Abstract
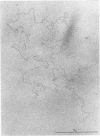
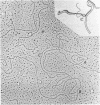
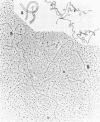
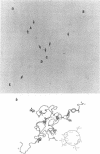
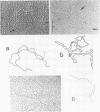
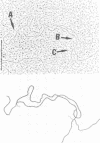
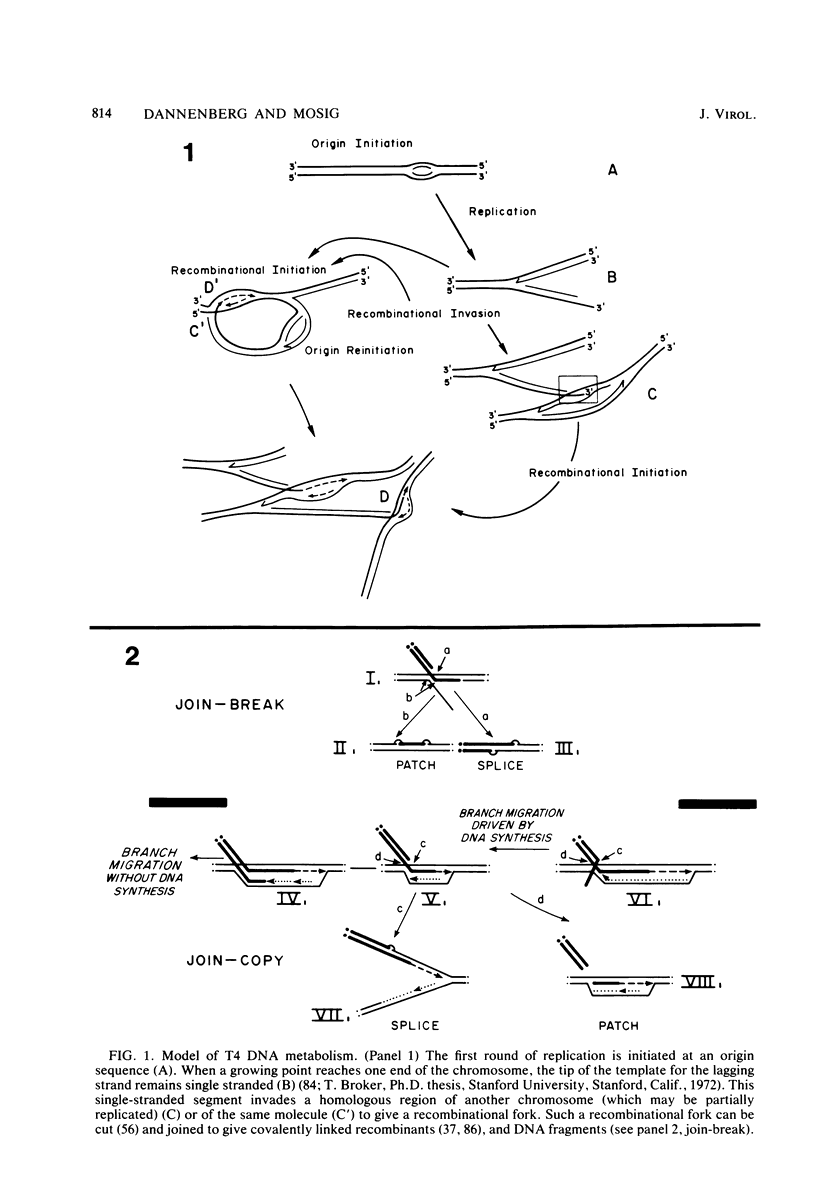
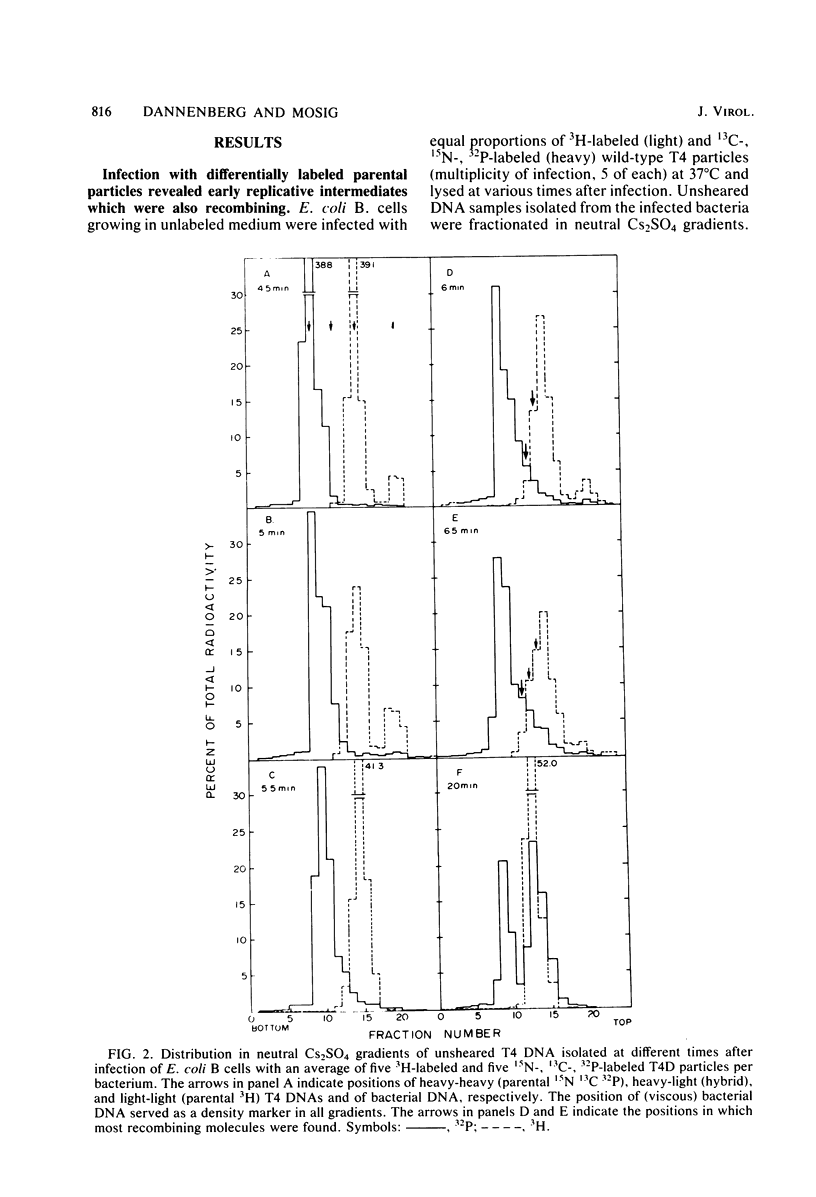
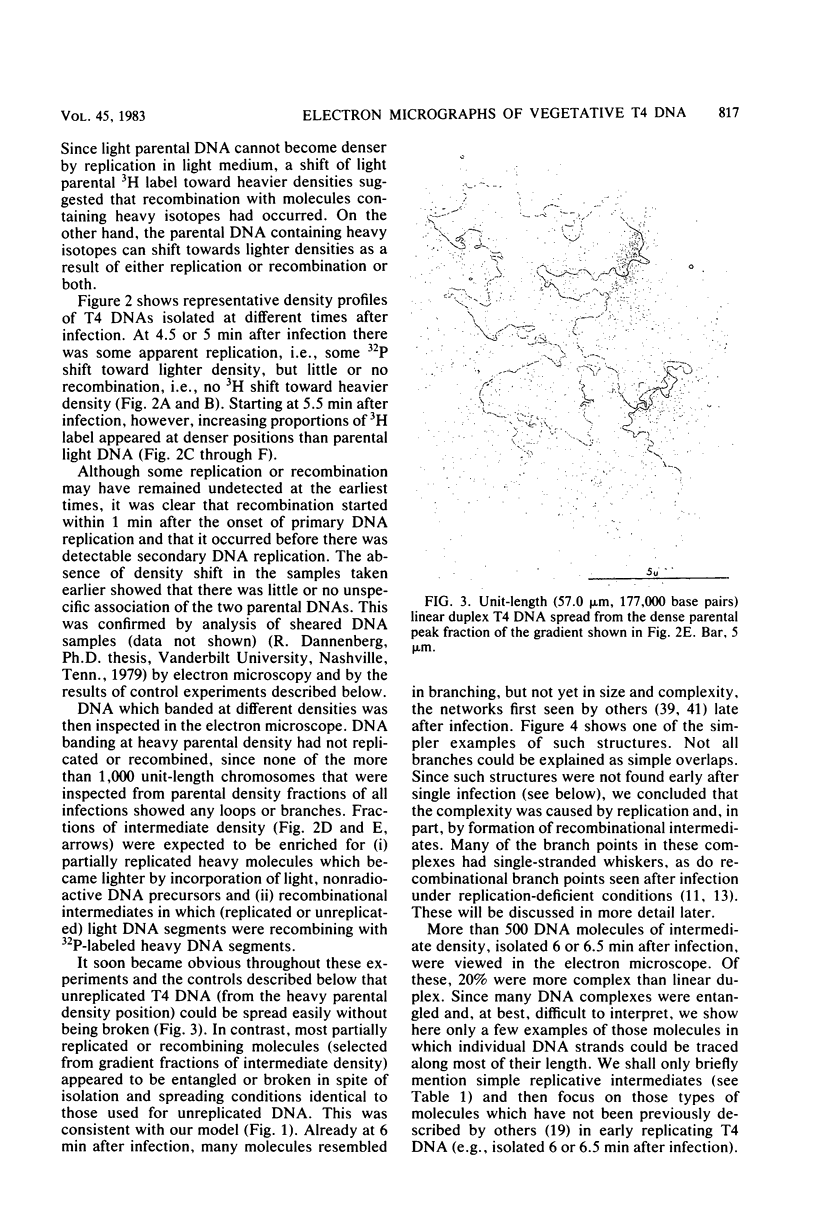
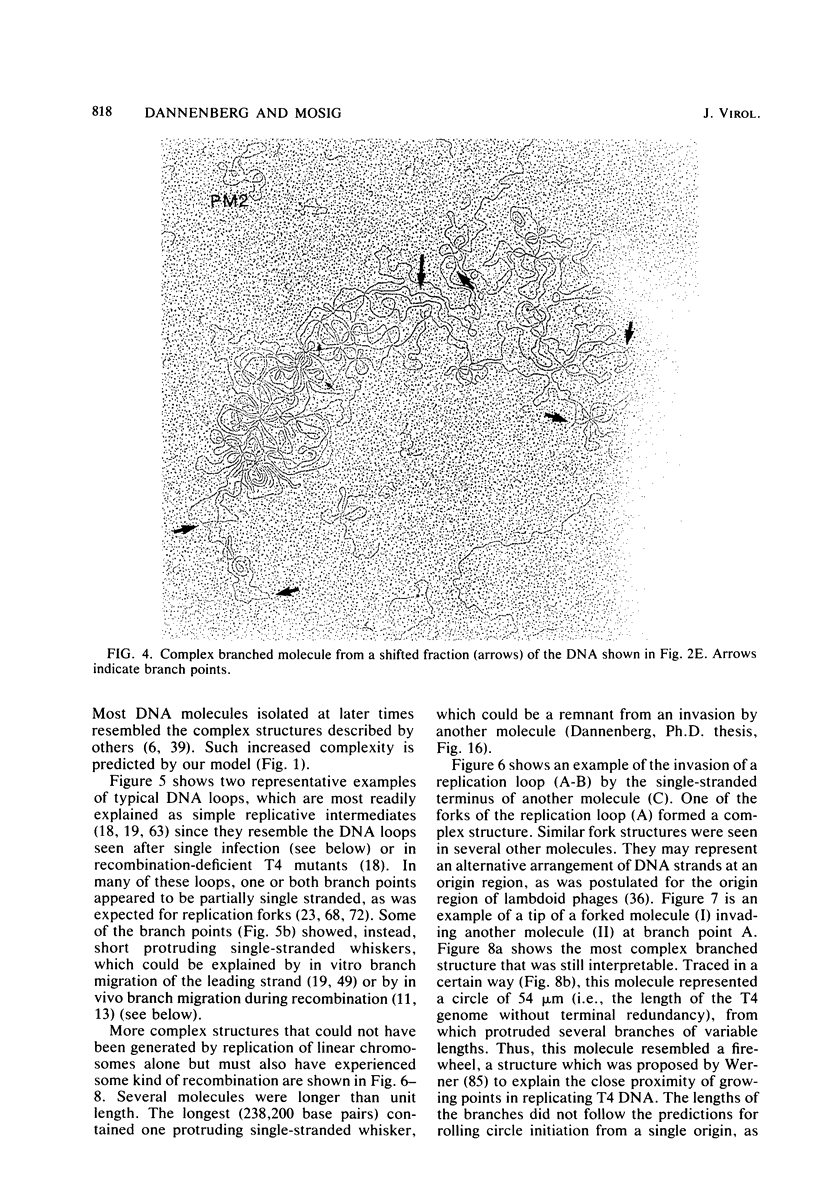
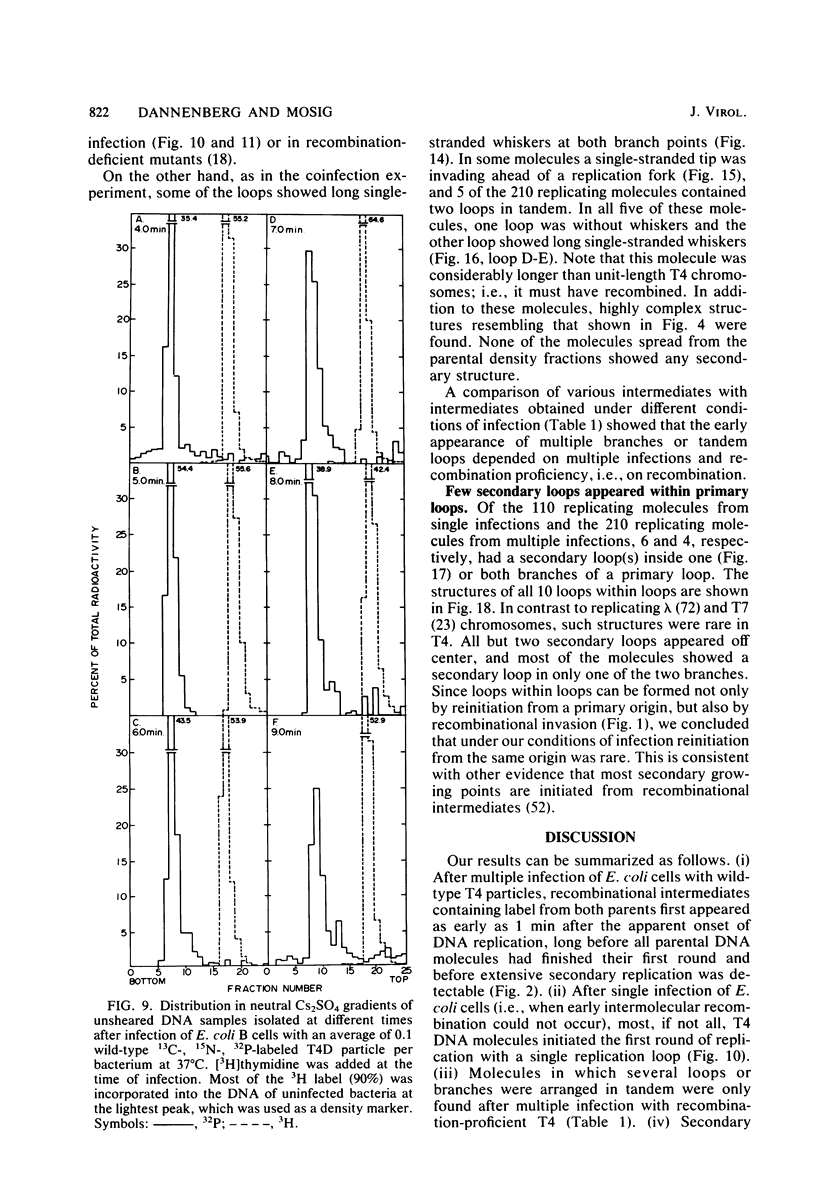
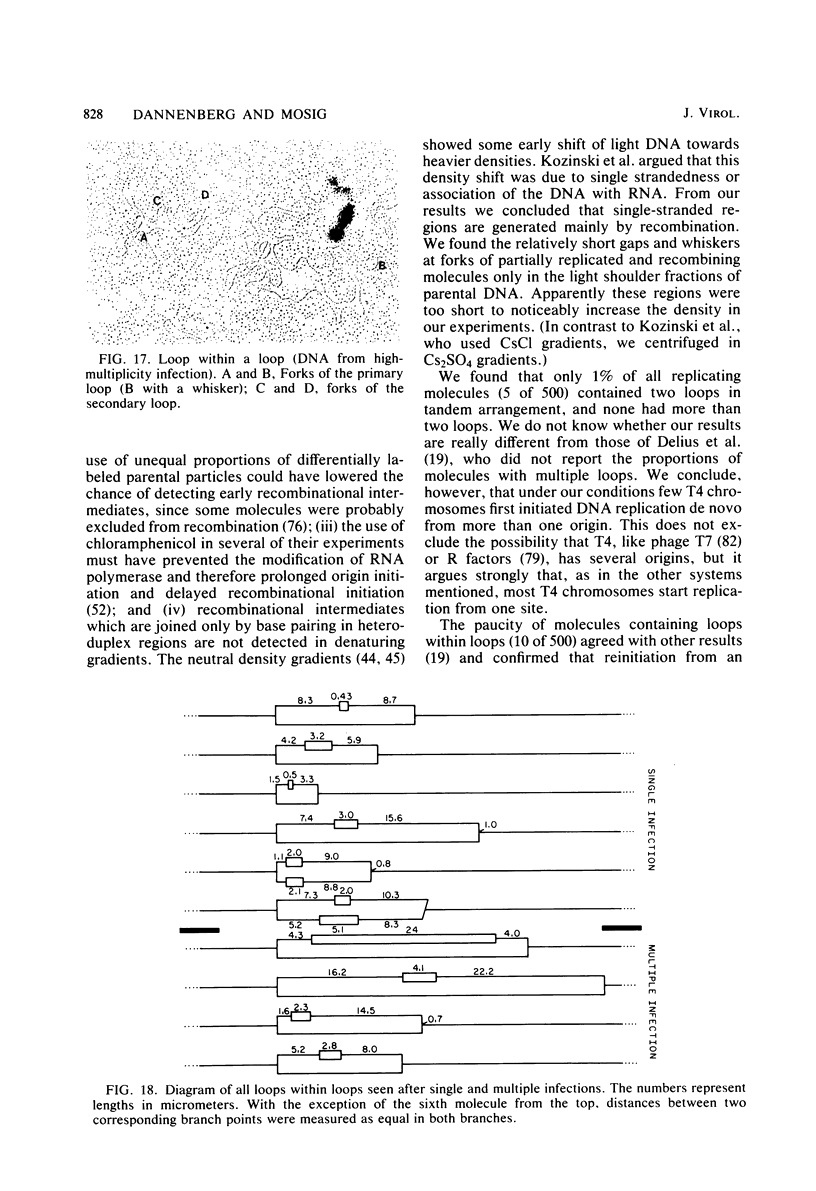
We investigated, by density gradients and subsequent electron microscopy, vegetative T4 DNA after single or multiple infection of Escherichia coli with wild-type T4. Our results can be summarized as follows. (i) After single infection (i.e., when early intermolecular recombination could not occur), most, if not all, T4 DNA molecules initiated the first round of replication with a single loop. (ii) After multiple infection, recombinational intermediates containing label from both parents first appeared as early as 1 min after the onset of replication, long before all parental DNA molecules had finished their first round and before secondary replication was detectable. (iii) At the same time, in multiple infections only, complex, highly branched concatemeric T4 DNA first appeared. (iv) Molecules in which two loops or several branches were arranged in tandem were only found after multiple infections. (v) Secondary loops within primary loops were seen after both single and multiple infections, but they were rare and many appeared off center. Thus, recombination in wild-type T4-infected cells occurred very early, and the generation of multiple tandem loops or branches in vegetative T4 DNA depended on recombination. These results are consistent with the previous finding (A. Luder and G. Mosig, Proc. Natl. Acad. Sci. U.S.A. 79:1101-1105, 1982) that most secondary growing points of T4 are not initiated from origin sequences but from recombinational intermediates. By these and previous results, the various DNA molecules that we observed are most readily explained as intermediates in DNA replication and recombination according to a model proposed earlier to explain various other aspects of T4 DNA metabolism (Mosig et al., p. 277-295, in D. Ray, ed., The Initiation of DNA Replication, Academic Press, Inc., New York, 1981).
Full text
PDF

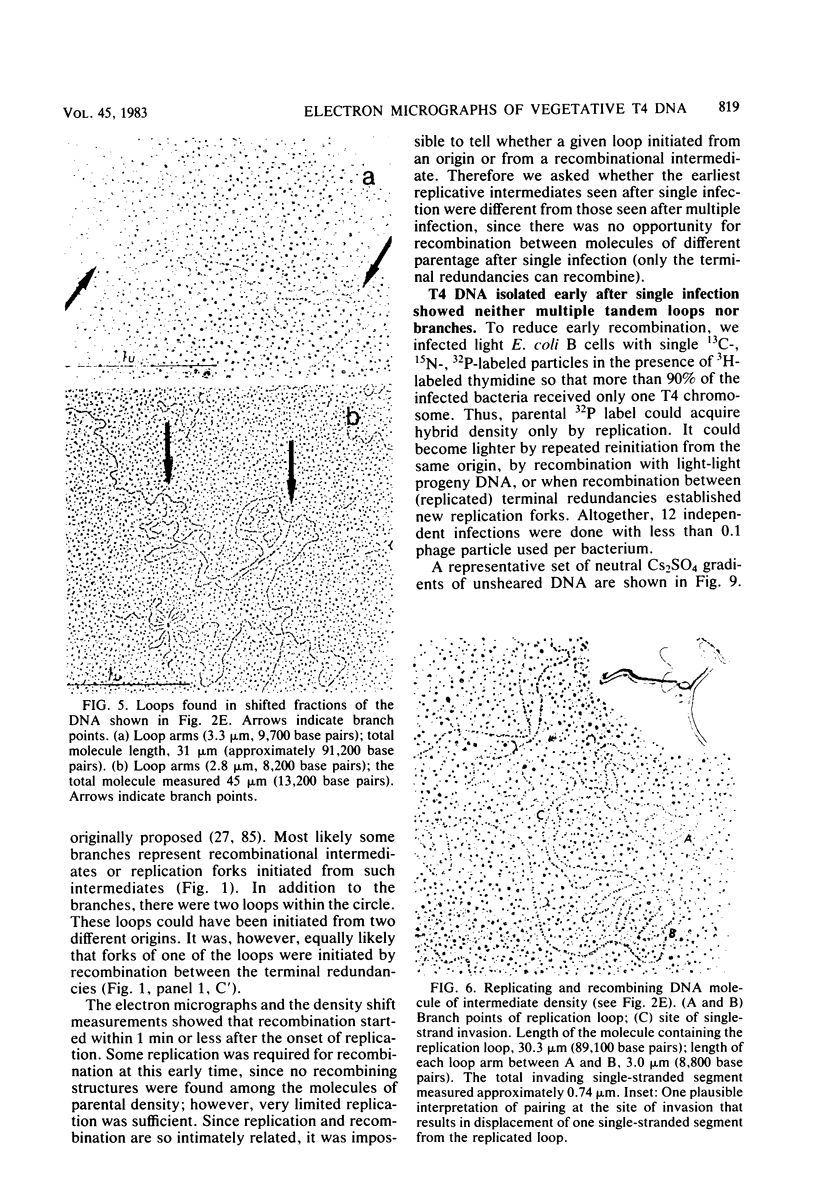

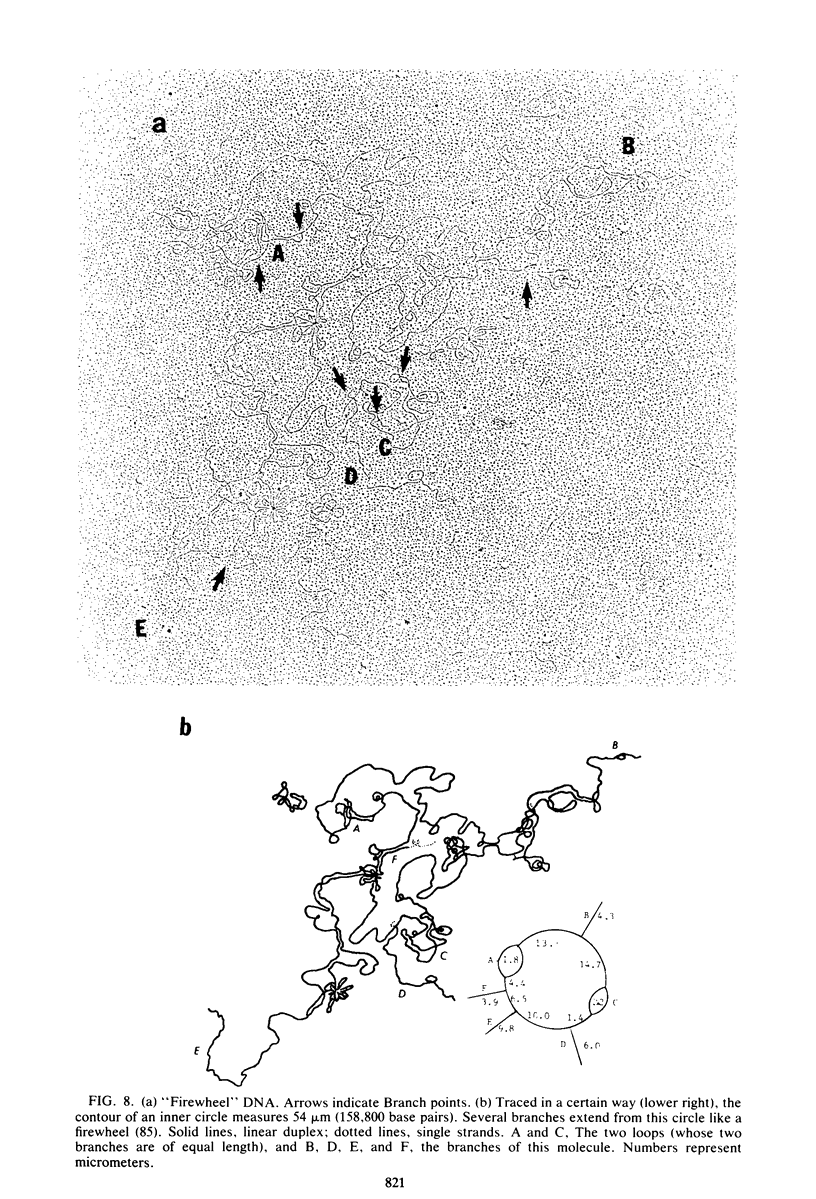
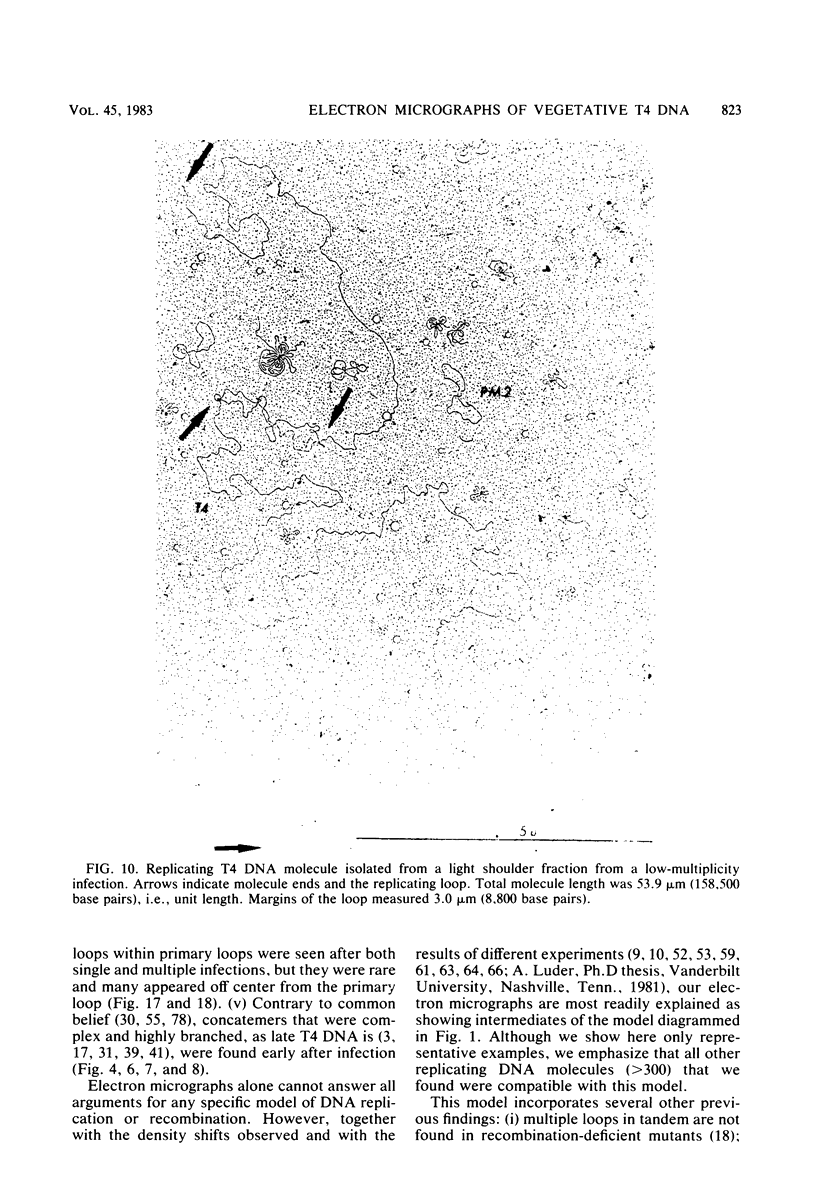
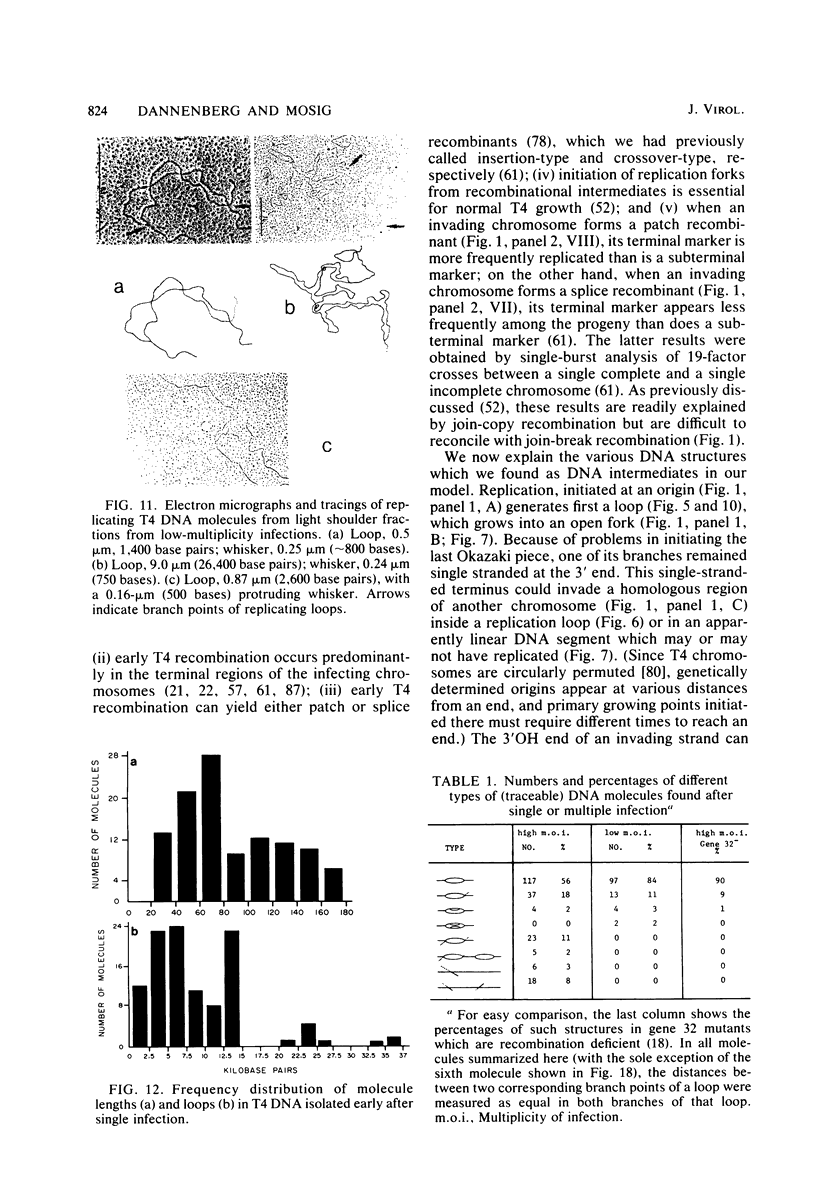
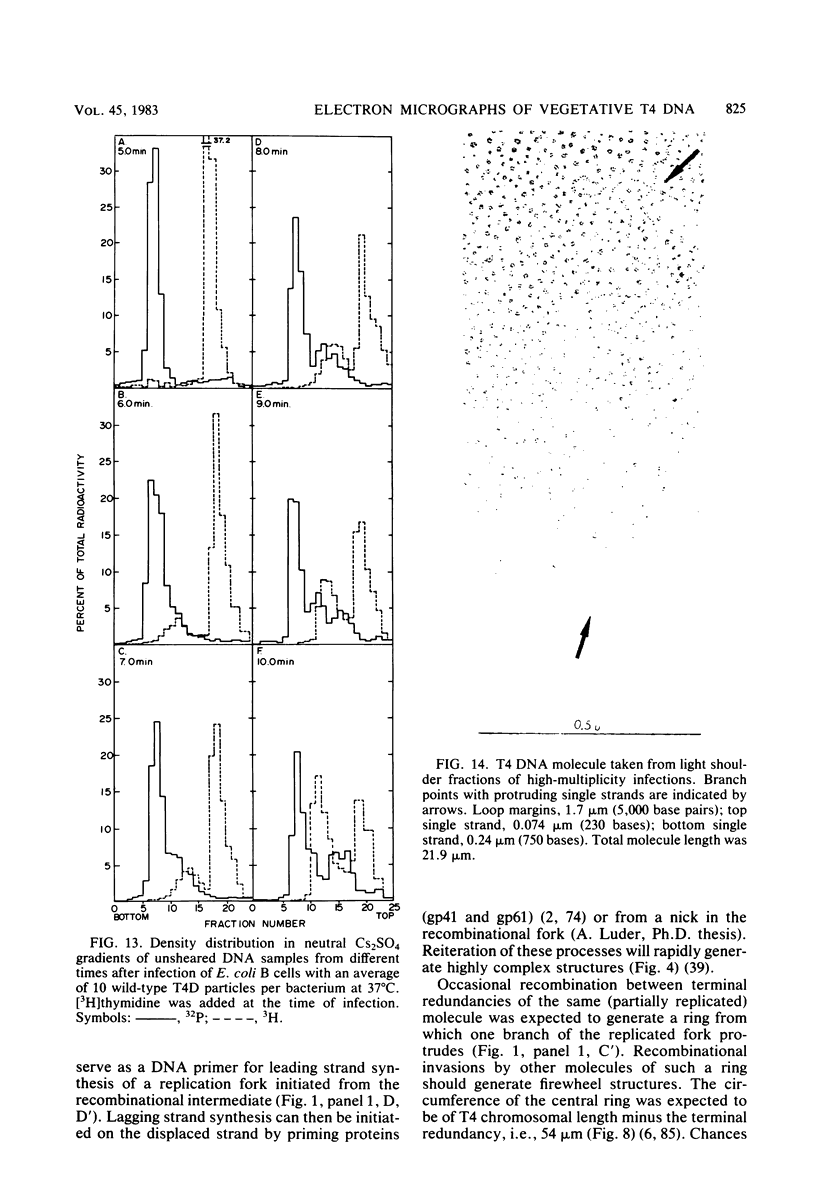
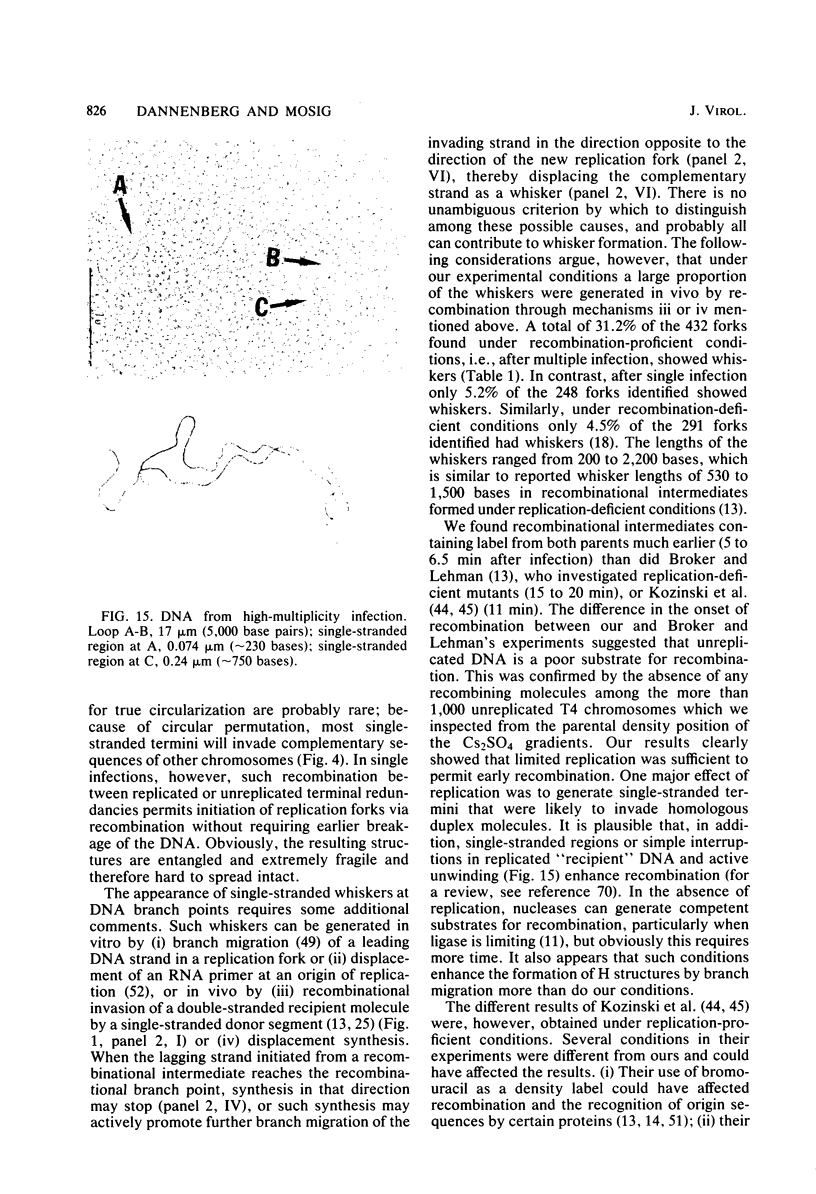
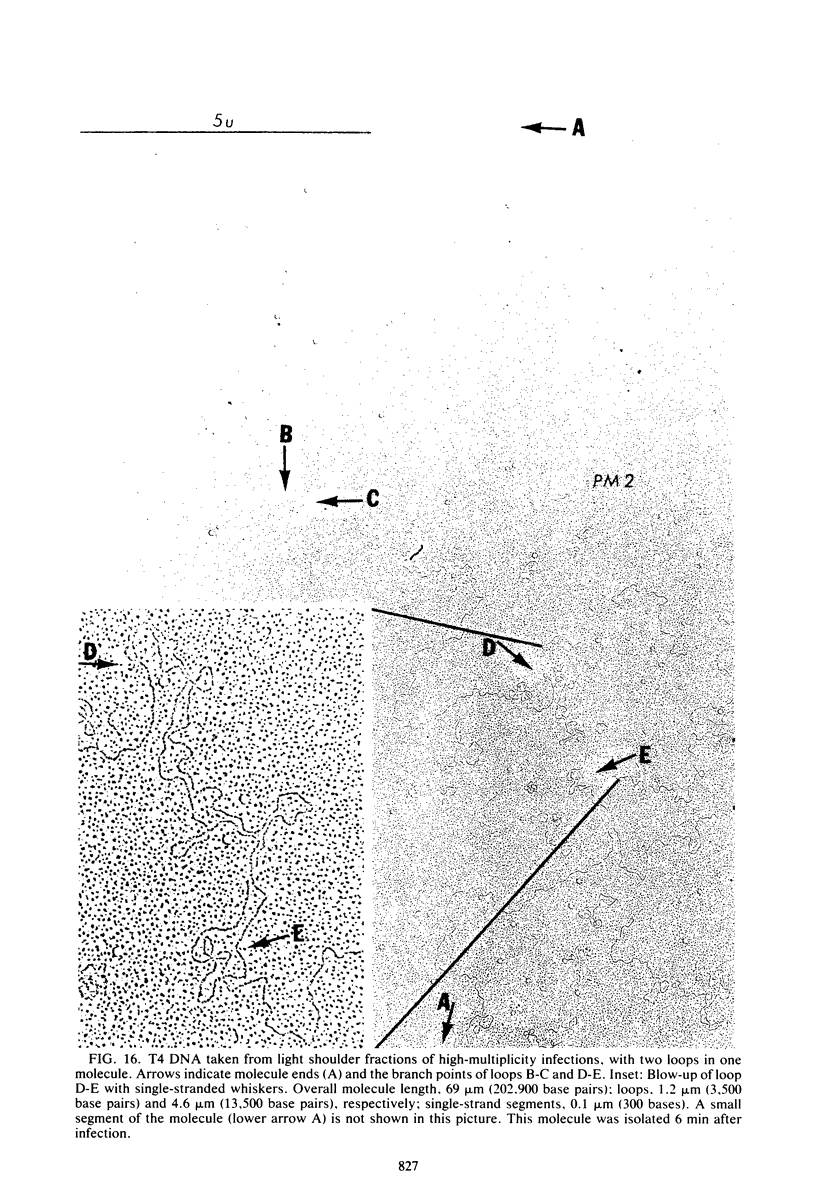



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Anraku N., Anraku Y., Lehman I. R. Enzymic joining of polynucleotides. 8. Structure of hybrids of parental T4 DNA molecules. J Mol Biol. 1969 Dec 28;46(3):481–492. doi: 10.1016/0022-2836(69)90191-0. [DOI] [PubMed] [Google Scholar]
- Anraku N., Tomizawa J. Molecular mechanisms of genetic recombination of bacteriophage. V. Two kinds of joining of parental DNA molecules. J Mol Biol. 1965 Jul;12(3):805–815. doi: 10.1016/s0022-2836(65)80329-1. [DOI] [PubMed] [Google Scholar]
- Bernstein C., Bernstein H. Coiled rings of DNA released from cells infected with bacteriophages T7 or T4 or from uninfected Escherichia coli. J Virol. 1974 Jun;13(6):1346–1355. doi: 10.1128/jvi.13.6.1346-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T., Zinder N. D. Genotypes produced by individual recombination events involving bacteriophage f1. J Mol Biol. 1971 May 28;58(1):133–151. doi: 10.1016/0022-2836(71)90237-3. [DOI] [PubMed] [Google Scholar]
- Botstein D., Matz M. J. A recombination function essential to the growth of bacteriophage P22. J Mol Biol. 1970 Dec 28;54(3):417–440. doi: 10.1016/0022-2836(70)90119-1. [DOI] [PubMed] [Google Scholar]
- Breschkin A. M., Mosig G. Multiple interactions of a DNA-binding protein in vivo. I. Gene 32 mutations of phage T4 inactivate different steps in DNA replication and recombination. J Mol Biol. 1977 May 15;112(2):279–294. doi: 10.1016/s0022-2836(77)80144-7. [DOI] [PubMed] [Google Scholar]
- Breschkin A. M., Mosig G. Multiple interactions of a DNA-binding protein in vivo. II. Effects of host mutations on DNA replication of phage T4 gene 32 mutants. J Mol Biol. 1977 May 15;112(2):295–308. doi: 10.1016/s0022-2836(77)80145-9. [DOI] [PubMed] [Google Scholar]
- Broker T. R. An electron microscopic analysis of pathways for bacteriophage T4 DNA recombination. J Mol Biol. 1973 Nov 25;81(1):1–16. doi: 10.1016/0022-2836(73)90243-x. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Doermann A. H. Molecular and genetic recombination of bacteriophage T4. Annu Rev Genet. 1975;9:213–244. doi: 10.1146/annurev.ge.09.120175.001241. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- Carlson K. Multiple initiation of bacteriophage T4 DNA replication: delaying effect of bromodeoxyuridine. J Virol. 1973 Aug;12(2):349–359. doi: 10.1128/jvi.12.2.349-359.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Berger H. Mutations affecting genetic recombination in bacteriophage T4D. II. Genetic properties. Virology. 1978 Jul 1;88(1):62–70. doi: 10.1016/0042-6822(78)90110-1. [DOI] [PubMed] [Google Scholar]
- Curtis M. J., Alberts B. Studies on the structure of intracellular bacteriophage T4 DNA. J Mol Biol. 1976 Apr 25;102(4):793–816. doi: 10.1016/0022-2836(76)90292-8. [DOI] [PubMed] [Google Scholar]
- DOERMANN A. H., BOEHNER L. AN EXPERIMENTAL ANALYSIS OF BACTERIOPHAGE T4 HETEROZYGOTES. I. MOTTLED PLAQUES FROM CROSSES INVOLVING SIX RII LOCI. Virology. 1963 Dec;21:551–567. doi: 10.1016/0042-6822(63)90227-7. [DOI] [PubMed] [Google Scholar]
- Dannenberg R., Mosig G. Semiconservative DNA replication is initiated at a single site in recombination-deficient gene 32 mutants of bacteriophage T4. J Virol. 1981 Dec;40(3):890–900. doi: 10.1128/jvi.40.3.890-900.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A. H., Parma D. H. Recombination in bacteriophage T4. J Cell Physiol. 1967 Oct;70(2 Suppl):147–164. doi: 10.1002/jcp.1040700411. [DOI] [PubMed] [Google Scholar]
- Doermann A. H. T4 and the rolling circle model of replication. Annu Rev Genet. 1973;7:325–341. doi: 10.1146/annurev.ge.07.120173.001545. [DOI] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A., Geiduschek E. P., Epstein R. H., Metter E. J. Capsid size and deoxyribonucleic acid length: the petite variant of bacteriophage T4. J Virol. 1970 Dec;6(6):865–876. doi: 10.1128/jvi.6.6.865-876.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. S. On the mechanism of integration of transforming deoxyribonucleate. J Gen Physiol. 1966 Jul;49(6):183–196. doi: 10.1085/jgp.49.6.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Chandler M. On the molecular mechanisms of transposition. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4858–4862. doi: 10.1073/pnas.78.8.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Sherratt D. J. Sequence analysis at IS1 insertion sites: models for transposition. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1257–1261. doi: 10.1101/sqb.1979.043.01.142. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., MELECHEN N. E. Synthesis of phage-precursor nucleic acid in the presence of chloramphenicol. Virology. 1957 Feb;3(1):207–236. doi: 10.1016/0042-6822(57)90034-x. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Mattson T., Kozinski A. W. Origins of phage T4 DNA replication as revealed by hybridization to cloned genes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6137–6141. doi: 10.1073/pnas.76.12.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M., Mattson T., Kozinski A. W. Late events in T4 bacteriophage DNA replication. III. Specificity of DNA reinitiation as revealed by hybridization to cloned genetic fragments. J Virol. 1982 May;42(2):422–431. doi: 10.1128/jvi.42.2.422-431.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S., Pettijohn D. E. Properties of condensed bacteriophage T4 DNA isolated from Escherichia coli infected with bacteriophage T4. J Virol. 1976 Sep;19(3):1012–1027. doi: 10.1128/jvi.19.3.1012-1027.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlett N. V., Berger H. Mutations altering genetic recombination and repair of DNA in bacteriophage T4. Virology. 1975 Feb;63(2):539–567. doi: 10.1016/0042-6822(75)90326-8. [DOI] [PubMed] [Google Scholar]
- Harshey R. M., McKay R., Bukhari A. I. DNA intermediates in transposition of phage Mu. Cell. 1982 Jun;29(2):561–571. doi: 10.1016/0092-8674(82)90172-6. [DOI] [PubMed] [Google Scholar]
- Henderson D., Weil J. Recombination-deficient deletions in bacteriophage lambda and their interaction with chi mutations. Genetics. 1975 Feb;79(2):143–174. doi: 10.1093/genetics/79.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R. Molecular aspects of genetic exchange and gene conversion. Genetics. 1974 Sep;78(1):273–287. doi: 10.1093/genetics/78.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C. C., Buckley P. J., Carlson K. M., Kozinski A. W. Multiple and specific initiation of T4 DNA replication. J Virol. 1973 Jul;12(1):130–148. doi: 10.1128/jvi.12.1.130-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Boone L. R., Skalka A. M. Retroviral DNA H structures: displacement-assimilation model of recombination. Cell. 1982 Aug;30(1):53–62. doi: 10.1016/0092-8674(82)90011-3. [DOI] [PubMed] [Google Scholar]
- Kemper B., Brown D. T. Function of gene 49 of bacteriophage T4. II. Analysis of intracellular development and the structure of very fast-sedimenting DNA. J Virol. 1976 Jun;18(3):1000–1015. doi: 10.1128/jvi.18.3.1000-1015.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Davidson N. Electron microscope heteroduplex study of sequence relations of T2, T4, and T6 bacteriophage DNAs. Virology. 1974 Jan;57(1):93–111. doi: 10.1016/0042-6822(74)90111-1. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Doermann A. H., Kozinski P. B. Absence of interparental recombination in multiplicity reconstitution from incomplete bacteriophage T4 genomes. J Virol. 1976 Jun;18(3):873–884. doi: 10.1128/jvi.18.3.873-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B., James R. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. I. Tertiary structure of early replicative and recombining deoxyribonucleic acid. J Virol. 1967 Aug;1(4):758–770. doi: 10.1128/jvi.1.4.758-770.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Ling S. K., Hutchinson N., Halpern M. E., Mattson T. Differential amplification of specific areas of phage T4 genome as revealed by hybridization to cloned genetic segments. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5064–5068. doi: 10.1073/pnas.77.9.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Leung D., Behme M. T., Ebisuzaki K. Effect of DNA delay mutations of bacteriophage T4 on genetic recombination. J Virol. 1975 Jul;16(1):203–205. doi: 10.1128/jvi.16.1.203-205.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. The effect of 5-bromouracil on recombination of phage lambda. Virology. 1976 Jul 15;72(2):530–535. doi: 10.1016/0042-6822(76)90184-7. [DOI] [PubMed] [Google Scholar]
- Luder A., Mosig G. Two alternative mechanisms for initiation of DNA replication forks in bacteriophage T4: priming by RNA polymerase and by recombination. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1101–1105. doi: 10.1073/pnas.79.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. C., Breschkin A. M., Mosig G. Origin and direction of bacteriophage T4 DNA replication. II. A gradient of marker frequencies in partially replicated T4 DNA as assayed by transformation. J Mol Biol. 1971 Sep 14;60(2):213–233. doi: 10.1016/0022-2836(71)90289-0. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Minner C., Bernstein H., Bernstein C. DNA elongation rates and growing point distributions of wild-type phage T4 and a DNA-delay amber mutant. J Mol Biol. 1976 Oct 5;106(4):963–981. doi: 10.1016/0022-2836(76)90346-6. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr Replication and molecular recombination of T-phage. Annu Rev Microbiol. 1975;29:355–376. doi: 10.1146/annurev.mi.29.100175.002035. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Kemper B., Hays J., Weisberg R. A. T4 endonuclease VII cleaves holliday structures. Cell. 1982 Jun;29(2):357–365. doi: 10.1016/0092-8674(82)90152-0. [DOI] [PubMed] [Google Scholar]
- Mosig G. A preferred origin and direction of bacteriophage T4 DNA replication. I. A gradient of allele frequencies in crosses between normal and small T4 particles. J Mol Biol. 1970 Nov 14;53(3):503–514. doi: 10.1016/0022-2836(70)90080-x. [DOI] [PubMed] [Google Scholar]
- Mosig G., Ehring R., Schliewen W., Bock S. The patterns of recombination and segregation in terminal regions of T4DNA molecules. Mol Gen Genet. 1971;113(1):51–91. doi: 10.1007/BF00335007. [DOI] [PubMed] [Google Scholar]
- Mosig G., Ghosal D., Bock S. Interactions between the maturation protein gp17 and the single-stranded DNA binding protein gp32 initiate DNA packaging and compete with initiation of secondary DNA replication forks in phage T4. Prog Clin Biol Res. 1981;64:139–150. [PubMed] [Google Scholar]
- Mosig G., Luder A., Garcia G., Dannenberg R., Bock S. In vivo interactions of genes and proteins in DNA replication and recombination of phage T4. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):501–515. doi: 10.1101/sqb.1979.043.01.056. [DOI] [PubMed] [Google Scholar]
- Mosig G., Revel H. R. Expression of genes in incomplete T4 genomes. Virology. 1967 Mar;31(3):397–401. doi: 10.1016/0042-6822(67)90218-8. [DOI] [PubMed] [Google Scholar]
- Mosig G., Werner R. On the replication of incomplete chromosomes of phage T4. Proc Natl Acad Sci U S A. 1969 Oct;64(2):747–754. doi: 10.1073/pnas.64.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti S., Bernstein H. The DNA-delay mutants of bacteriophage T4. J Virol. 1974 Oct;14(4):860–871. doi: 10.1128/jvi.14.4.860-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powling A., Knippers R. Recombination of bacteriophage T7 in vivo. Mol Gen Genet. 1976 Nov 24;149(1):63–71. doi: 10.1007/BF00275961. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Genetic recombination: strand transfer and mismatch repair. Annu Rev Biochem. 1978;47:847–880. doi: 10.1146/annurev.bi.47.070178.004215. [DOI] [PubMed] [Google Scholar]
- Salditt M., Braunstein S. N., Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. X. Improved techniques for the purification of bacteriophage PM2. Virology. 1972 Apr;48(1):259–262. doi: 10.1016/0042-6822(72)90133-x. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P. Differential transmission of amber and wild-type alleles of bacteriophage T4 in mixedly infected cells of Escherichia coli. Virology. 1970 May;41(1):52–65. doi: 10.1016/0042-6822(70)90053-x. [DOI] [PubMed] [Google Scholar]
- Stahl F. W. Special sites in generalized recombination. Annu Rev Genet. 1979;13:7–24. doi: 10.1146/annurev.ge.13.120179.000255. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Electron microscopy of DNA: determination of absolute molecular weights and linear density. Mol Gen Genet. 1977 Sep 9;154(3):299–303. doi: 10.1007/BF00571286. [DOI] [PubMed] [Google Scholar]
- Teifel J., Schmieger H. The influence of host DNA replication on the formation of infectious and transducing Mu-particles. Mol Gen Genet. 1981;184(2):308–311. doi: 10.1007/BF00272922. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE H. L. A THEORY OF CROSSING-OVER BY MEANS OF HYBRID DEOXYRIBONUCLEIC ACID. Nature. 1963 Sep 14;199:1034–1040. doi: 10.1038/1991034a0. [DOI] [PubMed] [Google Scholar]
- WOMACK F. C. AN ANALYSIS OF SINGLE-BURST PROGENY OF BACTERIA SINGLY INFECTED WITH A BACTERIOPHAGE HETEROZYGOTE. Virology. 1963 Oct;21:232–241. doi: 10.1016/0042-6822(63)90262-9. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Werner R. Initiation and propagation of growing points in the DNA of phage T4. Cold Spring Harb Symp Quant Biol. 1968;33:501–507. doi: 10.1101/sqb.1968.033.01.058. [DOI] [PubMed] [Google Scholar]