Vassilakos A, Cohen-Doyle MF, Peterson PA, Jackson MR, Williams DB. The molecular chaperone calnexin facilitates folding and assembly of class I histocompatibility molecules. EMBO J. 1996;15:1495–1506. [PMC free article] [PubMed] [Google Scholar]
- Wada I, Rindress D, Cameron PH, Ou W-J, Doherty JJ, II, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJ, M. SSRα and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- Wiley DC, Skehel JJ. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–395. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wolin SL, Walter P. Ribosome pausing and stacking during translation of a eukariotic mRNA. EMBO J. 1988;7:3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapun A, Petrescu SM, Rudd PM, Dwek RA, Thomas DY, Bergeron JJM. Conformation independent binding of monoglucosylated ribonuclease B to calnexin. Cell. 1997;88:29–38. doi: 10.1016/s0092-8674(00)81855-3. [DOI] [PubMed] [Google Scholar]