Abstract
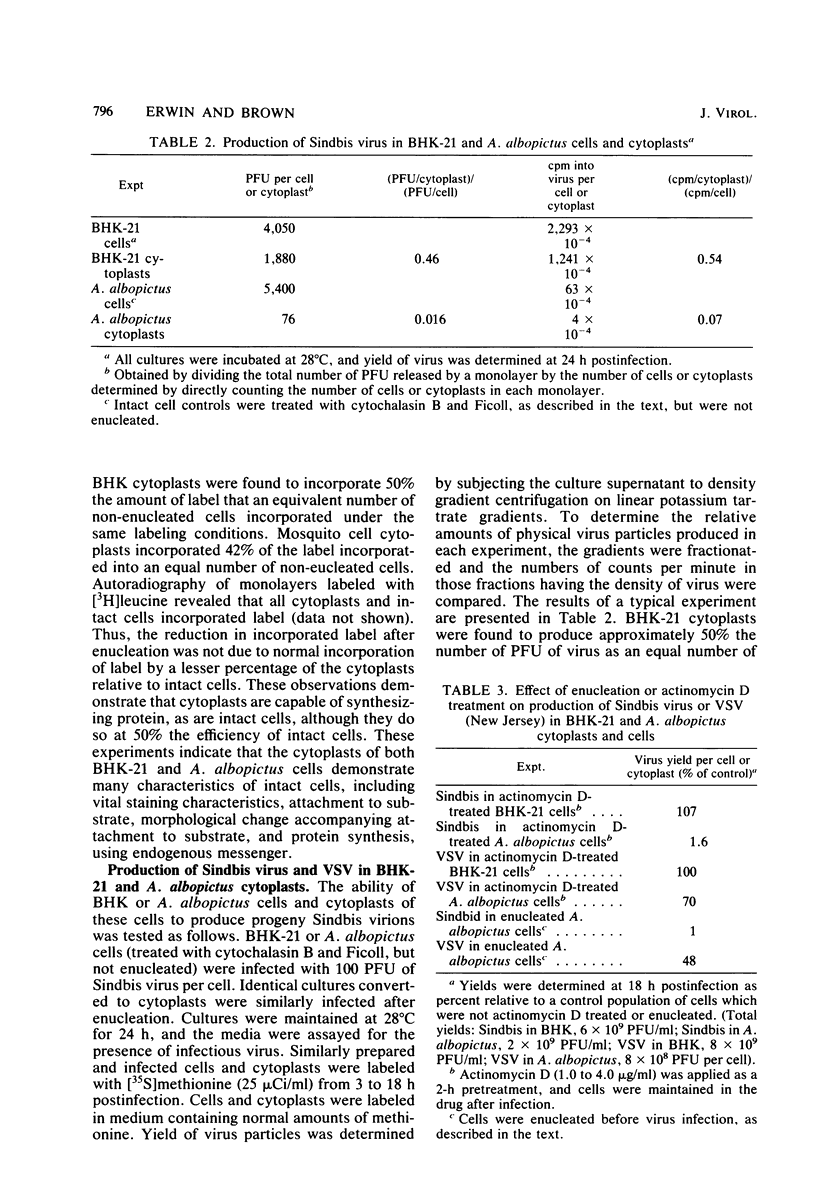
The ability of Sindbis virus to grow in enucleated BHK-21 (vertebrate) and Aedes albopictus (invertebrate) cells was tested to determine the dependence of this virus upon nuclear function in these two phylogenetically unrelated hosts. Although both cell types could be demonstrated to produce viable cytoplasts (enucleated cells) which produced virus-specific antigen subsequent to infection. BHK cytoplasts produced a significant number of progeny virions, whereas mosquito cytoplasts did not. The production of vesicular stomatitis virus in mosquito cells was not significantly reduced by enucleation. That such a host function was not essential for vesicular stomatitis virus growth in insect cells is supported by the observation that the production of this virus by mosquito cells is not actinomycin D sensitive. This result agrees with a previously published report in which it was shown that Sindbis virus maturation in invertebrate cells is inhibited by actinomycin D, indicating a possible requirement for host cell nuclear function (Scheefers-Borchel et al., Virology, 110:292-301, 1981).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baric R. S., Carlin L. J., Johnston R. E. Requirement for host transcription in the replication of Sindbis virus. J Virol. 1983 Jan;45(1):200–205. doi: 10.1128/jvi.45.1.200-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K., Mann E., Edwards J., Brown D. T. Effects of chloroquine and cytochalasin B on the infection of cells by Sindbis virus and vesicular stomatitis virus. J Virol. 1981 Mar;37(3):1060–1065. doi: 10.1128/jvi.37.3.1060-1065.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Pennington T. H. Virus development in enucleate cells: echovirus, poliovirus, pseudorabies virus, reovirus, respiratory syncytial virus and Semliki Forest virus. J Gen Virol. 1975 Feb;26(2):183–196. doi: 10.1099/0022-1317-26-2-183. [DOI] [PubMed] [Google Scholar]
- Gliedman J. B., Smith J. F., Brown D. T. Morphogenesis of Sindbis virus in cultured Aedes albopictus cells. J Virol. 1975 Oct;16(4):913–926. doi: 10.1128/jvi.16.4.913-926.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A., Koo R., Stollar V. Evolution and properties of Aedes albopictus cell cultures persistently infected with sindbis virus. Virology. 1977 Oct 1;82(1):69–83. doi: 10.1016/0042-6822(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Kos K. A., Osborne B. A., Goldsby R. A. Inhibition of group B arbovirus antigen production and replication in cells enucleated with cytochalasin B. J Virol. 1975 Apr;15(4):913–917. doi: 10.1128/jvi.15.4.913-917.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinoski F., Stollar V. Inhibition of Sindbis virus replication in Aedes albopictus cells by virazole (ribavirin) and its reversal by actinomycin: a correction. Virology. 1980 Apr 30;102(2):473–476. doi: 10.1016/0042-6822(80)90117-8. [DOI] [PubMed] [Google Scholar]
- Ortin J., Vińuela E. Requirement of cell nucleus for African swine fever virus replication in Vero cells. J Virol. 1977 Mar;21(3):902–905. doi: 10.1128/jvi.21.3.902-905.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Kirkpatrick J. B. Mass enucleation of cultured animal cells. Methods Cell Biol. 1973;7:189–202. doi: 10.1016/s0091-679x(08)61777-x. [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Novel antiviral activity found in the media of Sindbis virus-persistently infected mosquito (Aedes albopictus) cell cultures. J Virol. 1979 Jan;29(1):51–60. doi: 10.1128/jvi.29.1.51-60.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Role of extracellular virus on the maintenance of the persistent infection induced in Aedes albopictus (mosquito) cells by Sindbis virus. J Virol. 1977 Sep;23(3):554–561. doi: 10.1128/jvi.23.3.554-561.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977 Jul 15;80(2):390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- Scheefers-Borchel U., Scheefers H., Edwards J., Brown D. T. Sindbis virus maturation in cultured mosquito cells is sensitive to actinomycin D. Virology. 1981 Apr 30;110(2):292–301. doi: 10.1016/0042-6822(81)90061-1. [DOI] [PubMed] [Google Scholar]
- Stollar V. Inhibition of Sindbis virus replication in Aedes albopictus cells deprived of methionine. Virology. 1978 Dec;91(2):504–507. doi: 10.1016/0042-6822(78)90400-2. [DOI] [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]