Abstract
Objectives
To assess the feasibility of collecting sexual behaviour data during HIV surveillance in antenatal care (ANC) clinics, and to establish whether these data can provide information about the correlates of HIV infection in this population.
Methods
Sexual behaviour surveys were conducted in the context of two HIV sentinel surveillance rounds in 11 ANC clinics in north west Tanzania between 2000 and 2002. Responses of individual women were anonymously linked to their HIV status. Three clinic catchment areas overlapped with a community based longitudinal study, which provided independent estimates of HIV prevalence and sexual behaviour. Changes between rounds and differentials between clinics were assessed and a two level logistic regression model used to identify behavioural and contextual correlates of HIV in 3689 women under 25 years of age.
Results
Women attending clinics were willing to participate in the study. The sexual behaviour data obtained were internally consistent and tallied reasonably well with sexual behaviour data collected in the community overlapping the clinic catchment. Clear relations emerged between HIV infection and measures of sexual exposure: OR 1.20 (95% CL 1.12 to 1.28) for each year of premarital exposure and 1.09 (1.04 to 1.16) for each year after first marriage; background prevalence OR 1.15 (1.04 to 1.26) associated with each percentage point increase in background prevalence at the clinic; and certain partnership variables such as partner's age OR 0.58 (0.45 to 0.76) if partner less than 10 years older.
Conclusion
Conducting sexual behaviour surveys in the context of ANC clinics surveillance is feasible and yields useful data.
Keywords: HIV prevalence, sexual behavioural, surveillance, Tanzania, antenatal care
In generalised epidemics, HIV estimates are usually based on antenatal care (ANC) surveillance, testing residual blood left over from routine syphilis screening.1 HIV test results cannot be linked back to individual women, but simple characteristics (age, parity, education, marital status, and area of residence) are recorded to provide a more informative breakdown of observed patterns and trends. However, our capacity to analyse prevalence trends attributable to sexual behaviour change is limited by the lack of linked behavioural information.2 The objectives of this study are to assess the feasibility of collecting sexual behaviour data during HIV surveillance in ANC clinics, and to establish whether these data can provide information about the correlates of HIV infection in this population.
Setting
The study site is in the Mwanza region of north west Tanzania. Settlements in the area range from remote rural villages to highly urbanised areas, such as Mwanza (population 300 000), officially designated a city in 2000. The Sukuma are the main ethnic group—the majority practise subsistence agriculture, selling surplus produce in roadside markets and urban areas.
The long history of HIV research in this part of Tanzania provides good background data. Prevalence was measured in the city ANC clinics3,4,5,6,7 and in Kisesa ward which hosted a longitudinal community based study since 19948,9 providing HIV prevalence comparisons between pregnant women and the community.10 Estimates obtained from these sources suggest prevalence in the city has been fairly stable since 1994, at around 12%, whereas in the adjacent semirural part of Magu district, prevalence increased steadily from 6% to 8% in the same time.4,5,6,7,8,11
Methods
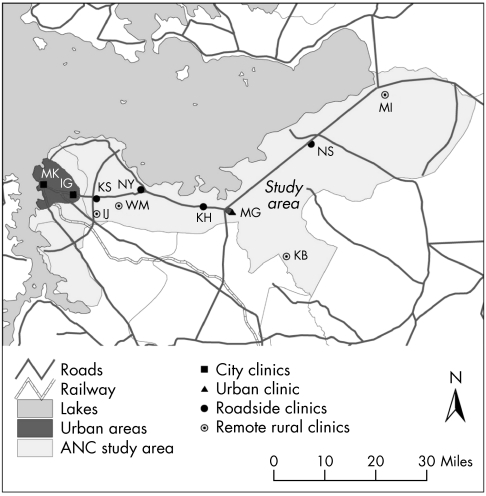
Two rounds of an experimental surveillance study were conducted in 2000–2 in 11 ANC clinics: two in Mwanza city, one in the district hospital in Magu town, four in rural roadside settlements, and four in remote rural areas of Magu district (fig 1). Data collection lasted 3 months in each annual clinic round. Every woman attending ANC clinics for the first time in the current pregnancy was eligible for inclusion in the study (every third woman in the busy city centre clinic). Special training was provided to health workers conducting the interviews and to those collecting specimens. Ethical approval was given by the Tanzanian National Institute for Medical Research.
Figure 1 Location of ANC clinic facilities participating in experimental surveillance. Key to clinic names: MK, Makongoro; IG, Igoma; KS, Kisesa; WS, Welamasonga; IJ, Igekamaja; NY, Nyanguge; KH, Kahangara; MA, Magu; KB, Kabila; NS, Nassa; ML, Mkula.
Verbal consent was obtained for interviews, conducted before all clinical procedures. Refusals were not recorded directly, but a check was made on the client volume of participating clinics in the weeks in which interviews took place. Tanzanian ANC protocols state that blood should routinely be taken from all pregnant women for syphilis screening, and treatment offered as necessary. This procedure was followed in the study clinics, and partner notification encouraged. Women gave separate verbal consent to the phlebotomist to use residual blood from the syphilis screening for HIV testing, on the understanding that neither they nor the phlebotomist would learn the result. The protocol differed in this respect from standard national surveillance procedures, where informed consent is not required. Dried blood spots were used for HIV tests, using Vironostika HIV‐MIXT (Organon, Boxtel, Netherlands), ELISA, and Enzygnost HIV1/HIV2 (Behring, Marburg, Germany) for confirmation of positive results.
The questionnaire collected information on previous ANC use, current residence, mobility, socioeconomic background, pregnancy history, marital history, recent sexual behaviour, partner's sexual behaviour, family planning use and sexually transmitted disease (STD) signs and symptoms. Women received syndromic treatment if they reported symptoms of STD other than syphilis. There were no programmes to prevent mother to child transmission (PMTCT) in the study clinics, but voluntary counselling and testing (VCT) was available at Makongoro and Magu clinics—around 23 participants who wanted to know their HIV status were directed there. The supervisor visited each clinic fortnightly to collect completed questionnaires. Data were entered in duplicate; automatic data editing included imputation for certain missing or out of range values, based on consistency with other information (for example, age at marriage derived from current age and marriage duration).
Stata 8.112 was used for statistical analyses. χ2 tests were used to assess the significance of differences in the distribution of risk factors at clinic level, and standard errors were adjusted for clinic level clustering. Length of exposure to risk was measured as the difference between current age and age at first sex. For never married women exposure was exclusively premarital; for ever married women it was broken down into premarital exposure and exposure after first marriage. Analysis was restricted to women under 25, since most infections in this age group are fairly recent, and more strongly related to recent behaviour.13 Continuous forms were used for variables representing exposure time and clinic prevalence.
We hypothesised that HIV status was determined by factors operating at two levels: individual (level 1) and community (level 2). Fixed effects logistic regression was used in a multilevel model to identify the relative contribution of individual level correlates of HIV infection in the light of background HIV prevalence and behaviour patterns.14 Each clinic was treated as a primary sampling unit and robust standard errors were calculated to allow for clustering, which is also reflected in the adjusted Wald tests. Level 2 variables were HIV and syphilis prevalence; average values of behavioural and sociodemographic indicators for women attending each clinic; and a categorical variable describing clinic location. Co‐linear combinations of variables were excluded, and variables that did not show a relation with HIV infection either in univariate or multivariate regression with significance less than 30% are not reported.
Results
A total of 7032 women (3689 under 25) participated in the study; 1634 and 2055 were interviewed in rounds 1 and 2, respectively. Only seven questionnaires could not be matched with serological results because of identification code errors. No systematic count was kept of refusals, but staff recall fewer than five women declining the syphilis test. A comparison of numbers interviewed with numbers registered in eight ANC clinics where registers were available for inspection showed 2180 registered and 2182 interviewed in round 1; and 2264 registered and 2626 interviewed in round 2. The excess is the result of inclusion of women who went for their first ANC clinics visit to a clinic that was not participating in the study, and then switched to a study clinic.
Overall, unweighted HIV prevalence was 10.2% in women under 25 and 10.7% in all women, and changed little between rounds. Prevalence was highest in city clinics and lowest in those serving rural areas, but age patterns were similar across sites, with rapid increases in the early twenties, and declines after age 35. The only risk factor to show a substantial overall change between rounds was syphilis prevalence, increasing from 11.1% to 16.7% (χ2(1) = 23.4, pr<0.0001).
Notable differences were observed in socioeconomic and behavioural characteristics of women attending clinics in different sites (table 1). Women attending city clinics had much shorter residence duration; proportions with secondary education and in polygamous marriages show steady gradients from remote rural to city clinics. The proportion sexually active before 15 was highest in remote clinics followed by city clinics. Higher fractions of women attending city and roadside clinics were past users of condoms and contraceptives. Syphilis prevalence was higher in rural clinics. Women in city and roadside clinics are more likely to report more than one sexual partner in the course of the last year, but higher proportions of women in rural clinics report that their male partners had more than one partner—partly explained by higher rates of polygamy in these areas.
Table 1 Characteristics of ANC clinic users under 25 by clinic site: percentage of total (n).
| Characteristic | Rural | Urban | p Value for χ2 | ||
|---|---|---|---|---|---|
| Remote | Roadside | Town | City | ||
| Number of clinics | 4 | 4 | 1 | 2 | |
| Number of women | 705 | 1042 | 656 | 1286 | |
| HIV prevalence | 6.0 (42) | 10.3 (107) | 9.0 (59) | 13.0 (167) | 0.017 |
| Residence | |||||
| Resident <1 year | 27.7 (195) | 31.8 (331) | 32.2 (211) | 51.9 (668) | |
| Resident 1–5 years | 24.8 (175) | 21.1 (220) | 22.3 (146) | 29.3 (377) | 0.000 |
| Resident 5+ years | 7.4 (52) | 7.1 (74) | 7.9 (52) | 6.3 (81) | |
| Always at current residence | 40.1 (283) | 40.0 (417) | 37.7 (247) | 12.4 (160) | |
| Current urban residents | 0.7 (5) | 0.2 (2) | 46.0 (302) | 96.5 (1241) | 0.000 |
| Previously urban resident | 10.2 (72) | 14.7 (153) | 24.7 (162) | 55.9 (719) | 0.000 |
| Age | |||||
| Age <18 | 10.1 (71) | 10.8 (113) | 9.5 (62) | 11.4 (147) | 0.520 |
| Marriage | |||||
| Never married | 21.6 (152) | 20.2 (210) | 11.7 (77) | 35.8 (460) | |
| Currently married | 76.2 (537) | 77.5 (808) | 87.5 (574) | 62.9 (809) | 0.055 |
| Formerly married | 2.3 (16) | 2.3 (24) | 0.8 (5) | 1.3 (17) | |
| In polygamous marriage | 11.2 (79) | 10.0 (104) | 6.1 (40) | 3.9 (50) | 0.028 |
| Education | |||||
| None | 21.1 (149) | 13.9 (145) | 16.5 (108) | 11.4 (147) | |
| Incomplete primary | 24.0 (169) | 22.8 (238) | 17.1 (112) | 19.4 (249) | 0.008 |
| Completed primary | 53.2 (375) | 59.7 (622) | 61.1 (401) | 61.9 (796) | |
| Some secondary | 1.7 (12) | 3.6 (37) | 5.3 (35) | 7.4 (95) | |
| Occupation | |||||
| Peasant | 97.2 (685) | 86.0 (896) | 81.6 (535) | 20.5 (263) | 0.000 |
| Other STD | |||||
| Positive syphilis (RPR) | 15.3 (108) | 21.3 (222) | 9.3 (61) | 10.5 (135) | 0.057 |
| Self reported STI | 3.3 (23) | 6.5 (68) | 4.6 (30) | 10.2 (131) | 0.000 |
| Start of sexual activity | |||||
| Sex before age 15 | 10.5 (74) | 7.4 (77) | 4.1 (27) | 8.2 (105) | 0.003 |
| Sex before marriage | 55.3 (390) | 67.4 (702) | 65.5 (430) | 71.5 (919) | 0.171 |
| Sexual precautions | |||||
| Ever used family planning | 5.1 (36) | 8.9 (93) | 6.1 (40) | 16.6 (213) | 0.001 |
| Ever used condom | 7.7 (54) | 15.6 (163) | 13.3 (87) | 18.7 (241) | 0.044 |
| Sexual partners | |||||
| Partner <10 years older | 47.0 (331) | 53.1 (553) | 63.4 (416) | 44.2 (568) | |
| Partner 10+ years older | 9.4 (66) | 10.7 (104) | 10.0 (70) | 10.3 (133) | 0.098 |
| Partner age not known | 43.7 (308) | 36.9 (384) | 25.9 (170) | 45.5 (585) | |
| More than 1 partner last year | 5.2 (37) | 9.5 (99) | 4.7 (31) | 13.1 (168) | 0.055 |
| Partner had >1 partner last year | 23.7 (167) | 26.4 (275) | 14.3 (94) | 18.8 (242) | 0.009 |
RPR, rapid protein reagin; STI, sexually transmitted infections.
Correlates of HIV infection: individual level factors
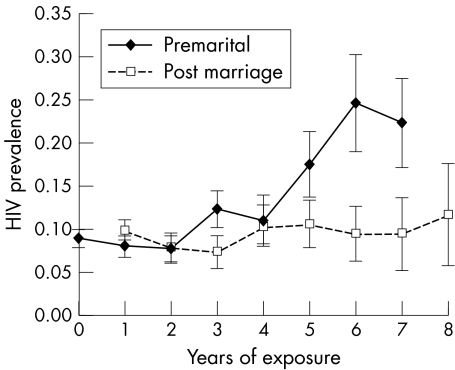
Logistic regression results assessing the influence of selected variables on HIV status are shown in table 2. Figure 2 shows that in young women lengthy premarital exposure is riskier than equivalent years of exposure after marriage. Current marital status loses its explanatory value when past exposure time in different marital status categories is controlled. In the crude analysis, working in subsistence agriculture and attending a remote rural clinic are associated with low odds of infection, whereas past or current urban residence and having a secondary education carry increased risk, only past urban residence retains a strong association with increased risk in the multivariate model.
Table 2 Crude and adjusted odds ratios for HIV infection in 3689 women under 25.
| Explanatory variables | Crude | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Individual level | ||||||
| Residence | ||||||
| Current urban (1550) | 1.59 | 1.15 to 2.19 | 0.008 | 1.18 | 0.69 to 2.02 | 0.532 |
| Past urban (1106) | 1.77 | 1.39 to 2.25 | 0.000 | 1.44 | 1.12 to 1.87 | 0.006 |
| Resident less than 1 year (1405) | 1.13 | 0.85 to 1.52 | 0.373 | 1.11 | 0.82 to 1.49 | 0.476 |
| Marriage | ||||||
| Never married (899) | 1.37 | 1.01 to 1.89 | 0.85 | 0.61 to 1.26 | ||
| Currently married (2728) | 1 | 0.115 | 0.743 | |||
| Formerly married (62) | 1.23 | 0.46 to 3.27 | 1.09 | 0.41 to 2.94 | ||
| Education | ||||||
| None (548) | 1 | 1 | ||||
| Incomplete primary (768) | 1.46 | 0.86 to 2.50 | 0.104 | 1.49 | 0.86 to 2.61 | 0.288 |
| Complete primary (2194) | 1.32 | 0.93 to 1.86 | 1.30 | 0.88 to 1.92 | ||
| Some secondary (179) | 2.00 | 1.05 to 3.79 | 1.51 | 0.77 to 2.97 | ||
| Occupation | ||||||
| Peasant (2379) | 0.60 | 0.45 to 0.80 | 0.002 | 0.73 | 0.57 to 0.94 | 0.016 |
| Sexual precautions | ||||||
| Ever used family planning (382) | 1.31 | 1.05 to 1.64 | 0.021 | 0.90 | 0.71 to 1.13 | 0.312 |
| Ever used condom (545) | 1.85 | 1.27 to 2.69 | 0.003 | 1.52 | 0.96 to 2.40 | 0.071 |
| Other STI | ||||||
| Positive syphilis (526) | 1.22 | 0.84 to 1.76 | 0.281 | 1.22 | 0.87 to 1.72 | 0.225 |
| Self reported STD (252) | 1.52 | 1.02 to 2.27 | 0.040 | 1.24 | 0.82 to 1.89 | 0.289 |
| Sexual partners | ||||||
| Partner <10 years older (1868) | 0.60 | 0.48 to 0.75 | 0.000 | 0.58 | 0.45 to 0.76 | 0.001 |
| More than 1 partner last year (335) | 1.38 | 0.79 to 2.40 | 0.237 | 1.04 | 0.58 to 1.89 | 0.885 |
| Partner had more than 1 partner (778) | 1.23 | 0.93 to 1.62 | 0.144 | 1.16 | 0.89 to 1.50 | 0.261 |
| Sexual exposure | ||||||
| Age* | 1.11 | 1.07 to 1.15 | 0.000 | (co‐linear) | ||
| Years premarital exposure* | 1.15 | 1.09 to 1.22 | 0.000 | 1.20 | 1.12 to 1.28 | 0.000 |
| Years since first marriage* | 0.97 | 0.93 to 1.02 | 0.263 | 1.09 | 1.04 to 1.16 | 0.003 |
| Age at sexual debut* | 1.00 | 0.90 to 1.06 | 0.904 | 1.10 | 1.02 to 1.17 | 0.011 |
| Clinic level | ||||||
| Type of site | ||||||
| Rural remote (705) | 1 | |||||
| Rural roadside (1042) | 1.81 | 1.09 to 2.99 | 0.000 | 1.26 | 0.90 to 1.77 | 0.857 |
| Urban town (656) | 1.56 | 1.34 to 1.80 | 1.02 | 0.62 to 1.67 | ||
| Urban city (1286) | 2.36 | 1.87 to 2.96 | 0.59 | 0.17 to 2.04 | ||
| Risk of infected partner | ||||||
| HIV percentage prevalence* | 1.08 | 1.05 to 1.12 | 0.000 | 1.15 | 1.04 to 1.26 | 0.009 |
| Syphilis percentage prevalence* | 0.98 | 0.93 to 1.03 | 0.368 | 0.96 | 0.83 to 1.08 | 0.200 |
| Behaviour profile | ||||||
| Mean partners in last year* | 1.98 | 0.72 to 5.47 | 0.172 | 3.36 | 0.93 to 10.94 | 0.093 |
| Percentage with no premarital sex* | 0.99 | 0.98 to 1.00 | 0.180 | 0.96 | 0.92 to 1.01 | 0.089 |
| Mobility | ||||||
| Percentage resident <1 year* | 1.02 | 1.01 to 1.04 | 0.010 | 1.02 | 1.00 to 1.04 | 0.035 |
*Continuous variables.
Figure 2 HIV prevalence in ANC clinic attenders aged less than 25 years, by duration of exposure before and after first marriage.
Having a partner less than 10 years older was associated with low odds of infection, even when other explanatory variables were considered. However, partner's age was incompletely reported: 23% of young married women did not know their husband's age; 89% of single women and 40% of the previously married did not know the age of their partner. Those who did not know their partner's age had similar crude odds ratios (OR) for HIV infection to those whose partner was more than 10 years older (1.7 and 1.8 respectively) and similar adjusted OR (1.5 and 1.6), which justified grouping them together in the analysis. Women who knew their partners were younger might have lower odds of HIV infection because they have more stable relationships and know their partners better, or because their partners have been less exposed to infection risk.
Other sexual behaviour variables (such as having more than one sexual partner) which did not have a strong association with HIV status, either alone or in a multivariate model, nevertheless had OR which suggested that they acted in the expected direction. The exception is age at sexual debut, which is not important in the crude analysis, but is positively correlated with HIV risk in the full model.
Condom use is positively related to HIV risk, and is also a marker for risky behaviour: only 11% of women with one sexual partner last year had ever used a condom, compared to 30% of those with two sexual partners, or 39% of those with three or more (χ2(2) = 218, pr<0.0001). The correlation of condom use with partner's risk behaviour was weaker: 11% of women who believed themselves to be their husband's only sexual partner had ever used a condom, compared to 15% of those who believed their husbands had other partners (χ2(1) = 12, pr<0.0001). Condoms are not generally regarded as a method of family planning: 70% of 880 women who reported having used condoms said they had never used family planning.
Correlates of HIV infection: clinic level factors
Remote rural clinic location was associated with a lower OR in the crude analysis; however, when background prevalence is controlled for clinic location becomes unimportant. Syphilis prevalence and clinic level behaviour measures were not associated with increased risk, but the community level measure of mobility appeared influential in both crude and adjusted models. The most important clinic level factor with respect to individual risk is the overall prevalence of HIV in clients of all ages. When this is controlled even some of the individual level factors, such as client's current residence lose their explanatory value.
Although no relation emerged between HIV infection and individual level mobility (residence change in past year), mobility may be important as a clinic level variable (proportion resident less than a year). This may be because of a specific change in one clinic: Kisesa clinic recorded a large increase in the proportions previously resident in an urban area (from 5% to 22%) and in HIV prevalence (from 10% to 26%). Between rounds a temporary housing centre was established in the neighbourhood for road building workers, whose partners used the clinic facilities. In Kisesa centre, but not in other clinics, there was a strong positive association between HIV status and individual recent residence change.
Syphilis prevalence increased markedly between rounds in just three clinics: Magu (from 2% to 15%), Makongoro (3% to 9%), and Nyanguge (13% to 32%), and these three clinics also reported around 30% higher numbers of interviews and blood tests than first visit ANC clinics registrations. However there were no corresponding increases in sexual behaviour risk indicators, and HIV prevalence actually declined slightly (from 12.3% to 10.5%) in these clinics. Excluding round 2 sessions in these three clinics did not alter the OR in the multivariate analysis.
Comparison with population based estimates
Table 3 compares measures from the population cohort study in Kisesa ward,8 the catchment area for Kisesa, Welamasonga, and Igekamaja clinics with results from ANC clinics surveillance. This table includes women of all ages, and although numbers in the ANC clinics sample are rather small, the general agreement is quite good, particularly for HIV. With respect to sexual behaviour, differences between remote and roadside locations are the same in both data sources, and ANC clinic clients report similar levels of condom use and non‐marital partnerships to women in the community, but slightly higher levels than women interviewed in the community based survey who gave birth in 1999–2000.
Table 3 Comparison of ANC clinics surveillance results with community based survey, Kisesa Ward.
| Community based survey, 1999–2000 | ANC clinic surveillance 2000 | |||
|---|---|---|---|---|
| Both sexes | Women | Women who gave birth 1999–2000 | ||
| Number | ||||
| Remote | 1753 | 969 | 310 | 151 |
| Roadside | 1993 | 1196 | 338 | 186 |
| HIV prevalence, % | ||||
| Remote | 5.5 | 5.7 | 4.8 | 4.6 |
| Roadside | 11.0 | 12.4 | 11.7 | 11.3 |
| Ever use a condom, % | ||||
| Remote | 9.9 | 3.1 | 3.6 | 2.7 |
| Roadside | 17.6 | 6.8 | 8.9 | 10.2 |
| Non‐marital partner last year, % | ||||
| Remote | 6.5 | 4.1 | 3.3 | 4.6 |
| Roadside | 9.9 | 6.0 | 3.9 | 8.1 |
| Median age at first sex | ||||
| Remote | 15.9* | 15.7* | 15.5† | |
| Roadside | 16.5* | 16.1* | 15.7† | |
*Calculated using current status data; †calculated from retrospective report.
Discussion
Sexual behaviour data collected in ANC clinics were reasonably complete, plausible, and internally consistent. Statistical analysis showed the most important risk factors were duration of exposure to sexual activity, especially premarital sexual activity, having a partner who was more than 10 years older (and thus exposed for longer), past residence in an urban area and current residence in the catchment area of a clinic with high HIV prevalence. The relation between urban residence and HIV risk is a common finding, and other studies have noted the importance of large age differences between sexual partners in facilitating the spread of HIV.15
Decomposing the effect of age on HIV infection into duration of sexual exposure before and after marriage is an improvement on analytical procedures that consider only current marital status. No information was available about time spent after the end of their first marriage by pregnant women who had been widowed, separated, or remarried. It is likely that years of exposure in these categories carry different levels of risk to exposure within first marriage—in future studies it would be useful to include questions on age at end of marriage to further refine measurement of exposure time.
Many studies have shown HIV prevalence in ANC clinics corresponds closely with prevalence for both sexes in clinic catchment areas,10,16 but use of a clinic level variable to measure the risk of encountering an infected partner is also an important methodological advance.17,18 Other clinic level variables did not add to the explanatory power of our model, even though there were clear behavioural differences between clinic populations,19 but geographical interpretation of the results is limited by the fact that some of the women came from outside the notional clinic catchment areas
Ethical and practical objections20 have been raised to the use of surveys about sexual behaviour in ANC clinics settings, on the grounds that introducing non‐routine procedures (such as interviews) that do not directly benefit the mother, entails the need for obtaining informed consent, which increases refusals and decreases representativeness. In this study refusal rates were extremely low for both specimen collection and interview. The possibility remains that some women may have avoided using these clinics during the study, though probably in smaller numbers than those attracted by the availability of syphilis testing.
In round 2, interviewed numbers were higher than registered numbers, because women who attended a non‐study ANC clinic earlier in their pregnancy were not registered as first time attenders in study clinics. Switching ANC clinics during pregnancy was thought to be associated with provision of syphilis tests in the study clinics, knowledge about the availability of these tests having spread to adjacent clinics between the two rounds. Representativeness of these results may have been compromised by inclusion of women whose nearest ANC clinic was outside the study area, particularly in the second round, although overall syphilis prevalence is almost identical in those women who were recorded as repeat attenders in round 2 compared to those attending for the first time.
The clinics participating in this study are a convenience sample—not chosen to be representative of Mwanza and Magu districts. Their catchment populations are not defined, so it is not possible derive an estimate of HIV prevalence for the community as a whole by weighting the data. However, the close correspondence between ANC clinics and community data collected in Kisesa ward validates these reports, and suggests that denial of risk behaviours does not happen to a greater extent in women interviewed in ANC clinics than in other survey settings. ANC clinic women actually report slightly higher rates of risky behaviour—for example, higher proportions with a non‐marital partner last year—possibly because many marriages occur when pregnancy is confirmed after an ANC clinic visit. Complete denial of sexual activity, an error that biases estimates of age at first sex in community based interviews of young women,2 does not affect ANC clinics data collection. The plausible regression analysis results, showing a strong relation between infection risk and sexual exposure time before and after marriage suggests that the retrospective reporting of ages and dates is of reasonable quality.
The correlation between HIV and syphilis infections was not as strong as we had expected. Syndromic management of STI has been available in this area since 1997, which may have depressed the proportion of active syphilis cases. Routine testing and treatment of syphilis in ANC clinics before this study was concentrated in the urban clinics where HIV prevalence is higher.
Behavioural interviews in ANC clinics could be part of a second generation surveillance1 strategy, making sexual behaviour data collection an inexpensive, routine activity. Data on sociodemographic and behavioural characteristics of clinic populations may help to explain changes over time or persistent differences between clinics. Multilevel statistical analysis would be possible at national level if individual data from participating clinics were pooled, rather than adding aggregate tables.
Key messages
Behavioural surveillance in antenatal clinics is feasible, ethical, and efficient and can be used to produce reliable data in a routine fashion
Sexual behaviour data from antenatal clinic attenders in HIV sentinel surveillance clinics can be individually linked to HIV status data, and used to interpret HIV results, helping to explain interclinic differences and, eventually, time trends
Statistical analyses of the relation of HIV status to sociodemographic and behavioural data should incorporate measures of exposure to risk, both at the community level (for example, average prevalence in the clinic population) and at the individual level (for example, time duration since start of sexual activity)
As PMTCT programmes are introduced into selected antenatal clinics, the characteristics of pregnant women who participate in surveillance may change and this will affect the validity of HIV trend data from these sites.21,22 Background behavioural information will make it possible to identify such changes and adjust surveillance data from PMTCT clinics to make it more representative.17
Given the interesting findings that have emerged from this modest survey, it would be useful to repeat a similar exercise in the same clinics after a longer interval, during which larger changes may occur in behaviour and HIV prevalence. Interpretation of ANC clinic surveillance in other parts of Tanzania could be improved by adding simple questions to existing protocols, to yield indicators of sexual risk behaviour without appearing unduly intrusive in the ANC clinic context.
Acknowledgements
The National Institute for Medical Research (Mwanza branch) was able to carry out this research thanks to financial support from the TANESA programme and from the World Health Organization.
Contributors
MU, principal investigator in charge of the whole study, including liason with NIMR laboratory; YK, field supervisor, responsible for training, logistics, coordination; RI, statistician, data cleaning, editing, and management; GM, (previous) director of Kisesa cohort study, supplied comparison data; BM, district medical officer (Mwanza) facilitating work in ANC clinics, staff management (interviewers and phlebotomists), setting up syphilis testing facility in clinics; KM, district medical officer (Magu) facilitating work in ANC clinics, staff management (interviewers and phlebotomists), setting up syphilis testing facility in clinics; TB, technical advice, design of study protocol; TC, technical advice, ethical aspects, and coordination with WHO second generation surveillance project; ES, data analysis, statistical advice, poster design for conference presentation; BZ, data analysis, writing first draft of this manuscript. In addition, all authors read and commented on the draft versions of the paper, contributed to the editing process and approved the final draft.
Abbreviations
ANC - antenatal care
PMTCT - prevention of mother to child transmission
RPR - rapid protein reagin
STD - sexually transmitted diseases
STI - sexually transmitted infections
VCT - voluntary counselling and testing
Footnotes
The authors declare that they have no conflict of interest in connection with this paper.
Ethical approval was given by the Tanzanian National Institute for Medical Research, Dar es Salaam.
References
- 1.UNAIDS/WHO Working Group of Global HIV/AIDS and STI Surveillance Guidelines for second generation HIV surveillance. Geneva: UNAIDS, 2000
- 2.Zaba B, Pisani E, Slaymaker E, Boerma T. Age at first sex: understanding recent trends in African demographic surveys. Sex Transm Infect 200480(Suppl II)ii28–ii35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borgdorff M, Barongo L, van Jaarsveld E.et al Sentinel surveillance for HIV‐1 infection: how representative are blood donors, outpatients with fever, anaemia, or sexually transmitted diseases, and antenatal clinic attenders in Mwanza Region, Tanzania? AIDS 19937567–572. [PubMed] [Google Scholar]
- 4.Changalucha J, Grosskurth H, Mwita W.et al Comparison of HIV prevalences in community‐based and antenatal clinic surveys in rural Mwanza. AIDS 200216661–665. [DOI] [PubMed] [Google Scholar]
- 5.Kigadye R M, Klokke A, Nicoll A.et al Sentinel surveillance for HIV‐1 among pregnant women in a developing country: 3 years' experience and comparison with a population serosurvey. AIDS 19937849–855. [DOI] [PubMed] [Google Scholar]
- 6.Mayaud P, Gill D K, Weiss H A.et al The interrelation of HIV, cervical human papillomavirus, and neoplasia among antenatal clinic attenders in Tanzania. Sex Transm Infect 200177248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley M, Munguti K, Grosskurth H.et al Sexual behaviour patterns and other risk factors for HIV infection in rural Tanzania: a case‐control study. AIDS 199711237–248. [DOI] [PubMed] [Google Scholar]
- 8.Boerma J T, Urassa M, Senkoro K P.et al Spread of HIV infection in a rural area of Tanzania. AIDS 1999131233–1240. [DOI] [PubMed] [Google Scholar]
- 9.Zaba B W, Carpenter L M, Boerma J T.et al Adjusting ante‐natal clinic data for improved estimates of HIV prevalence among women in sub‐Saharan Africa. AIDS 2000142741–2750. [DOI] [PubMed] [Google Scholar]
- 10.Boerma J T, Gregson S, Nyamukapa C.et al Understanding the uneven spread of HIV within Africa: comparative study of biologic, behavioral, and contextual factors in rural populations in Tanzania and Zimbabwe. Sex Transm Dis 200330779–787. [DOI] [PubMed] [Google Scholar]
- 11.Grosskurth H, Mosha F, Todd J.et al Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet 1995346530–536. [DOI] [PubMed] [Google Scholar]
- 12. Stata/SE 8.1 for Windows [computer program]. Version 8.1. College Station, USA: Stata Corporation, 2003
- 13.Zaba B, Boerma J T, White R. Monitoring the AIDS epidemic using HIV prevalence data for young women attending antenatal clinics: prospects and problems. AIDS 2000141633–1645. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein H, Leyland A. eds. Further topics in multi‐level modelling. Multilevel modelling of health statistics. Chapter 12. London: Wiley, 2004
- 15.Luke N. Age and economic asymmetries in the sexual relationships of adolescent girls in sub‐Saharan Africa. Studies in Family Planning. 2003 20033467–86. [DOI] [PubMed] [Google Scholar]
- 16.Kwesigabo G, Killewo J Z, Urassa W.et al Monitoring of HIV‐1 infection prevalence and trends in the general population using pregnant women as a sentinel population: 9 years experience from the Kagera region of Tanzania. J Acquir Immune Defic Syndr 200023410–417. [DOI] [PubMed] [Google Scholar]
- 17.Zaba B, Slaymaker E, Urassa M.et al The role of behavioural data in HIV surveillance. AIDS 2005 [DOI] [PubMed]
- 18.Auvert B, Buve A, Ferry B.et al Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub‐Saharan Africa with different levels of HIV infection. AIDS 200115(suppl 4)S15–S30. [DOI] [PubMed] [Google Scholar]
- 19.Urassa M, Kumogola Y, Isingo R.et al Integrated ante‐natal HIV and behavioural surveillance in Tanzania. Paper presented at: XV International AIDS Conference, 2004; Bangkok, Thailand,
- 20.Centers for Disease Control and Prevention (CDC) Global AIDS Program (GAP), World Health Organization (WHO), Joint United Nations Programme on HIV/AIDS (UNAIDS), United States Agency for International Development (USAID) Session 3: Linking behavioural and HIV surveillance. Paper presented at: New strategies for HIV/AIDS surveillance in resource‐constrained countries, 2004; Addis Ababa, Ethiopia,
- 21.Hladik W, Masupu K, Roels T.et al Prevention of mother‐to‐child transmission and voluntary counselling and testing programme data: what is their utility for HIV surveillance? AIDS 200519(suppl 2)S19–S24. [DOI] [PubMed] [Google Scholar]
- 22.Mpairwe H, Muhangi L, Namujju P.et al HIV Risk perception and prevalence in a program for prevention of mother‐to‐child HIV transmission comparison of women who accept voluntary counseling and testing and those tested anonymously. J Acquir Immune Defic Syndr 200539354–358. [DOI] [PubMed] [Google Scholar]