Abstract
The effects of the bioactive polyacetylenes, falcarinol and falcarindiol, present in carrots, celery, celeriac and other umbelliferous vegetables, on the stress responses in primary myotube cultures, were studied. Biphasic responses on cellular stress responses in myotube cultures were investigated by exposing them to various concentrations of falcarinol and falcarindiol for 24 h before testing effects of 100 μM H2O2 on the intracellular formation of reactive oxygen species (ROS), transcription of the antioxidative enzyme cytosolic glutathione peroxidase (cGPx), and the heat shock proteins (HSP) HSP70 and HO1. At low concentrations (1.6 to 25 μM) polyacetylenes caused a slightly accelerated intra-cellular ROS formation, increased cGPx transcription and decreased HSP70 and HO1 transcription. The increased cGPx transcription may be interpreted as an adaptive response to the increased ROS formation and may have caused a reduced demand for the protective functions of the HSPs. ROS formation, however, was substantially decreased after pre-incubation with both polyacetylenes at 50 and 100 μM, the cGPx transcription was reduced and the HSP70 and HO1 transcription increased, indicating a need for the protective and repairing functions of the HSPs. In conclusion, pre-incubation with low concentrations of both polyacetylenes prior to H2O2 exposure induced a cytoprotective effect whereas higher concentrations had adverse effects.
Keywords: falcarinol, falcarindiol, polyacetylenes, stress response, primary myotubes, biphasic
INTRODUCTION
Epidemiological studies indicate some correlation between high intake of fruit and vegetables and certain beneficial health effects (Steinmetz and Potter 1991; Block et al. 1992; Maynard et al. 2003). This has lead to a great interest in studying effects of single compounds originating from fruit and vegetables, e.g. bioactive compounds such as caroteonoids (The Alpha-Tocopherol B-CCPSG 1994) and flavonoids (Duthie and Dobson 1999). Other highly bioactive, but less abundant compounds in fruit and/or vegetables that may contribute to overall effects, include the aliphatic C17-polyacetylenes falcarinol and falcarindiol (Figure 1), which are mainly present in carrots, celery, celeriac and other umbelliferous vegetables (Zidorn et al. 2005). Falcarinol is bioavailable in humans (Christensen and Brandt 2006) and the C17-polyacetylenes of the falcarinol type has shown biological characteristics such as anti-inflammatory (Alanko et al. 1994; Liu et al. 1998), immune stimulatory (Hansen et al. 1986), anti-platelet-aggregatory effects (Teng et al. 1989) as well as cytotoxicity (Bernart et al. 1996; Zidorn et al. 2005). In the present study we investigated the effects of falcarinol and falcarindiol on the stress responses in primary myotube cultures isolated from porcine semimembranosus muscle. The muscle cell was chosen as a model because it is the most abundant cell type in the human body constituting 40% of the body weight. Also this cell type is a good model for studying oxidative stress responses since oxidative stress is regularly induced in muscle cells under physical exercise. In a pilot study we have investigated stress conditions that trigger a moderate and reversible cellular stress response, as determined by HSP70 and HO1 m-RNA expression, and we found that exposure to 100 μM H2O2 for 1 hour induced a background level of stress from which both decreasing and accelerating stress effects may be determined.
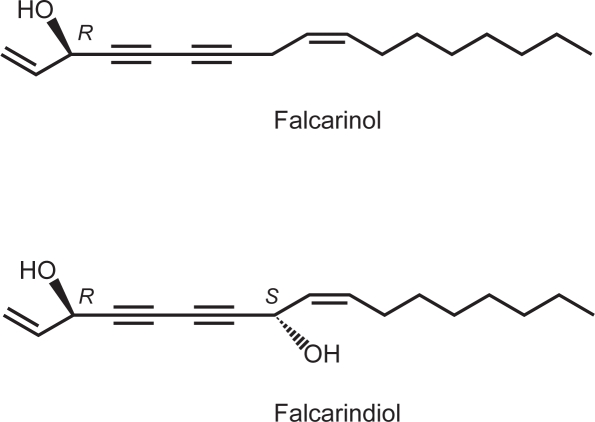
FIGURE 1.
Chemical structures of the polyacetylenes falcarinol [(3R)-heptadeca-1,9(Z)-diene-4,6-diyne-3-ol] and falcarindiol [(3R, 8S)-heptadeca-1,9(Z)-diene-4,6-diyne-3,8-diol] isolated from carrots.
MATERIALS AND METHODS
Isolation of Falcarinol and Falcarindiol
Four kilograms of carrot roots (cv. Bolero) were ground and extracted with 5 l ethyl acetate (EtOAc) for 24 h, at room temperature in the dark. The EtOAc phase was collected by decanting, and the carrots re-extracted with another 5 l EtOAc for 24 h. The combined extracts were filtered, dried over anhydrous Na2SO4, and concentrated in vacuo (35 ºC) under dim light. The extract (12 g) was chromatographed on silica gel, eluting with n-hexane, n-hexane–EtOAc (v/v) (9:1, 4:1, 7:3, 3:2, 1:1, 2:3, 1:4), and finally EtOAc (Kidmose et al. 2004). Fractions containing crude falcarinol and falcarindiol, respectively, were combined, and the individual polyacetylenes further purified by preparative reversed phase (RP) high-performance liquid chromatography (HPLC) on a Dionex Summit Preparative HPLC system (Dionex Denmark A/S, Rødovre, Denmark) controlled by Chromeleon (version 6.50) software, and equipped with a HPLC pump (P680), solvent rack (SOR-100), and a diode array detector (UV D340U) operating from 200–595 nm. Separations of polyacetylenes were performed on a Develosil ODS-HG-5 HPLC column (RP-18, 250 ↔ 20 mm i.d., Nomura Chemical Co., Seto, Japan), at 25 ºC, using the following stepwise gradient: CH3OH−H2O [0 min (20:80), 50–60 min (100:0), 70–80 min (20:80)], yielding 45 mg falcarinol and 60 mg falcarindiol, respectively. Detection wavelength: 203 nm. Flow rate: 5 ml/min. Injection volume: 25 ml. Acquisition off at 70 min. Purity of polyacetylenes were > 96%, as determined by analytical RP-HPLC (Christensen and Kreutzmann 2007). Falcarinol and falcarindiol was obtained as colorless oils and identified by optical rotation, UV, mass spectrometry (MS) [gas chromatography (GC)–MS (EI, 70 eV)], one-dimensional and two dimensional nuclear resonance spectroscopy (NMR) [1H- and 13C-NMR, 1H–1H- and 1H–13C-COSY] and the complete spectral data set corresponded fully with literature values (Lemmich 1981; Czepa and Hofmann 2003; Kobæk-Larsen et al. 2005).
Porcine primary myoblast and myotube cultures
Myotube cultures were derived from porcine primary satellite cells isolated from M. semimembranosus of female pigs at an age of six weeks. The original method of Bischoff was used with some modifications (Bischoff 1974; Theil et al. 2006). Muscle tissue was excised, stripped for visible fat and connective tissue, placed in ice-cold transport medium (1% glucose, 500 IU/ml of penicillin, 500 μg/ml of streptomycin sulfate, 15 μg/ml of amphotericin and 100 μg/ml of gentamycin in Ca2+ and Mg2+ free phosphate buffered saline (PBS)), and transferred to a laminar flow bench. The muscle tissue was finely chopped with a pair of scissors and digested for 20 min, in 20 ml PBS (Ca2+ free) containing 1% glucose, 1.5 mg/ml collagenase II, 0.25% trypsin and 0.01% DNAse. As much as possible of the digestion medium was aspirated and another 20 ml digestion medium was added and left to digest for another 20 min. This procedure was repeated to give a total of 3 x 20 min digestion and a total volume of approximately 60 ml digest. Following digestion the cells were transferred to a Primary Growth Medium (PGM, DMEM (Dulbecco’s modified Eagle’s medium, Life Technologies, Naperville, IL) with 10% foetal calf serum (FCS, Life Technologies, Naperville, IL) and 10% horse serum (HS) supplemented with antibiotics: 100 IU/ml penicillin and 100 μg/ml streptomycin sulfate, 3 μg/ml amphotericin B, 20 μg/ml gentamycin), triturated 10 times (stripette, Costar, Cat.no. 4101, VWR, Aarhus Denmark), centrifuged at 630 x g for 8 min at 4°C, resuspended, and filtered through a 200 μm and then a 50 μm Nytex filter. Percoll gradients (20% Percoll) were used to enrich the relative proportion of satellite cells in the cell suspension (Ortenblad et al. 2003). Cells were kept in liquid nitrogen until use, where cells were thawed at 37°C and equally distributed into 24- (∼ 60.000 cells/cm2) or 96-well (∼ 90.000 cells/cm2) plates, coated with matrigel (1:50 v/v) and grown in PGM (95% air and 5% CO2 at 37°C). For cell viability experiments cells were grown approximately 3 days to 80% of confluence before assaying, but for analysis of mRNA expression and DCFH2 (2’,7’ dichlorodihydroflourescein) oxidation cells were fused into myotubes prior to the experiment. Cells were made to fuse after approximately 4 days of proliferation by switching to DMEM with 10% FCS, 1 μM insulin and antibiotics for 24 h, and then to DMEM containing 5% FCS, 1 μM insulin, antibiotics and 1 μM cytosine arabinosid (fusion medium) for 72 h. The latter medium was changed after 48 h of incubation. Cells were fully differentiated after approximately 3 days in fusion medium.
Experimental setup, RNA extraction and real time RT-PCR analyses
Differentiated myotubes in 24 well plates were exposed to various concentrations (6.25-50 μM) of falcarinol or falcarindiol in the fusion medium. After 24 h fusion medium including falcarinol or falcarindiol was aspirated and the cells were washed with 1 ml/well KCl buffer (150 mM KCl, 1.3 mM CaCl2, 0.5 mM MgCl2 and 10 mM Hepes) before exposure to 100 μ M H2O2 in KCl buffer for 1 h at 37°C in 95% air and 5% CO2. KCl buffer including H2O2 was aspirated, cells washed and left at 37°C in 95% air and 5% CO2 in fusion medium for 18 h. Cells were washed twice in PBS, harvested in 0.25% trypsin, and stored at -80°C until extraction. RNA was extracted from the cells using the RNeasy mini kit (Qiagen, Albertslund, Denmark) and reverse transcribed with oligo-dT primers and Superscript II RNase H reverse transcriptase kit (Invitrogen, Taastrup, Denmark). Reverse Transcribed material (1 μl) was amplified with TaqMan Universal PCR Master Mix (Applied Biosystems, Stockholm, Sweden). Primers and probe were designed specifically for each gene by using Primer Express 2.0 software (Applied Biosystems, Stockholm, Sweden) and either forward primer, minor groove binding (MGB) probe or reverse primer was designed to anneal to an exon boundary. Exon structures reported for humans or mouse (HO1) were used. Details of primer/probe design and runs of real time RT-PCR are given in Table 1. Amplicon length was tested after real time RT-PCR analysis on a 2% agarose gel and only one PCR product was amplified per gene and the amplicon length agreed with the predicted length based on the nucleotide sequences (data not shown). Quantity of mRNA was detected by gene specific MGB or TAMRA probes labelled with FAMTM fluorophore in the 5’ end and a non-flourescent quencher in the 3’ end. For PCR, 40 cycles at 95°C for 15 s and 60°C for 60 s were applied to amplify the PCR products. A selected sample was diluted serially and analyzed in triplicate to test linearity and efficiency of the PCR amplifications. Furthermore, control wells with either water or genomic DNA was used as negative controls. All samples were analyzed in duplicates using the ABI 7900HT sequence detection system (Applied Biosystems, Stockholm, Sweden). The sequences of forward primers, MGB probes and reverse primers were as follows:
TABLE 1.
Accession numbers, amplicon location, amplicon length, range of Ct values in samples and slope of standard curves of the analyzed genes.
| Gene | Accession No. | Amplicon location (exon-exon) | Amplicon Length (bp) | Range of Ct in samples
|
Slope of std. curve | |
|---|---|---|---|---|---|---|
| Falcarinol | Falcarindiol | |||||
| HSP70 | M69100 | 7–8 | 86 | 22.7–26.7 | 25.7–28.8 | −3.43 |
| HO1 | AC091316 | 2–3 | 77 | 24.1–27.3 | 24.7–29.1 | −3.26 |
| cGPx | AF532927 | 1–1 | 76 | 25.5–28.5 | 24.5–27.4 | −3.41 |
| GAPDH | AF017079 | 2–3 | 76 | 23.8–25.7 | 23.5–25.4 | −3.33 |
Heat shock protein 70 (HSP70): 5’-GGCAAGGCCAACAAGATCAC-3’, 5’-ACAAGGGCCGCCTGAGCAAGG-3’, 5’-TTCTCAGCCTCCTGCACCAT-3’
Heme oxygenase 1 (HO1): 5’-GCTGAGAATGCCGAGTTCATG-3’,5’-CAGAAGGGCGAGGTCACCCGAGA-3’,5’-GACGCCATCACCAGCTT AAAG-3’
Glutathion peroxidase (cGPx): 5’-CAAGGTGCTGCTCATTGAGAAC-3’, 5’-AGCATCGCTCTGAGGCACAACGGT-3’,5’-CAGGTCATTCATCTGGGTG TAGTC-3’
Glyceraldehyde 3 phosphate dehydrogenase (GAPDH): 5’- GTCGGAGTGA ACGGATTTGG -3’, 5’- CGCCTGGTCACCAGGGCTGCT -3’,5’- CAATG TCCACTTTGCCAGAGTTAA -3’
To evaluate mRNA quantities, data was obtained as Ct values (the cycle number at which logarithmic plots cross a calculated threshold line) according to the manufacturer’s guidelines, and used to determine ΔCt values (ΔCt = Ct of the target gene – Ct of the housekeeping gene (GAPDH)). To exclude potential bias because of averaging data that had been transformed through the equation 2−ΔΔCt, all statistics were performed at the ΔCt values. The quantitative expression of target genes was expressed relative to the level observed for cells without polyacetylene addition by calculating the ΔΔCt values (ΔCt observed at a given polyacetylene concentration -ΔCt observed for cells without polyacetylene addition) and by using the formula: Relative quantity = 2−ΔΔCt. Within each compound every concentration was added to 4 wells (n = 4) from which RNA was isolated and each isolate was analyzed for mRNA expression in duplicate.
Experimental setup and DCFH2 oxidation analyses (ROS formation)
Differentiated myotubes in 96 well plates were exposed to various concentrations (1.6–100 μM) of falcarinol or falcarindiol in PGM for 18 h. Myotubes were washed twice in 200 μL/well Krebs-Hepes buffer (KHB)(118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 4.2 mM NaHCO3, 1.3 mM CaCl2, 1.2 mM MgSO4, 10 mM Hepes, and 10 mM D-glucose) before they were loaded with H2DCF-DA (10 μM) (2’,7’ dichlorodihy-droflourescein diacetate, Molecular Probes, Inc. Eugene, OR) in KHB for 2 h at 37 ºC (95% air, 5% CO2). Buffer was aspirated and myotubes were washed twice with 200 μL/well to remove excess extracellular H2DCF-DA. H2O2 (100 μM in KCl buffer) was added to the cells and the intracellular DCFH2 oxidation was determined every 4 min directly in the culture plate by fluorescence from 2,7-dichlorofluorescein (DCF) at excitation and emission wavelengths of 490 and 515 nm, respectively, at 34 ºC with a microtiter plate reader (Perkin-Elmer LS50B fluorometer, Beaconsfield, U.K.), and a custom-made thermostating element from Mikrolab, (Aarhus, Denmark) for approximately 6 h. Data were corrected for background signal from wells without cells but otherwise treated similarly. All concentrations tested of falcarinol and falcarindiol were determined in triplicate wells (n = 3).
Experimental setup and analyses of myoblast viability
Viability of porcine primary myoblasts was evaluated in 96 well plates by WST-1 (Roche, Hvidovre, Denmark) a formazan salt which is cleaved by mitochondrial dehydrogenase of viable cells. The relative amount of viable cells was determined by incubating the cells with 10 μl/well WST-1 for 4 h and measuring the absorbance at 450 nm (Oksbjerg et al. 2000). Data was corrected for the absorbance at 630 nm and background absorbance of the medium alone. All concentrations tested of falcarinol and falcarindiol were determined in quadruplicate wells (n = 4).
Statistics
The data from the experiment was analyzed by the MIXED procedure in SAS (SAS Institute, Cary, NC, USA). The absorption measured after WST-1 addition (indicating cell viability) and expression of the heat shock proteins were analyzed in a model with the concentration of falcarinol/falcarindiol as a fixed effect and replication by falcarinol/falcarindiol as a random effect. Although the ROS production was measured at several time points only one time point was analyzed statistically according to the model given for the other traits. Data is presented as LSMeans ± SEM.
RESULTS
mRNA expression
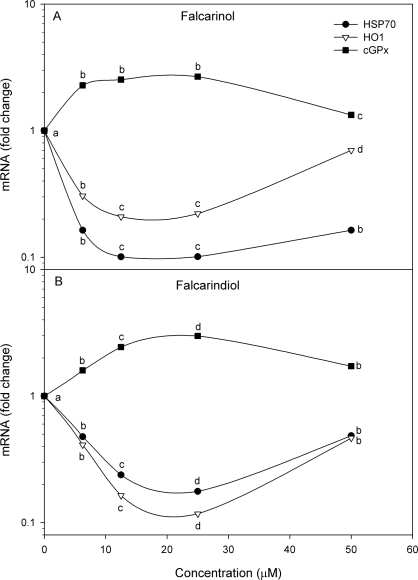
The mRNA expression of both the heat shock proteins HSP70 and HO1 of H2O2-stressed myotubes were lower in myotubes pre-incubated with 6.25 μM falcarinol (Figure 2A) or falcarindiol (Figure 2B) compared to control cells exposed to H2O2 without any pre-treatment of the polyacetylenes. Increasing the concentration of the polyacetylenes in the pre-incubation to 12.5 μM further decreased the mRNA expression of both heat shock proteins whereas a further increased concentration to 25 μM caused no (falcarinol) or only slightly (falcarindiol) further reduction in the mRNA expression of HSP70 and HO1. However, both heat shock proteins had increased mRNA expression when pre-incubated with 50μM of either polyacetylene compared to that of the expression at 25 μM reaching at least the levels of cells pre-incubated with only 6.25 μM polyacetylene. The mRNA expression of glutathione peroxidase (cGPx) followed the reverse pattern as it increased at polyacetylene concentrations of 25 μM or below and declined to at least the level of cells pre-incubated with only 6.25 μM polyacetylene when preincubated with 50 μM polyacetylene.
FIGURE 2.
LSMeans (n = 4) of the relative mRNA expression in primary porcine myotubes exposed to 100 μM H2O2 for 1 h after 24 h pre-exposure to falcarinol (A) or falcarindiol (B) at concentrations ranging from 6.25 μM to 50 μM. Cells were harvested for RNA extraction 18 h after stress exposure. Within each gene (HSP70: Heat shock protein 70, HO1: Hemoxygenase 1, cGPx: glutathione peroxidase), within each compound points without a common letter are significantly different (P < 0.05).
DCFH2 oxidation
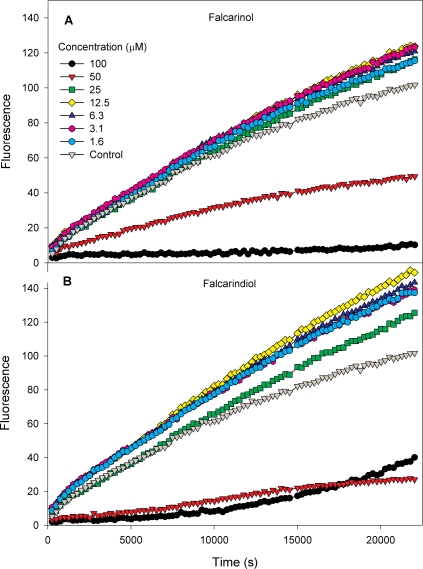
The intracellular oxidation of DCFH2 in myotubes exposed to H2O2 with or without pre-incubation with polyacetylenes is shown in Figure 3 with continuous measurements every 4 minutes, and a selected time point at 4 h is illustrated in Figure 4. In control myotubes exposed to H2O2 without pre-incubation with polyacetylenes the DCFH2 oxidation increased over the assay period, as illustrated in Figure 3. Myotubes pre-incubated with either falcarinol or falcarindiol in the concentration range from 1.6–12.5 μM accelerated the DCFH2 oxidation (Figure 3) resulting in an increased value at all time points (Figure 4). However, upon pre-incubation with 50 or 100 μM of either polyacetylene the oxidation DCFH2 was decreased compared to that of the control myotubes exposed to H2O2 without any polyacetylene pre-incubation
FIGURE 3.
LSMeans (n = 3) of oxidation of intracellular 2,7-dichloroflourescein in primary porcine myotubes exposed to 100 μM H2O2 after 24 h pre-exposure to falcarinol (A) or falcarindiol (B) at concentrations ranging from 1.6 μM to 100 μM.
FIGURE 4.
LSMeans (n = 3) and SEM of oxidation of intracellular 2,7-dichloroflourescein in primary porcine myotubes exposed to 100 μM H2O2 for 4 h (one selected time-point from Figure 3) after 24h pre-exposure to falcarinol (A) or falcarindiol (B) at concentrations ranging from 1.6 μM to100 μM. Within each polyacetylene, points without a common letter are significantly different (P < 0.05).
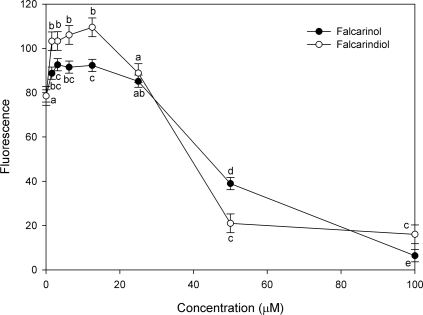
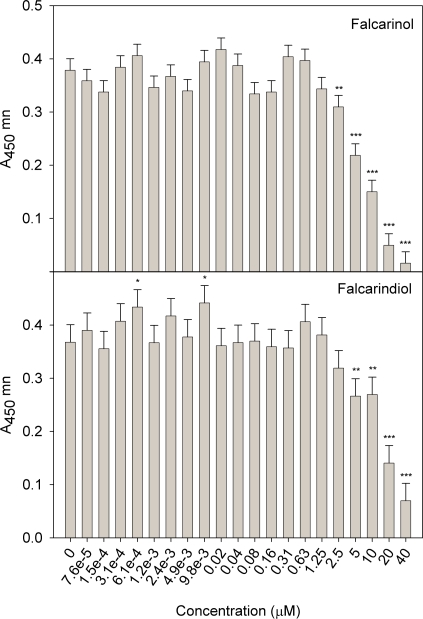
Myoblast viability
Viability of myoblasts in the presence of various concentrations of the polyacetylenes falcarinol and falcarindiol are shown in Figure 5 A and B, respectively. Compared to the control myoblasts without polyacetylene addition the viability of myoblasts was significantly reduced in the presence of falcarinol and falcarindiol in concentrations above 2.5 and 5 μM, respectively. At lower concentrations (9.8 and 0.61 nM) of falcarindiol the myoblast viability was significantly increased, and even though the concentrations in between were not significantly increased this may indicate a similar trend as previously indicated in studies on other cell types (Hansen et al. 2003; Young et al. 2007) where proliferation was increased at low concentrations of falcarinol.
FIGURE 5.
LSMeans (n = 4) and SEM of A 450 nm (WST-1 assay) expressing viable myoblasts upon exposure to falcarinol (A) or falcarindiol (B) for 48 h at concentrations ranging from 7.6 10−5 μM to 40μM. Values significantly different from control are indicated by * P < 0.05 or *** P < 0.001.
DISCUSSION
In order to allow the investigation of biphasic responses of isolated plant compounds on cellular stress responses in myotube cultures we exposed myotubes to a mild and reversible stress condition. The myotubes showed an increased intracellular ROS production (DCFH2 oxidation) when exposed to 100 μM H2O2, and this cellular ROS formation may be comparable to that of contracting muscle cells under moderate physical exercise which acts as health beneficial signals to increase the defense systems i.e. up-regulation of the anti-oxidative enzymes (Gomez-Cabrera et al. 2008). Gomez-Cabrera et al. also argued that only at the point where the amount of ROS generation exceeds the capacity of the cellular defense mechanisms the cells are adversely affected by the oxidative stress. Hence, the extent of intracellular ROS generation would determine if the ROS has an overall beneficial or harmful effect on the cells. The isolated plant compounds falcarinol and falcarindiol have proven to be very bioactive (Hansen et al. 1986; Bernart et al. 1996; Zidorn et al. 2005; Christensen and Brandt 2006); and in addition these compounds may interfere with the intracellular ROS production, quenching of these or ROS signalling functions. In the present study pre-exposure to low concentrations (< 25 μM) of falcarinol and falcarindiol accelerated the intracellular oxidation of DCFH2, indicating an increased formation of reactive oxygen species (ROS) within the myotubes (Figures 3 and 4) and this co-occurred with increased m-RNA expression of the anti-oxidative enzyme glutathione peroxidase (cGPx), which in this context may be regarded as an indicator of an adaptive action towards the increased intracellular ROS content. This adaptive action also co-occurred with a decreased mRNA expression of the heat shock proteins HSP70 and HO1. The HSP70 is cytoprotective and acts as a chaperone protein assisting the folding of newly produced proteins as well as solubilising denatured protein aggregates, restore protein function and assist clearance of damaged proteins (Kiang and Tsokos 1998), whereas HO1 is a heme degrading enzyme that binds to metalloporphyrins (Maines 1988). Thus, the need for protective and repairing actions of the HSPs seemed to be reduced at low concentrations of polyacetylenes compared to control cells exposed to H2O2 without pre-exposure to polyacetylenes. The decreased need for cellular HSPs may be a reflection of a reduced demand for protection and repair within the myotubes as a consequence of less damaging actions due to the adaptation (e.g. increased cGPx) to the moderately increased ROS. Hence, polyacetylenes in the low concentration range seem to have a protective role as they trigger parts of the defense mechanisms through a slightly increased ROS generation. Other studies have also shown suppression of the HSP60 and HSP70 response in lymphocytes challenged with H2O2 (Khassaf et al. 2003). DCFH2 oxidation decreases below that in the control myotubes when exposed to poly-acetylene concentrations above 25 μM, accompanied by decreased glutathione peroxidase mRNA expression and an increased mRNA expression of the HSPs compared to that in myotubes pre-exposed to poly-acetylenes at lower concentrations. The mechanism by which high concentrations of polyacetylenes affect the myotubes may be either by causing a reduction in the generation of ROS or by quenching the already produced ROS, leading to a hampered initiation of parts of the defense mechanisms within the myotubes as indicated by the decreased cGPx mRNA expression, and a simultaneously increased demand for the protective/repairing HSPs as the mRNA expressions for these proteins increase. This effect may be comparable to the negative effects of antioxidants in the context of moderate exercise, where these compounds hamper adaptive actions towards ROS as recently discussed by Jackson (2008) and Gomez-Cabrera et al. (2008). Alternatively, the high concentrations of falcarinol and falcarindiol used in the present study may have had a distinct toxic effect on the myotubes, by which several cellular responses and pathways are being compromised. These biphasic effects may be encompassed by the hormesis concept, which is defined by a U-shaped dose-response relationship (Calabrese 2003), e.g. a low dose stimulatory effect combined with a high dose inhibitory effect (Calabrese and Baldwin 2003). This phenomenon was also proven in the CaCo-2 cells exposed to falcarinol where DNA strand breakage and expression of the apoptosis indicator caspase-3 active protein was reduced at low concentrations of falcarinol and increased at high concentrations (Young et al. 2007). The bell-shaped does-response found in proliferation assays of bovine mammary epithelial cells (Hansen et al. 2003) and CaCo-2 cells (Young et al. 2007) exposed to falcarinol, supports the indication of increased viability of myoblasts in a narrow low concentration-range and a decreased viability at high doses. In conclusion, pre-incubation of myotubes with low concentrations of the polyacetylenes falcarinol and falcarindiol prior to H2O2 exposure induced a protective effect as it triggered parts of the defense mechanisms whereas higher concentrations of the polyacetylenes had adverse effects.
Acknowledgments
We thank The Danish Research Agency, Ministry of Science Technology and Innovation for financial support.
REFERENCES
- Alanko J, Kurahashi Y, Yoshimoto T, Yamamoto S, Baba K. Panaxynol, a polyacetylene compound isolated from oriental medicines, inhibits mammalian lipoxygenases. Biochem Pharmacol. 1994;48:1979–1981. doi: 10.1016/0006-2952(94)90598-3. [DOI] [PubMed] [Google Scholar]
- Bernart MW, Cardellina JH, Balaschak MS, Alexander MR, Shoemaker RH, Boyd MR. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J Nat Prod. 1996;59:748–753. doi: 10.1021/np960224o. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Enzymatic liberation of myogenic cells from adult rat muscle. Anat Rec. 1974;180:645–661. doi: 10.1002/ar.1091800410. [DOI] [PubMed] [Google Scholar]
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer pervention: A review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. The maturing of hormesis as a credible dose-response model. Nonlinearity Biol Toxicol Med. 2003;1:319–343. doi: 10.1080/15401420390249907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: The dose-response revolution. Ann Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Christensen LP, Brandt K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J Pharm Biomed Anal. 2006;41:683–693. doi: 10.1016/j.jpba.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Christensen LP, Kreutzmann S. Determination of polyacetylenes in carrot roots (Daucus carota L.) by high-performance liquid chromatography coupled with diode array detection. J Sep Sci. 2007;30:483–490. doi: 10.1002/jssc.200600325. [DOI] [PubMed] [Google Scholar]
- Czepa A, Hofmann T. Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree. J Agric Food Chem. 2003;51:3865–3873. doi: 10.1021/jf034085+. [DOI] [PubMed] [Google Scholar]
- Duthie SJ, Dobson VL. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur J Nutr. 1999;38:28–34. doi: 10.1007/s003940050043. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Hansen L, Hammershøy O, Boll PM. Allergic contact-dermatitis from falcarinol isolated from Scheffleraarboricola. Contact Dermatitis. 1986;14:91–93. doi: 10.1111/j.1600-0536.1986.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Purup S, Christensen LP. Bioactivity of falcarinol and the influence of processing and storage on its content in carrots (Daucus carota L) J Sci Food Agric. 2003;83:1010–1017. [Google Scholar]
- Jackson MJ. Free radicals generated contracting muscle: By-products of metabolism or key regulators of muscle function? Free Radic Biol Med. 2008;44:132–141. doi: 10.1016/j.freeradbiomed.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol -London. 2003;549:645–652. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat Shock Protein 70 kDa: Molecular Biology, Biochemistry, and Physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Kidmose U, Hansen SL, Christensen LP, Edelenbos M, Larsen E, Norbaek R. Effects of genotype, root size, storage, and processing on bioactive compounds in organically grown carrots (Daucus carota L.) J Food Sci. 2004;69:S388–S394. [Google Scholar]
- Kobæk-Larsen M, Christensen LP, Vach W, Ritskes-Hoitinga J, Brandt K. Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-induced preneoplastic lesions in the rat colon. J Agric Food Chem. 2005;53:1823–1827. doi: 10.1021/jf048519s. [DOI] [PubMed] [Google Scholar]
- Lemmich E. Constituents of Umbelliferous Plants .23. the Absolute-Configuration of the Acetylenic-Compound Falcarindiol. Phytochem. 1981;20:1419–1420. [Google Scholar]
- Liu JH, Zschocke S, Reininger E, Bauer R. Inhibitory effects of Angelica pubescens f. biserrata on 5-lipoxygenase and cyclooxygenase. Planta Med. 1998;64:525–529. doi: 10.1055/s-2006-957507. [DOI] [PubMed] [Google Scholar]
- Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- Maynard M, Gunnell D, Emmett P, Frankel S, Smith GD. Fruit, vegetables, and antioxidants in childhood and risk of adult cancer: the Boyd Orr cohort. J Epidemiol Community Health. 2003;57:218–225. doi: 10.1136/jech.57.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksbjerg N, Petersen JS, Sorensen IL, Henckel P, Vestergaard M, Ertbjerg P, Moller AJ, Bejerholm C, Stoier S. Long-term changes in performance and meat quality of Danish Landrace pigs: a study on a current compared with an unimproved genotype. Anim Sci. 2000;71:81–92. [Google Scholar]
- Ortenblad N, Young JF, Oksbjerg N, Nielsen JH, Lambert IH. Reactive oxygen species are important mediators of taurine release from skeletal muscle cells. Am J Physiol Cell Physiol. 2003;284:C1362–C1373. doi: 10.1152/ajpcell.00287.2002. [DOI] [PubMed] [Google Scholar]
- Steinmetz KA, Potter JD. Vegetables, fruit and cancer. I. Epidemiology. Cancer Cause Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- Teng CM, Kuo SC, Ko FN, Lee JC, Lee LG, Chen SC, Huang TF. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim Biophys Acta. 1989;990:315–320. doi: 10.1016/s0304-4165(89)80051-0. [DOI] [PubMed] [Google Scholar]
- The Alpha-Tocopherol B-CCPSG Effect of vitamin E and beta-carotene on the incidence of lung-cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- Theil PK, Sorensen IL, Nissen PM, Oksbjerg N. Temporal expression of growth factor genes of primary porcine satellite cells during myogenesis. Anim Sci J. 2006;77:330–337. [Google Scholar]
- Young JF, Duthie SJ, Milne L, Christensen LP, Duthie GG, Bestwick CS. Biphasic effect of falcarinol on CaCo-2 cell proliferation, DNA damage and apoptosis. J Agric Food Chem. 2007;55:618–623. doi: 10.1021/jf0616154. [DOI] [PubMed] [Google Scholar]
- Zidorn C, Johrer K, Ganzera M, Schubert B, Sigmund EM, Mader J, Greil R, Ellmerer EP, Stuppner H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J Agric Food Chem. 2005;53:2518–2523. doi: 10.1021/jf048041s. [DOI] [PubMed] [Google Scholar]