Abstract
The introduction of NAATs has revolutionised chlamydial diagnostics and these tests are now the standard of care. However, as with all new technologies, they have also presented new challenges. This review attempts to answer some of the questions that have been raised, particularly by groups about to embark on implementing a screening programme. Laboratory tests are continually changing but it is hoped that the paper provides a useful update of the current situation.
Keywords: chlamydia, testing, amplification, screening
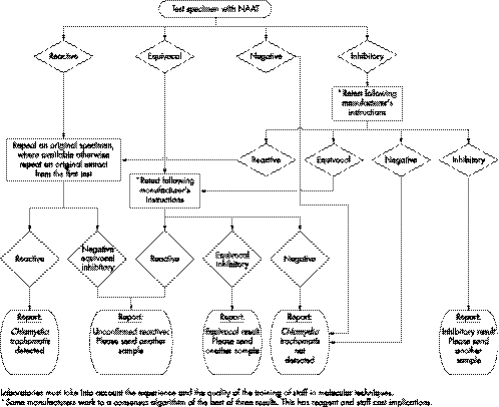
Nucleic acid amplification tests (NAATs) can increasingly offer improved opportunities for the diagnosis of sexually transmitted infections (STI).1 Funding has been allocated in all strategic health authority areas of the United Kingdom to facilitate the Department of Health recommended change from enzyme immunosorbent assays (EIA) to NAATs for diagnosis of genital chlamydial infection. In addition, NAATs must be used when offering opportunistic testing within the national chlamydia screening programme.2 Extension of testing may be accompanied by problems associated with screening low prevalence populations. It is therefore timely to consider a testing algorithm (fig 1),3 bearing in mind that no test is 100% sensitive or specific. This article discusses the issues which should be taken into consideration when testing for Chlamydia trachomatis.
Figure 1 Chlamydia infection, testing by nucleic acid amplification tests (NAATS), minimum testing algorithm.
Two NAATs currently in widespread use are the Beckton Dickinson Probetec which uses strand displacement amplification (SDA) technology and the Roche Cobas Amplicor polymerase chain reaction (PCR) which is now being superseded by a real time PCR method, the Roche Cobas TaqMan CT. In addition, Aptima Combo 2 (Gen‐Probe) is available, which utilises transcription mediated amplification (TMA) now combined with magnetic target capture.
When deciding which platform to use, caution is needed in the interpretation of performance data. Accepted practice is to evaluate a new test against a reference standard, a “gold standard.” Historically, this was cell culture for C trachomatis but this lacks sensitivity and there is currently no accepted “gold standard” comparator test. In this situation the simplest and most practical approach is to use a “composite reference standard.”4 This is derived by combining results from two or more existing tests and, in the case of C trachomatis, results from different specimen types from individual patients can be used. These results define the “infected patients” who are taken as the true positives within the study population.
Sensitivity of a test is the percentage of true positives that are found positive by that test. Some (usually small) exaggeration of sensitivity claims can arise if a rigorous “gold standard” approach is not used.5
Specificity is the percentage of true negatives that are called negative by the new test. Any positive by the new test that is not also positive by the “gold/infected patient” standard is by definition a false positive and reduces the specificity claim.
The predictive value of a negative test (PVN) (the likelihood that a negative in the test is a true call of an uninfected patient) and the predictive value of a positive test (PVP) (the likelihood that a positive in the test being evaluated is a true call of an infected patient) are related to both sensitivity and specificity. The PVP will vary depending on the population under test since it sets false positives in the new test (expected to occur at a constant rate) against true positives (which will be fewer in a low prevalence group).
(Note on terminology: We have chosen for clarity of meaning to refer to predictive value of a positive test, PVP, rather than the term positive predictive value, PPV, which has been widely used) (see table 1).
Table 1 Illustration of predictive value of a positive test (PVP) in high and low prevalence populations.
| Prevalence of chlamydia in population | 10% (high) | 2% (low) | |
| True positives among 1000 women | 100 | 20 | |
| True positives detected by the test (sensitivity = 92.6%) | 92 | 18 | |
| False positives given by the test (specificity = 99.4 (0.6 false positives per 100) | 6 | 6 | |
| Predictive value positive (PVP) = true positives/total positive results | 92/98 (94%) | 18/24 (75%) |
However, if the new test has in fact a greater sensitivity than the current “gold standard,” any extra true positives that it detects will, by evaluation protocol definition, be classified as false positive with consequent adverse effects in specificity and PVP calculation.
Thus, where there are expectations that the new test could/should be more sensitive than the current “gold standard,” PVPs should be regarded as reflecting the “worst case” scenario rather than an actual likelihood of an accurate diagnosis. McAdam has suggested a practical approach for trying to resolve accurately this inherent difficulty with assessing test performance of NAATs.6 Additional direct evidence should be sought under controlled trial conditions to determine what proportion of the positives in the new test can actually be verified by obtaining extra information. This may comprise results from repeat specimens or additional tests, not dependent on the same NAAT technology under investigation, and on the patient or clinical correlations, which for an STI we believe should also include a positive partner diagnosis.
Confirmatory/repeat testing
The algorithm suggested (fig 1) advocates repeat testing of positives. This improves specificity by countering processing errors but at the expense, which is usually judged acceptable, of a small reduction in sensitivity caused by specimens with a low organism load being missed at retest. It introduces another category of report “unconfirmed positive” allowing considered discussion with the patient on issues of additional tests and/or the need for treatment and partner follow up. The significance of confirmatory testing in C trachomatis screening is discussed in the published recommendations from CDC.7
Some manufacturers have designed their assays to minimise back contamination of amplicon, Roche employs an “AMPerase” enzyme and Gen‐Probe use an alternative magnetic capture target which is downstream of the sequence that is amplified. However, with the current state of technology and with the possibility of false positives caused by errors in processing, the use of repeat testing of residual sample is advocated to ensure that the initial result can be duplicated. Numbers of specimens that fail to confirm should be monitored, as this can be a good indication of possible contamination. Very occasionally this approach can reveal inherent, batch related problems with a test.8
Repeat testing, even in the same system, is recommended therefore to give a reproducibly positive result. However, unless a different platform is used, this approach cannot be regarded as confirmatory testing. Although, ideally, a second platform should be used, this may not be practical for several reasons including lack of access and incompatibility of samples. For example, the addition of the “urine preservation pouch” in the SDA assay may make samples unsuitable for use in the PCR. Samples could be aliquoted before testing to allow for second tests, although this is time consuming. There may be buffer incompatibility problems in applying a second test to swab extracts. Clearly, buffer compatibility must be established with dilutions of positive specimens before the routine adoption of confirmation by a second platform (Scragg et al, 2005, personal communication). There is evidence that the SDA has a lower analytical sensitivity than PCR which means that SDA may not be suitable for the confirmation of PCR results.9 There are also data to suggest that Aptima Combo 2 has a higher sensitivity than the other two assays discussed.10 However, this system does have its own confirmatory assay with matching sensitivity.
Specimens giving equivocal results
Gen‐Probe and Roche Cobas (but not TaqMan) describe a grey zone for results obtained and give an algorithm for the investigation of such specimens. Becton Dickinson point out that the MOTA (method other than acceleration) score reading is a measure of signal strength and not of organism load. They recommend that a low positive be defined as a specimen that gives a MOTA score of between 2000 and 9999. For such results, they suggest that “supplemental testing may be useful for verifying the presence of C trachomatis.” It is preferable to retest specimens in this range with an alternative test—for example, PCR. If the primary test is repeatedly equivocal, a second sample should be requested.
Specimens containing inhibitors of amplification
Reported rates of inhibition have varied over a substantial range and can occasionally reach levels that compromise the sensitivity of the assay. It is not known if such high rates represent specimen associated factors or are caused by a problem with certain batches of kits. Rates should be regularly monitored and investigated if they reach more than 10%. Both PCR and SDA incorporate internal standards to check that amplification has taken place in any given specimen. In both cases, use of the standard is optional but is advisable for specimen types (for example, urine) prone to inhibition. Gen‐Probe provides evidence that after their magnetic target capture process, inhibitors are removed and are therefore not an issue in their assay. There are plans for this methodology to be introduced into the other systems.
Specimen types and processing
Care should be taken to follow the manufacturer's recommendations regarding swab types, urine volumes, and times since last voiding. Transport of specimens should also be carried out following the manufacturer's instructions. It is imperative that laboratory staff members receive adequate training in using these tests particularly when processing urine samples.11 In general, specimen type is limited to cervical swabs, male urethral swabs, and first catch urine from both sexes. However, evidence is accumulating in the literature12,13 to support the use of self taken vulvovaginal swabs and this type of sample is validated for use in the Gen‐Probe assay.
Other specimens may be encountered such as:
rectal swabs
pharyngeal swabs
ulcer swabs and bubo aspirates from patients with lymphogranuloma venereum
conjunctival swabs from both babies and adults.
Although not validated by the manufacturer, it is likely that the high sensitivity and specificity of NAATs provides the optimum approach to testing other specimen types such as rectal and pharyngeal swabs.14 With such “off label” use, a previous DNA extraction step (to combat inhibitory effects) should be considered for DNA based tests as well as confirmatory testing on an alternative platform. Laboratories providing a service for such specimen types must collate their own validation document and may consider issuing a disclaimer with the results.
Key messages
Assessing NAAT use in low prevalence populations
A confirmatory algorithm for chlamydia testing
Suitability of chlamydia specimen types
There has been debate about the use of NAATs in medicolegal cases that, in the past, have relied on culturing the organism. However, facilities for culture are no longer available at many laboratories and as stated previously, the high sensitivity and specificity of NAATS offers the best approach. This approach has been endorsed in the 2006 draft revised BASHH “National guideline for the management of genital tract infection with C trachomatis,” which is shortly to go out for consultation (P Horner, personal communication).15 This suggests taking two samples—one for the initial test and the second for confirmation on an independent platform. This would address the issue of specimen collection kit incompatibility between platforms. The Royal College of Pathologists also recognise that micro‐organism detection using NAATs, instead of culture, will be used in medicolegal cases. They have issued guidance on the storage of such specimens.16 Given the complexities highlighted above, the significance of these test results will need careful interpretation particularly bearing in mind that, as stated previously, no test is 100% sensitive or specific.
(The Chlamydia Diagnosis Forum is not aware of any precedent for acceptance of results of chlamydia NAATs as legal evidence but it is monitoring the situation.)
Acknowledgements
The Chlamydia Diagnosis Forum would like to thank Dr Alan Herring, a founder member of the group for his help and advice, and Dr Linda Lazarus of the Expert Advice Support Office for her continuing support.
Members of the HPA Chlamydia Diagnosis Forum
Dr C Carder, University College Hospital NHS Foundation Trust; Dr S Corden, University Hospital of Wales; Dr P Horner, Milne Sexual Health Centre, Bristol; Professor C Ison, HPA Centre for Infections; Dr H Mallinson (Chair), University Hospital Aintree; Dr S O'Connell, HPA Laboratory, Southampton General Hospital; Dr M Sillis, The Norfolk and Norwich University Hospital NHS Trust; Dr S Skidmore, Princess Royal Hospital, Telford; Dr A Todd, North Cumbria Acute Hospitals NHS Trust; Dr G Underhill, St Mary's Hospital, Portsmouth.
Contributors
The Chlamydia Diagnosis Forum (Health Protection Agency) recognised the need for information to be brought together on issues surrounding the expanding use of nucleic acid amplification testing. Members of the CDF have compiled this paper and the authors have, with continued consultation, prepared the manuscript for publication.
Abbreviations
EIA - enzyme immunosorbent assays
MOTA - method other than acceleration
NAATs - nucleic acid amplification tests
PCR - polymerase chain reaction
PVN - predictive value of a negative test
PVP - predictive value of a positive test
SDA - strand displacement amplification
STI - sexually transmitted infections
TMA - transcription mediated amplification
Footnotes
*Members listed at end of paper.
Competing interest: The authors have no competing interests to declare.
References
- 1.Gaydos C A, Quinn T C. Urine nucleic acid amplification tests for the diagnosis of sexually transmitted infections in clinical practice. Curr Opin Infect Dis 20051855–66. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health www.dh.gov.uk/then search for 32219
- 3.Health Protection Agency National standard method: chlamydia infection—testing by nucleic acid amplification tests VSOP 37. London: Health Protection Agency, 2005, http://www.hpa‐standardmethods.org.uk/documents/vsop/pdf/vsop37.pdf
- 4.Alonzo T A, Pepe M S. Using a combination of reference tests to assess the accuracy of a new diagnostic test. Stat Med 1999182987–3003. [DOI] [PubMed] [Google Scholar]
- 5.Hadgu A. Discrepant analysis is an inappropriate and unscientific method. J Clin Microbiol 2000384301–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdam A J. Discrepant analysis: how can we test a test? J Clin Microbiol 2000382027–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson R E, Newhall W J, Papp J R.et al Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections. MMWR Recomm Rep. 2002;18;1–38. [PubMed]
- 8.Mallinson H, Hopwood J, Mutton K. Resolution of performance problem with Abbott LCx Chlamydia trachomatis assay? Issues of repeat testing for confirmation of chlamydial infection. Sex Transm Infect 200278225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalker V J, Vaughan H, Patel P.et al External quality assessment for detection of Chlamydia trachomatis. J Clin Microbiol 2005431341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schachter J, Hook E W, Martin D H.et al Confirming positive results of nucleic acid amplification tests (NAATs) for Chlamydia trachomatis: All NAATs are not created equal. J Clin Microbiol 2005431372–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schachter J, McCormack W, Chernesky M A.et al Vaginal swabs are appropriate specimens for diagnosis of genital tract infection with Chlamydia trachomatis. J Clin Microbiol 2003413784–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray R H, Wawer M J, Girdner J.et al Use of self‐collected vaginal swabs for detection of Chlamydia trachomatis infection. Sex Transm Dis 19982545. [DOI] [PubMed] [Google Scholar]
- 13.Oakeshott P, Hay P, Hay S.et al Detection of Chlamydia trachomatis infection in early pregnancy using self‐administered vaginal swabs and first pass urines: a cross‐sectional community‐based survey. Br J Gen Pract 200252830–832. [PMC free article] [PubMed] [Google Scholar]
- 14.Lister N A, Tabrizi S N, Fairley C K.et al Validation of Roche Cobas Amplicor assay for detection of Chlamydia trachomatis in rectal and pharyngeal specimens by an omp1 PCR assay. J Clin Microbiol 200442239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horner P J, Caul E O, on behalf of the British Association of Sexual Health and HIV Clinical Effectiveness Group Clinical effectiveness guideline for the management of Chlamydia trachomatis genital tract infection, 2002. Available from http://www.bash.org/guidelines/2002/c4a_0901c.pdf
- 16.Ridgway G, Bigrigg A, Carder C, on behalf of the Royal College of Pathologists et al National guidelines on a standardized proforma for ‘chain of evidence' specimen collection and on retention and storage of specimens for medicolegal purposes, 2005. Available at http://www.rcpath.org/resources/pdf/ChainofEvidenceMay05.pdf