Abstract
Objectives
To evaluate the effectiveness and cost effectiveness of syndromic sexually transmitted infection (STI) packages on appropriate treatment and preventive management during primary care consultations.
Methods
Cluster randomised trial of 37 Durban primary care clinics randomised to use syndromic packages (containing antibiotics, condoms, partner notification cards, and written information) or not. We assessed outcomes using simulated patients who reported STI symptoms and recorded how they were managed, before and after implementation (269 and 256 simulated patient consultations). We adjusted for baseline values and intra‐clinic correlation of outcomes statistically. We used health department information to estimate the extra resources needed to provide the packages to 20 clinics for 1 year and their costs.
Results
Simulated patients in intervention clinics were more likely to receive appropriate syndromic STI management (correct treatment plus condoms offered plus partner notification cards offered; prevalence rate ratio 2.3; 95% confidence intervals (CI) 1.6 to 3.0) and to receive more STI advice and information (odds ratio 1.5; 95% CI 1.01 to 2.1). Women were less likely to receive appropriate syndromic STI management. The intervention increased STI information provision in women more than in men. The extra cost per extra patient appropriately managed was $1.51.
Conclusions
Syndromic packages improved syndromic STI management at a reasonable cost and should be used more widely.
Keywords: sexually transmitted infections, syndromic management, primary care, South Africa
Syndromic treatment of sexually transmitted infection (STI) can be effective in settings where primary care workers have limited diagnostic skills and facilities, if the symptoms are reasonably sensitive and specific indicators of STI, and if the infective organisms are responsive to the drugs used.1 STI treatment also provides opportunities to prevent transmission of HIV and other STIs through promotion of condom use and contact tracing.2,3,4 But in southern Africa primary care opportunities to treat STIs, promote condom use, and trace sexual contacts are often missed,4,5,6,7,8,9,10,11,12 especially for women.5,9,11,12 South Africa's Kwa Zulu Natal province has the country's highest HIV prevalence.13 In 2004, 7.8% of the provincial population aged over 15 years was treated for STI, and 3.4% of primary care visits were for STIs.13 A major study found that 25% of Kwa Zulu Natal women had Trichomonas vaginalis, Neisseria gonorrhoeae, Chlamydia trachomatis, or Treponema pallidum at any time, half of whom were symptomatic.14 In such high prevalence settings, positive predictive values of STI syndromes tend to be higher, and thus syndromic treatment is more appropriate, compared to lower prevalence settings. But Kwa Zulu Natal has relatively few primary care resources.13 Over 80% of South Africans are dependent on government provided primary care, which is mostly provided by nurse practitioners working in clinics, supervised by doctors and employed by provincial or local health authorities.
An earlier randomised trial in Kwa Zulu Natal showed that correct syndromic management was increased by provision of syndromic packages in five rural clinics, plus staff training and supervision.15 Health departments in the Kwa Zulu Natal city of Durban planned to provide these packages, which has not been done elsewhere in South Africa. This study aimed to estimate the effect of a syndromic package intervention, on treatment and comprehensive STI management, excluding training effects, and its cost and cost effectiveness, when implemented on a large scale in a South African city. Our hypothesis was that the intervention would improve syndromic STI management.
Methods
This cluster randomised controlled trial was conducted in Durban, Kwa Zulu Natal, from April 2002 to April 2003. The study population comprised all clinicians in all 115 government primary healthcare clinics in Durban. We randomly sampled 42 clinics, stratifying by local or provincial government department. STI care was provided by nurse practitioners in all 42 clinics, except for one clinic that also had a doctor. We aimed for eight simulated patient visits for each clinic at baseline and at follow up. Staff in three clinics did not consent to participate and two did not provide STI care, leaving 37 clinics in the sample. Follow up took place 2–3 months after starting the intervention. Clinicians and simulated patients could not be blinded because the syndromic packages could be seen in intervention clinics. Trial statisticians were not blinded. We had already reported baseline findings.11
We randomly allocated clinics to receive the intervention or not, stratifying by local or provincial authority, and by whether at baseline more or less than 35% of simulated patients were correctly managed. Within each stratum, clinics were ranked by the proportion correctly managed and then randomly allocated using a random number table and with a block size of four. A team member (MOB) who did not have contact with or knowledge of individual clinics performed the randomisation.
The intervention
The intervention consisted of pre‐packing and supplying the correct antibiotics for each STI syndrome in syndrome specific “syndromic packages,”4,15,16 including 10 condoms, a partner notification card, and an information leaflet. There were three different types of pack, containing antibiotics appropriate for either male urethral discharge, female vaginal discharge, or female lower abdominal pain.17 In the control clinics patients received standard care, supposedly following national antibiotic prescribing guidelines,17 and with condoms and partner notification cards provided at clinicians' discretion. Prescribed antibiotics for controls were issued at the clinics.
After the baseline study, all nurses in intervention and control clinics received a half day of training on syndromic STI management. The only difference was that intervention clinic nurses were taught how to use the syndromic packages, while control clinic nurses were trained in issuing correct drugs for each syndrome. Nurses in both arms of the trial also had 1½ days of training on attitudes to patients, which the baseline study had found to be a problem.11
Outcome measures
We assessed quality of syndromic STI care by using eight actors (simulated patients). They were males or females aged between 21 years and 29 years. All males reported symptoms of urethral discharge, half of females (at 55 baseline and 55 follow up visits) reported lower abdominal pain (symptomatic of pelvic inflammatory disease), and half of females reported vaginal discharge. Simulated patients were trained over 5 days, covering the clinical features, epidemiology and syndromic management of STIs, health seeking behaviours, attitudes to STIs and AIDS, condom issues, and how to simulate STI syndromes and record clinicians' behaviour. Scripts were not used. Immediately after each consultation, simulated patients completed forms recording details of the consultation process.
The primary outcome was whether the simulated patient was appropriately managed according to local guidelines, defined as correctly treated, and condoms offered, and a partner notification card offered. Correct treatment was defined as providing the correct dose and duration of ciprofloxacin (500 mg once) and doxycycline (100 mg twice daily for 7 days) for males and, for females, as ciprofloxacin (500 mg once), doxycycline (100 mg twice daily for 7 days), and metronidazole (400 mg twice daily for 7 days for pelvic inflammatory disease, or 2 g once for vaginal discharge).17
As secondary outcomes, scores of the quality of care were obtained by adding the values of component items: history taking (nine items), advice, and information on STIs (seven items), nurses' caring attitude (five items) and strength of agreement that the nurse was respectful, friendly, and caring (three items). Each score was the number of favourable items recorded, except that the three strength of agreement items were graded from 1 to 5 and added to produce a maximum possible score of 15. Scores had Cronbach's alphas of more than 0.7, showing good internal consistency.
The research proposal was approved by the research ethics committee of the South African Medical Research Council. Written, informed, individual consent to be visited by simulated patients was obtained from all professional nurses working in participating clinics.
Statistical analysis
The sample size of eight simulated patients in each of 40 clinics provided 80% power, at the 5% significance level, to detect a 20% difference in the proportion of simulated patients appropriately managed. This assumed that the inter‐cluster correlation coefficient would be 0.08, as in the earlier trial,15 and that the proportion correctly managed in the control arm would be 40%, as in this study at baseline (table 1).
Table 1 Prevalence of correct management, and history, STD information, and attitude scores, at baseline.
| Outcome | Control | Intervention |
|---|---|---|
| n/N (%) | n/N (%) | |
| Appropriate management* | 55/129 (42.6) | 51/140 (36.4) |
| Correct treatment provided† | 102/129 (79.1) | 111/140 (79.3) |
| Condoms provided | 66/129 (51.2) | 68/140 (48.6) |
| Partner notification card provided | 82/129 (63.6) | 85/140 (60.7) |
| History, STD advice and attitude scores | Mean, median (IQR) | Mean, median (IQR) |
| History taking (of 9 items)‡ | 3.6, 3 (2–5) | 3.1, 3 (2–4) |
| Advice and information on STDs (of 7 items)‡ | 3.7, 4 (2–5) | 3.6, 4 (2–5) |
| Nurses respectful attitude (of 3 items)¶ | 7.9, 9 (6–10) | 7.3, 8 (6–9) |
| Nurses caring attitude (of 5 items)‡ | 3.3, 4.5 (1–5) | 3, 3 (1–5) |
*All three subsequent outcomes (treatment, condoms, partner cards) present. †Correct dose and duration of ciprofloxacin and doxycycline for males, and of ciprofloxacin, doxycycline and metronidazole for females.17 ‡Number of items recorded. ¶Each item scored 1–5 so highest possible score = 3×5 = 15. IQR interquartile range.
Statistical analysis aimed to estimate the effects of the intervention on the quality of STI care to individual patients, using Stata software. Data were analysed at the level of simulated patient visits, adjusted for the stratified cluster randomisation design.
The effectiveness measures for binary outcomes were prevalence rate ratio and numbers needed to treat. As some simulated patients differed at baseline and follow up, linking baseline and post‐intervention results was not possible. A log binomial regression model was used, adjusting for provincial or local government clinic, the mean value of the same outcome variable in the same clinic at baseline, with robust adjustment for intra‐clinic correlation of the outcome.
Scores (numbers of items of STD information and advice or indicators of good attitude) were compared using ordinal logistic regression, adjusting for provincial or local government, the mean value of the same outcome variable in the same clinic at baseline, and intra‐clinic correlation of the outcome.
Differences in intervention effects between females and males were tested by adding trial arm‐sex interaction terms to regression models.
Economic evaluation
The economic evaluation was conducted from the perspective of government health services. We estimated the annual incremental cost to the Provincial Department of Health of resources needed to provide syndromic packages, using health department records, clinic utilisation statistics, interviews with service providers, and correspondence with the Department of Health central repackaging unit.
Additional resources to prepare syndromic packages comprised: (a) transportation, meals, per diems, and materials used for the training of providers, (b) supplies and materials used by the central repackaging unit (drug costs were not included as they would be used anyway), (c) time spent by health department staff delivering and receiving training, and (d) time spent by staff at the central repackaging unit to assemble the syndromic packages. The additional resources required for service provision, after training, was the additional time spent by intervention clinic staff on preparations and patient consultations. We calculated the expected time per day or per patient on each activity using numbers of providers and expected patient load in each clinic. The difference in expected time between intervention and control clinics was multiplied by numbers of days (preparation) or numbers of patients (assessment and counselling). Multiplying this by the average personnel cost per minute estimated the incremental value of clinic staff time required. No additional personnel were hired for the intervention, so staff costs represent a non‐monetary opportunity cost. Costs in South African rand were converted to US dollars based on the average inter‐bank exchange rate from April 2002–April 2003 of $0.10419 (£0.05; €0.08) per rand.18
Results
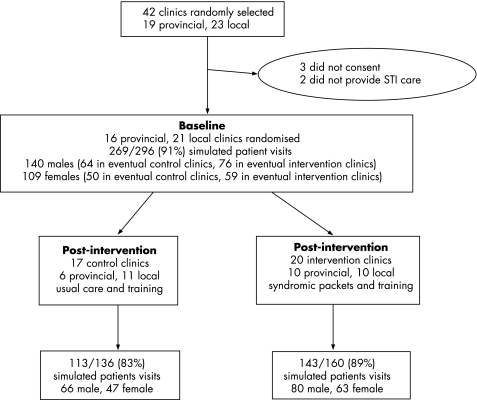
At baseline 269 simulated patient consultations were completed, of 296 (37×8) consultations planned (91%) (fig 1). At follow up 256 simulated patient visits were completed, of 296 planned (86%). Visits were fewer than planned because some actors left employment early or because their employment contracts ended before targets were met. Indicators of care quality were similar in intervention and control clinics at baseline (table 1).
Figure 1 Trial participants.
At follow up 108 of 143 (75.5%) intervention clinic patients and 39 of 113 (34.5%) control clinic patients were appropriately managed (adjusted prevalence ratio 2.18 (95% CI 1.6–3.0) (table 2). Simulated patients were 1.8 and 1.3 times as likely to received condoms and partner notification cards in intervention compared to control clinics. The proportion correctly treated was similar in the two arms. The numbers needed to treat show that for every 2.3–5.6 extra patients treated in intervention clinics, one extra patient attained the respective outcome.
Table 2 Prevalence of correct management at follow up, and effects of intervention on management.
| Control | Intervention | Prevalence ratio* | p Value* | NNT* | |
|---|---|---|---|---|---|
| n/N (%) | n/N (%) | PR (95% CI ) | No (95% CI} | ||
| Primary outcome: appropriate management (all three provided) | 39/113 (34.5) | 108/143 (75.5) | 2.18 (1.6 to 3.0) | <0.001 | 2.3 (1.8 to 3.3) |
| Correct treatment provided† | 95/113 (84.1) | 121/143 (84.6) | 1.06 (1.0 to 1.1) | 0.2 | 17.4 (0 to 7.3) |
| Condoms provided | 51/113 (45.1) | 117/143 (81.8) | 1.76 (1.3 to 2.3) | <0.0001 | 2.8 ( 2.1 to 4.5) |
| Partner notification card provided | 76/113 (67.3) | 121/143 (84.6) | 1.25 (1.1 to 1.4) | <0.0001 | 5.6 (3.7 to 11.2) |
*Adjusted for clustering of outcome at clinic level, for local or provincial authority and for clinic level prevalence of respective outcome at baseline using log binomial regression
†Correct dose and duration of ciprofloxacin and doxycycline for male, and of ciprofloxacin, doxycycline, and metronidazole for females.17
NNT, number needed to treat for one more patient to obtain the respective outcome.
Simulated patients attending intervention clinics were more likely to receive more advice and information about STIs (table 3). Perceived nurse attitudes did not differ. Sixty one percent (67/110) of females and 55% (80/146) of males were appropriately managed at follow up (p = 0.28). But the odds ratio of getting more STI advice and information in intervention clinics was larger for females 2.0 (95% CI 1.2 to 3.3) than for males 0.9 (95% CI 0.5 to 1.7) (p value for interaction term = 0.03). Intra‐cluster correlation coefficients for outcomes ranged from 0.01 to 0.03.
Table 3 STD information and attitude scores at follow up, and effects of intervention on scores (number of items recorded).
| Control | Intervention | Odds ratio* (95% CI) | p Value* | |
|---|---|---|---|---|
| Mean, median (IQR) | Mean, median (IQR) | |||
| STI advice and information (of 7 items)† | 3.5, 4 (2–5) | 3.9, 4 (2–6) | 1.5 (1.01 to 2.14) | 0.04 |
| Nurses respectful attitude (of 3 items)‡ | 8.7, 9 (7–12) | 8.5, 9 (7–12) | 1.1 (0.68 to 1.7) | 0.76 |
| Nurses caring attitude (of 5 items)† | 3.7, 5 (3–5) | 3.7, 5 (3–5) | 1.1 (0.62 to 1.9) | 0.79 |
IQR interquartile range.
*Odds ratio of having a higher score, adjusted for clustering of outcome at clinic level, for local or provincial authority, and for clinic level prevalence of respective outcome at baseline using ordinal logistic regression.
†Number of items recorded.
‡Each item scored 1–5 so highest possible score = 3×5 = 15.
In a secondary analysis we compared simulated patients who were actually given syndromic packages with simulated patients who were treated without packages. Treatment was correct in 95% of the former compared to 85% of the latter (p value for interaction term = 0.0091).
Mean duration of consultations did not differ between control and intervention clinics at baseline or after the intervention, but increased in both arms from 6.7 minutes to 9.0 minutes. Duration of consultation was not associated with correct treatment or correct management but was associated with more thorough history taking (p<0.001).
The estimated annualised incremental cost of the syndromic package intervention for the intervention clinics was $17 363. This comprised: (1) an annualised cost of $4035 for the training of providers (assuming a 2 year useful life for the training), (2) an additional $22 854 for the production of a 1 year supply of syndromic packages ($6791 for supplies and materials and $16 064 for the value of staff time), and (3) an annual net savings of $9526 for clinic staff time spent on the delivery of STI services. Net savings occurred because intervention clinic staff reported spending less time on preparation ($4307 saved) and on patient assessment ($8289 saved). Intervention clinic staff reported spending more time per patient on counselling (additional cost of $3070).
Based on trial outcomes (table 2) and a patient volume of 28 061 STI patients per year in the intervention clinics, we estimated that syndromic packages would result in 153 additional patients receiving the correct treatment and 11 508 additional patients receiving appropriate management per year (table 4). With an annualised incremental cost of $17 363, this yields an incremental cost effectiveness ratio of $113 per extra patient correctly treated and $1.51 per extra patient appropriately managed. This suggests that the intervention was not particularly cost effective in correcting treatment but was cost effective when condom provision and partner notification are also considered.
Table 4 Incremental cost effectiveness of syndromic package intervention.
| Outcome measure | Control clinics (%) | Intervention clinics (%) | Annualised incremental cost ($)* | Incremental effectiveness (no of patients)† | Incremental cost effectiveness ratio ($/patient) |
|---|---|---|---|---|---|
| Correct management | 34.5 | 75.5 | 17 363 | 11 508 | 1.51 |
| Correct treatment | 84.1 | 84.6 | 17 363 | 153 | 113 |
*This is the net value of all resources (monetary and non‐monetary) required to provide syndromic packages for 1 year to the 20 intervention clinics. It assumes the training has a useful life of 2 ears and is annualised with a 5% discount rate.
†Computed as number of patients with expected outcome when syndromic packages are used less number of patients with expected outcome in absence of syndromic packages. This estimate is based upon an estimated annual patient volume of 28 061 for the 20 intervention clinics.
Discussion
This study showed that syndromic packages improved the overall syndromic management of STIs by increasing the supply of condoms and partner notification cards, which were the greatest problems at baseline.10 It also increased the thoroughness of STI education, especially for females. Correct treatment did not differ between trial arms but was more frequent in patients who actually received syndromic packages.
This trial differed in several ways from the earlier trial reported by Harrison and others in 2000.15 Firstly, it evaluated the effect of the syndromic package, additional to training which control clinics received too. In the earlier trial only intervention clinics received STI training. Effects on appropriate management and information provision were similar in both trials, but we found no effect on correct treatment. This could be because our control clinics received training, or because correct treatment was already high at baseline (79%), compared to the previous trial (41%).15 Secondly, this trial was conducted in a large city whereas the earlier trial was conducted in a rural area with fewer services. Thirdly, this trial included more randomly sampled clinics and so had greater power and generalisability.
Our use of simulated patients probably caused less bias than assessment by observation, which could affect clinician behaviour, or exit interviews, which depend on patients' observation, memory, and candour. Drawbacks of simulated patients include the difficulty of simulating genital ulcer disease or discharge, possibly poor acting, and their cost.
This trial did not show that syndromic treatment was effective in curing or preventing STIs in actual patients. If the symptoms were insensitive or non‐specific, or if infective organisms were unresponsive to treatments, then syndromic treatment would not be effective.1 Use of real patients would enable laboratory assessment of under‐treatment and over‐treatment and treatment outcomes, assessment of sexual partners' treatment, and estimation of patient level costs and cost effectiveness.
Key messages
Providing primary care nurses with syndromic STI packages (containing antibiotics, condoms, partner notification cards, and information leafleted) improved syndromic STI management in Durban clinics
It was cost effective
It increased STI advice and information provision, especially among women
The cost and cost effectiveness of the intervention was reasonable, considering the public health benefits of syndromic STI treatment and prevention.1 If scaled up, packaging costs would increase but clinic workloads would probably decrease because of less preparation and better STI prevention. In other developing countries with STI epidemics, primary care staffing, organisation and costs may differ from this setting. But it seems likely that STI syndromic packages could be effective and cost effective in improving syndromic STI care and so should be more widely used.
Acknowledgements
The study was funded by Family Health International. We thank the editor and referees for helpful suggestions.
Contributors
MC conceived, initiated, and coordinated all aspects of the study and is guarantor of the paper; MOB and CC led the trial design and data analysis; DN coordinated the trial, including instruments development and training and supervision of simulated patients; FMN contributed to data analysis; RKH and EBR conducted the economic evaluation. All authors contributed to writing or commenting on the paper.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Dallabetta G A, Gerbase A C, Holmes K K. Problems, solutions, and challenges in syndromic management of sexually transmitted diseases. Sex Transm Infect 199874(suppl 1)S1–11. [PubMed] [Google Scholar]
- 2.Frieden T R, Das‐Douglas M, Kellerman S E.et al Applying public health principles to the HIV epidemic. N Engl J Med 20053532397–2402. [DOI] [PubMed] [Google Scholar]
- 3.Wasserheit J N. Epidemiologic synergy: interrelationships between HIV and other STDs. Sex Transm Dis 19921961–77. [PubMed] [Google Scholar]
- 4.Wilkinson D, Harrison A, Lurie M.et al STD syndrome packages: improving syndromic management of sexually transmitted diseases in developing countries. Sex Transm Dis 199926152–156. [DOI] [PubMed] [Google Scholar]
- 5.Harrison A, Wilkinson D, Lurie M.et al Improving quality of sexually transmitted disease case management in rural South Africa. AIDS 1998122329–2335. [DOI] [PubMed] [Google Scholar]
- 6.Chabikuli N, Schneider H, Blaauw D.et al Quality and equity of private sector care for sexually transmitted diseases in South Africa. Health Policy Plan 200217(Suppl)4046. [DOI] [PubMed] [Google Scholar]
- 7.Schneider H, Blaauw D, Dartnall E.et al STD care in the South African private health sector. S Afr Med J 200191151–156. [PubMed] [Google Scholar]
- 8.Connolly A M, Wilkinson D, Harrison A.et al Inadequate treatment for sexually transmitted diseases in the South African private health sector. Int J STD AIDS 199910324–327. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson D, Abdool Karim S S, Harrison A.et al Unrecognized sexually transmitted infections in rural South African women: a hidden epidemic. Bull World Health Organ 19997722–28. [PMC free article] [PubMed] [Google Scholar]
- 10.Mbofana F S, Brito F J, Saifodine A.et al Syndromic management of sexually transmitted diseases at primary care level, Mozambique. Sex Transm Infect 200278E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann M O, Colvin M S E, Nsibande D.et al Quality of primary care for sexually transmitted diseases in Durban, South Africa: influences of patient, nurse, organizational and socioeconomic characteristics. Int J STD AIDS 200415388–394. [DOI] [PubMed] [Google Scholar]
- 12.Boonstra E, Lindbaek M, Klouman E.et al Syndromic management of sexually transmitted diseases in Botswana's primary health care: quality of care aspects. Trop Med Int Health 20038604–614. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson D, Abdool Karim A A, Harrison A.et al Unrecognized sexually transmitted infections in rural South African women: a hidden epidemic. Bull World Health Organ 19997722–28. [PMC free article] [PubMed] [Google Scholar]
- 14.Health Systems Trust Health statistics. www.hst.org.za (accessed 25/04/2006)
- 15.Harrison A, Karim S A, Floyd K.et al Syndrome packets and health worker training improve sexually transmitted disease case management in rural South Africa: randomized controlled trial. AIDS 2000142769–2779. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs B, Kambugu F S, Whitworth J A.et al Social marketing of pre‐packaged treatment for men with urethral discharge (Clear Seven) in Uganda. Int J STD AIDS 200314216–221. [DOI] [PubMed] [Google Scholar]
- 17.South African National Department of Health Protocols for the management of a person with a sexually transmitted disease. Pretoria: South African National Department of Health, 1998
- 18.FXHistory: Historical currency exchange rates http://www.oanda.com/convert/fxhistory (accessed on 25 May 2005)