Abstract
Background/objective
Persistent or recurrent non‐gonococcal urethritis (NGU) is commonly observed in men attending sexually transmitted diseases clinics. The aim of this study was to determine the importance of Mycoplasma genitalium in this condition and to monitor the effect of treatment with macrolides.
Methods
78 male patients with persistent or recurrent symptomatic non‐chlamydial NGU after treatment with doxycycline 200 mg day 1 and 100 mg for the following 8 days were enrolled. The patients had a first void urine specimen examined for the presence of M genitalium DNA by an inhibitor controlled PCR. Treatment with azithromycin and/or erythromycin and/or repeat doxycycline was prescribed at the doctor's discretion to the M genitalium positive men. Microbiological cure rate was determined at a 3 week follow up visit.
Results
M genitalium was detected in 32 (41%) men and those infected had more often a high grade urethritis (>10 PMNLs/hpf) than those negative for M genitalium (p = 0.01). 22 men had been treated with azithromycin, 19 of whom received 1.5 g over 5 days and three received 1 g as a single dose. All 20 who came back after treatment were M genitalium negative. Only two of five erythromycin treated controlled cases were M genitalium negative after treatment compared to all six given azithromycin at inclusion (p = 0.12). Six of nine female partners were M genitalium positive; they were treated with 1.5 g azithromycin given over 5 days, and the four tested were M genitalium negative after treatment.
Conclusions
M genitalium is a common cause of persistent or recurrent urethritis among men treated with doxycycline and erythromycin appears to be less efficient than azithromycin in eradicating the infection.
Keywords: Mycoplasma genitalium , urethritis, tetracyclines, azithromycin
In many countries as well as in Sweden, doxycycline is the first choice for treatment of non‐gonococcal urethritis (NGU). According to current routine in the sexually transmitted infection (STI) clinics, treatment is usually given when urethritis is diagnosed on the basis of microscopy of a stained smear from the urethra. The number of polymorphonuclear leucocytes (PMNLs) defining urethritis is widely discussed, but usually >4 PMNLs per high power field (×1000) (hpf) is considered significant.1 PMNL counts between 5/hpf and 10/hpf are often considered low grade or borderline, and can in some patients represent a sign of infection, but may among other cases be normal.2,3 Haddow et al showed that the number of leucocytes was a strong predictor of infection with Chlamydia trachomatis although more than one third of the patients did not have urethritis.4 In contrast, a Scandinavian study showed that 90% of all patients with C trachomatis or Mycoplasma genitalium infection had urethritis and all of those with NGU had >10 PMNLs/hpf.5
In some settings, Neisseria gonorrhoeae is a common cause of urethritis. However, gonorrhoea has become a very uncommon disease in Sweden, with only about 600 reported cases per year (6.5/100 000 inhabitants), and the majority of the patients thus have NGU. The most common cause of this condition is C trachomatis, an infection that has increased substantially in Scandinavia over the last few years. Annually, about 30 000 cases of C trachomatis infection are diagnosed in Sweden. In a Swedish study, 28% of men with microscopic signs of infection were C trachomatis positive.6 However, more than half of the NGU patients are C trachomatis negative.6,7 These men have non‐specific urethritis (NSU), a condition that is not yet fully understood. Ureaplasmas may have a role in some patients but its high isolation rate in men without urethritis has given rise to much controversy. Ureaplasmaurealyticum seems to be more closely associated with NGU than U parvum,8 However, whereas this was found also a study from Scandinavia when univariate statistical analysis was applied, the use of logistic regression analysis showed that this apparent relation could be explained by a younger age of the patients with U urealyticum.9Trichomonas vaginalis might give rise to symptoms in some patients whereas M hominis appears to be without significance. Viruses, such as adenovirus, herpes simplex, and human papillomavirus, might be important in some cases, but the majority remains unexplained. The choice of antibiotic for NSU is usually the same as that for NGU in general—that is, doxycycline. Despite appropriate antibiotic therapy, 10%–20% of men return to the clinic because of persisting symptoms and show signs of infection.10,11 These patients often represent “problem cases” in the clinic. Frequently, different kinds of antibiotics are prescribed before the patients are cured. In a few men, antibiotics have only a limited effect, but most of these men are spontaneously cured after a varying time.
In recent years, M genitalium has been shown to be an important aetiological agent in non‐chlamydial, non‐gonococcal urethritis (NCNGU) accounting for approximately 21% of symptomatic urethritis in a recent Swedish cross sectional study.5M genitalium has been most thoroughly studied as a cause of urethritis and cervicitis,12 but it is most likely also a cause of upper genital tract infections.13,14 Tetracyclines are usually suboptimal,15 whereas azithromycin is likely to be the most effective antibiotic, when given as a total dose of 1.5 g over 5 days.15 Polymerase chain reaction (PCR) tests detecting M genitalium, which currently are the only diagnostic tools, have recently been introduced in Sweden, and it is the current recommendation to use these tests for “problem cases,” but still they are generally not recommended as screening tests in all men with urethritis.
The primary aim of the present study was to estimate the proportion of M genitalium positive cases among men initially treated with doxycycline who returned with symptomatic recurrent and/or persistent urethritis.
Patients and methods
Patients and clinical management
Men attending the STI clinic of the Karolinska Hospital, Stockholm, Sweden between April 2002 and June 2004 because of persisting or recurrent symptoms of urethritis were included if they had received treatment for urethritis with doxycycline 200 mg on day 1 and 100 mg on the 8 days following, but re‐attended the clinic. The men had microscopic signs of urethritis—that is, >4 PMNLs/hpf in a methylene blue stained urethral smear. A sexual history and information about previous STIs was collected and a clinical examination was carried out.
All doctors in the clinic were asked to collect a first void urine (FVU) specimen from these men for diagnosis of M genitalium. The samples were sent by regular mail to the laboratory in Copenhagen, Denmark. Regardless of the test result, patients were treated with antibiotics at the discretion of the treating doctor. When azithromycin was prescribed, it was given as 500 mg on day 1 and 250 mg on the 4 days following or as a 1 g single dose. Erythromycin was prescribed as 500 mg twice a day for 10 days. Some of the men received additional courses of doxycycline for various periods (200 mg the first day, and then 100 mg per day the rest of the course). A FVU specimen for detection of M genitalium was collected 3 weeks after antibiotic therapy for M genitalium positive cases.
If the patients were in a steady sexual relationship at the time of sampling, the partners of the M genitalium positive men were offered an examination and a M genitalium PCR test on a FVU specimen. The patients were informed not to have sex until the infection had been cleared and followed up, and their partners were tested. The study was approved by the ethics committee of the hospital.
Laboratory methods
M genitalium was detected in the FVU specimens using a PCR targeting the 16S rRNA gene and an internal control for inhibition as previously described.16 All positive results were confirmed with a PCR targeting the mgp adhesin gene.17,18
Statistical methods
For comparison of proportions, Fisher's exact test was used. Continuous variables were compared using the Mann‐Whitney test; all tests were performed using StatsDirect version 2,4,1.
Results
A total of 294 men had a diagnosis of NCNGU during the study period, of whom 78 (27%) returned to the clinic with symptomatic recurrent or persistent NGU and were included. M genitalium was detected in 32 (41%) of the men. Nine female partners of M genitalium positive men were examined and six (67%) were M genitalium positive. One of the nine women had been treated with doxycycline 2 weeks earlier, and this woman was M genitalium positive. The other women had not previously received treatment.
The median age of the men was 27 years for both M genitalium positive (range 20–47) and negative men (range 17–57) (p = 0.47), and the median age of the women was 25 (range 19–30) years. Only one of the men reported having had sex with other men, the remaining stated that they were heterosexual. Being in a steady relationship was reported by 36 (46%) of the men (mean duration 10 months) and 10 had been with the same partner for more than 5 years.
Any previous STI was reported by 38 (49%) of the men: C trachomatis infection by 26 (33%), gonorrhoea by five (6%), condylomas by 11 (14%), genital herpes by two (2%), and M genitalium by one (1%) of the men.
The median time that had elapsed since the doxycycline treatment was 2 months (range 0.25–17) for the M genitalium negative men and 1 month (range 0.25–12) for the M genitalium positive men (p = 0.047). Of the 57 men who came back within the first 3 months after treatment, 27 (47%) were M genitalium positive and there was no difference in the median follow up time (p = 0.25).
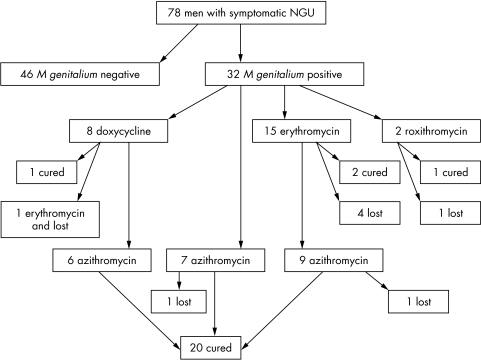
As shown in figure 1, a total of 22 men was treated with azithromycin, seven at the inclusion visit and 15 after they had received other antibiotics initially at the inclusion visit (doxycycline n = 6, erythromycin n = 9). Three men received azithromycin 1 g as a single dose, whereas the remaining 19 men received 1.5 g over 5 days. Two of the 22 men treated with azithromycin were lost to follow up, but all of the 20 men who returned were M genitalium PCR negative after treatment regardless of the dosage of azithromycin. Two of the 20 men had a slight urethral itch, but the remaining 18 were free of symptoms. As also shown in figure 1, erythromycin was prescribed at inclusion for 15 of the 32 M genitalium positive men. Four were lost to follow up and, of the remaining 11 men, only two (40%) of the five men who were examined microbiologically were cured. The nine men with clinical failure were subsequently treated with azithromycin. Erythromycin appeared to be less efficient than azithromycin since only two of five erythromycin treated patients were microbiologically cured, compared to six of the six men initially treated with azithromycin (p = 0.12). All of the six M genitalium positive female partners were prescribed azithromycin 1.5 g over 5 days, and the four women returning for follow up were M genitalium negative in the control test.
Figure 1 Flow chart detailing the number of men with recurrent or persistent non‐gonococcal urethritis (NGU) who were included in the study and followed up after various treatments.
At inclusion, 41 (52%) of the men had only low grade urethritis defined as 5–10 PMNLs/hpf. M genitalium was detected significantly more often in men with >10 PMNLs/hpf (57%) compared to those with low grade urethritis (27%) (p = 0.01).
A urethral swab for culture of bacteria was taken from 36 of the 46 M genitalium negative men. The test was negative or showed normal flora in 32 of the men. Four men were considered having significant growth of possible bacterial pathogens, one patient each with Haemophilus influenzae, H parainfluenzae, Escherichia coli, and anaerobic bacteria. Among the 32 M genitalium positive men, a urethral swab was taken from 20 men; in all cases it was negative or showed normal flora.
In all, 29 M genitalium negative men were followed up after an additional course of doxycycline or other antibiotics; 20 (69%) were clinically cured. The remaining nine men were treated with norfloxacin (n = 2), ciprofloxacin (n = 3), doxycycline (n = 1), metronidazole (n = 2), or azithromycin (n = 1). Of these, three were lost for follow up and six were cured.
Discussion
In this study, men who had received treatment for urethritis with doxycycline (200 mg day 1, and 100 mg the following 8 days), and who re‐attended the clinic because of persisting or recurring symptoms, were examined for the presence of M genitalium by PCR on FVU specimens. These represent 27% of men with NCNGU visiting the clinic during the study period. A high proportion of the men (41%) were M genitalium positive. Recently, Taylor‐Robinson et al presented data on 52 men with persistent or recurrent NGU.19 Of these, 11 (21%) were M genitalium PCR positive. The M genitalium positive men had not received less antibiotic treatment than the M genitalium negative cases.
The different doses and classes of antibiotics chosen at the first visit illustrate the different treatment traditions, and the lack of general guidelines for NSU in Sweden. M genitalium positive patients who received azithromycin responded well, none of these having to be treated again because of a positive M genitalium test or severe persisting symptoms. Among the M genitalium negative patients nine of 46 needed more than one additional course of antibiotics. A suspected causative agent was found only among a few of these men. The bacterial culture may not be sensitive enough nor does it have the ability to detect all relevant agents, of which we probably do not know all yet. Data for U urealyticum would have been interesting, but this agent was not tested for in the present study, partly because of data on patients from Sweden presented by Povlsen et al,9 but also because of the lack of an appropriate control group.
The M genitalium positive patients returned to the clinic with complaints earlier after treatment with doxycycline than did those in whom M genitalium was not detected. However, looking at men returning within the first 3 months after treatment removed the difference in follow up time and increased the M genitalium detection rate to 47%. This may be a result of the more severe symptoms often seen in M genitalium infected patients.5 Although a few patients may have been re‐infected after the initial treatment it would be difficult to explain the 47% M genitalium detection rate within the first 3 months. On the other hand, it may illustrate the fact that some patients are infected with M genitalium strains with decreased susceptibility or resistance to doxycycline. Those patients infected with resistant strains may experience no effect at all during the treatment and tend to come back early, whereas those infected with strains with decreased susceptibility tend to experience transient improvement followed by a relapse of the symptoms after discontinuation of the antibiotic treatment. In other studies, doxycycline 200 mg for 1 day and 100 mg for 8 days has been shown to have a very limited effect on M genitalium.15 Our study supports the notion that azithromycin given as a 5 day course is an effective treatment for M genitalium,15 although two patients treated with 1 g of azithromycin in a single dose were also cured. This is also in good agreement with recently presented data from a randomised trial comparing azithromycin 1 g with doxycycline 100 mg twice daily for 7 days,20 where it was shown that azithromycin eradicated M genitalium from 84% of the patients compared to a cure rate of 36% after doxycycline. However, erythromycin given as a 10 day course appeared less efficient than azithromycin. The fact that nine of 15 men initially treated with erythromycin came back illustrates the limited effect of this treatment. All six patients treated with azithromycin at inclusion and returning for follow up were cured both clinically and microbiologically compared to two of five cases prescribed erythromycin (p = 0.12). Unfortunately, only five of the erythromycin treated patients had FVU collected for M genitalium PCR, whereas the remaining patients were treated with azithromycin on basis of clinical findings. To some extent our findings are in agreement with the in vitro susceptibility studies of M genitalium that have shown azithromycin to be 10–100‐fold more potent than erythromycin.21 Furthermore, the prescribed amount used in this study (500 mg twice daily for 10 days) is indeed in the lower range of the recommended dosage but may, on the other hand, have improved the compliance.
In Sweden, all partners of patients with NCNGU are recommended to be tested and treated. The partners of our M genitalium positive men were also offered a partner check up, but only nine female partners attended the clinic, of whom eight were previously untreated. For the non‐attending partners we lack information about previous treatment. For C trachomatis infections, partner notification is mandatory by law in Sweden and partners are required to be tested. The lack of legal incitement in the case of M genitalium infections may explain the low partner attendance rate in our clinic. Six (67%) of the nine female partners were M genitalium PCR positive.
Until quite recently, laboratory tests for M genitalium have been unavailable outside research settings, and this is the case indeed in most countries, and therefore, most cases of NSU have been of unknown origin. The present study indicates that it would be highly relevant to test for M genitalium in selected cases such as patients with persistent or recurrent urethritis. Furthermore, controlled clinical trials aiming at determining the optimal treatment of persistent urethritis in the light of an expanded knowledge about the possible aetiological agents should be encouraged. Further studies are also urgently needed to investigate the pattern of complications of the infection; these could include epididymitis and sexually acquired reactive arthritis as well as upper genital tract infections in women. Partner studies are of course also important. Probably, the partners should be treated with azithromycin in the same way as the patients. At a later stage, detection of M genitalium should probably be included as a screening test in STI clinics.
Key messages
Mycoplasma genitalium is a common cause of recurrent or persistent symptomatic non‐chlamydial, non‐gonococcal urethritis (NCNGU) in men treated with doxycycline
Men with M genitalium more often have urethritis with >10 PMNL/hpf than men with NCNGU negative for M genitalium
Azithromycin is an effective treatment for M genitalium infection
Acknowledgements
Birthe Dohn is thanked for excellent technical assistance. The authors had no conflict of interest and no external financial support was received.
Contributors
AW initiated the study, examined and sampled most of the patients, collected all data, and wrote the first draft of the manuscript; JSJ was responsible for the M genitalium tests and provided major contributions to the analysis of the data and writing of the manuscript.
Abbreviations
FVU - first void urine
hpf - high power field
NCNGU - non‐chlamydial, non‐gonococcal urethritis
NGU - non‐gonococcal urethritis
NSU - non‐specific urethritis
PCR - polymerase chain reaction
PMNLs - polymorphonuclear leucocytes
STI - sexually transmitted infections
Footnotes
Conflict of interest: none.
References
- 1.Swartz S L, Kraus S J, Herrmann K L.et al Diagnosis and etiology of nongonococcal urethritis. J Infect Dis 1978138445–454. [DOI] [PubMed] [Google Scholar]
- 2.Smith R, Copas A J, Prince M.et al Poor sensitivity and consistency of microscopy in the diagnosis of low grade non‐gonococcal urethritis. Sex Transm Infect 200379487–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arya O P, Mallinson H, Andrews B E.et al Diagnosis of urethritis: role of polymorphonuclear leukocyte counts in gram‐stained urethral smears. Sex Transm Dis 19841110–17. [PubMed] [Google Scholar]
- 4.Haddow L J, Bunn A, Copas A J.et al Polymorph count for predicting non‐gonococcal urethral infection: a model using Chlamydia trachomatis diagnosed by ligase chain reaction. Sex Transm Infect 200480198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk L, Fredlund H, Jensen J S. Symptomatic urethritis is more prevalent in men infected with Mycoplasma genitalium than with Chlamydia trachomatis. Sex Transm Infect 200480289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Björnelius E, Lidbrink P, Jensen J S. Mycoplasma genitalium in non‐gonococcal urethritis ‐ a study in Swedish male STD patients. Int J STD AIDS 200011292–296. [DOI] [PubMed] [Google Scholar]
- 7.Johannisson G, Enström Y, Löwhagen G B.et al Occurrence and treatment of Mycoplasma genitalium in patients visiting STD clinics in Sweden. Int J STD AIDS 200011324–326. [DOI] [PubMed] [Google Scholar]
- 8.Deguchi T, Yoshida T, Miyazawa T.et al Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex Transm Dis 200431192–195. [DOI] [PubMed] [Google Scholar]
- 9.Povlsen K, Bjornelius E, Lidbrink P.et al Relationship of Ureaplasma urealyticum biovar 2 to nongonococcal urethritis. Eur J Clin Microbiol Infect Dis 20022197–101. [DOI] [PubMed] [Google Scholar]
- 10.Horner P, Thomas B, Gilroy C B.et al Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis 200132995–1003. [DOI] [PubMed] [Google Scholar]
- 11.Bowie W R, Alexander E R, Stimson J B.et al Therapy for nongonococcal urethritis: double‐blind randomized comparison of two doses and two durations of minocycline. Ann Intern Med 198195306–311. [DOI] [PubMed] [Google Scholar]
- 12.Jensen J S. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol 2004181–11. [DOI] [PubMed] [Google Scholar]
- 13.Cohen C R, Manhart L E, Bukusi E A.et al Association between Mycoplasma genitalium and acute endometritis. Lancet 2002359765–766. [DOI] [PubMed] [Google Scholar]
- 14.Simms I, Eastick K, Mallinson H.et al Associations between Mycoplasma genitalium, Chlamydia trachomatis, and pelvic inflammatory disease. Sex Transm Infect 200379154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falk L, Fredlund H, Jensen J S. Tetracycline treatment does not eradicate Mycoplasma genitalium. Sex Transm Infect 200379318–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen J S, Borre M B, Dohn B. Detection of Mycoplasma genitalium by PCR amplification of the 16S rRNA Gene. J Clin Microbiol 200341261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen J S, Uldum S A, Søndergård‐Andersen J.et al Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol 19912946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen J S, Björnelius E, Dohn B.et al Comparison of first void urine and urogenital swab specimens for detection of Mycoplasma genitalium and Chlamydia trachomatis by polymerase chain reaction in patients attending a sexually transmitted disease clinic. Sex Transm Dis 200431499–507. [DOI] [PubMed] [Google Scholar]
- 19.Taylor‐Robinson D, Gilroy C B, Thomas B J.et al Mycoplasma genitalium in chronic non‐gonococcal urethritis. Int J STD AIDS 20041521–25. [DOI] [PubMed] [Google Scholar]
- 20.Mroczkowski T F, Mena L, Nsumai M.et al A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium positive urethritis in men. 16th Biennial meeting of the International Society for Sexually Transmitted Diseases Research.Amsterdam, 2005, Abstract WP–107 [DOI] [PubMed]
- 21.Hannan P C. Comparative susceptibilities of various AIDS‐associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol 1998471115–1122. [DOI] [PubMed] [Google Scholar]