Abstract
Previous research has demonstrated that adding a very small gamma-ray dose to a small alpha radiation dose can completely suppress lung cancer induction by alpha radiation (a gamma-ray hormetic effect). Here we investigated the possibility of gamma-ray hormesis during low-dose neutron irradiation, since a small contribution to the total radiation dose from neutrons involves gamma rays. Using binucleated cells with micronuclei (micronucleated cells) among in vitro monoenergetic-neutron-irradiated human lymphocytes as a measure of residual damage, we investigated the influence of the small gamma-ray contribution to the dose on suppressing residual damage. We used residual damage data from previous experiments that involved neutrons with five different energies (0.22-, 0.44-, 1.5-, 5.9-, and 13.7-million electron volts [MeV]). Corresponding gamma-ray contributions to the dose were approximately 1%, 1%, 2%, 6%, and 6%, respectively. Total absorbed radiation doses were 0, 10, 50, and 100 mGy for each neutron source. We demonstrate for the first time a protective effect (reduced residual damage) of the small gamma-ray contribution to the neutron dose. Using similar data for exposure to gamma rays only, we also demonstrate a protective effect of 10 mGy (but not 50 or 100 mGy) related to reducing the frequency of micronucleated cells to below the spontaneous level.
Keywords: Gamma rays, X rays, Neutrons, Hormesis, Human Lymphocytes, Micronucleus
1. INTRODUCTION
There is no doubt that high doses of radiation are hazardous to cells and tissues. However, using varying biological endpoints (e.g., chromosomal damage, mutations, neoplastic transformation, and cancer), beneficial (not detrimental) effects of exposure to low doses of radiation have been found (Luckey 1982; Shadley and Wolff 1987; Bond et al. 1991; Luckey 1991; Cohen 1995; Azzam et al. 1996; Mitchel et al. 1999; Calabrese and Baldwin 2000; Hooker et al. 2004; Elmore et al. 2005; Feinendegen 2005; Day et al. 2006; Tubiana et al. 2006; Feinendegen et al. 2007). Nonetheless, information on such benefits is mainly limited to low doses of low linear-energy-transfer (LET) photons (i.e., X and γ rays) or combinations of low-LET gamma and high-LET alpha radiation (Scott and Di Palma 2006; Sanders and Scott 2008; Scott et al. 2008; Scott, 2008a,b). A large number of people are at risk of exposure to neutrons worldwide. For example, during 1988 it was estimated that approximately 7,000 people per year in the facilities of the Department of Energy (DOE) and about 6,000 research workers, including well loggers and reactor workers, were exposed to neutrons (Miller et al., 1999). Additionally, airline crew members and astronauts are at high risk of exposure to neutrons during space flights (Wilson and Townsend 1988).
Neutron interactions with matter involve absorption reactions. In a neutron absorption reaction the incident neutron is absorbed into the nucleus of an atom. Secondary radiations emerge with energies that depend on the intermediate excited nuclear configuration. The secondaries may be gamma rays from radioactive capture reactions (n, γ) and they can be nuclear-derived particles including protons, neutrons, or other heavier ions. The small low-LET gamma-ray contribution to the dose might cause radiation hormesis (i.e., adaptive response) with respect to reducing the harm associated with the other larger components to the total radiation dose, as has been demonstrated for combined alpha and gamma irradiation (Sanders 2007). Gamma-ray doses of 1 to 2 mGy appeared to completely prevent lung cancer induction in Wistar rats after inhaling the alpha emitter 239Pu in an insoluble dioxide form, for alpha radiation doses up to about 600 mGy (Sanders 2007; Scott et al. 2008; Scott 2008a). Spontaneous lung cancers also appeared to be prevented. The possibility that similar protective effect may occur during neutron irradiation has not previously been investigated.
To improve low-dose health risk assessment and biological dosimetry for neutron exposures, it is important to determine when hormetic effects from the gamma-ray contribution to the dose would be expected to occur. In this study of radiation-induced residual damage, as measured by micronucleated cells among irradiated human lymphocytes, we looked for evidence of hormetic effects (reduced micronucleated cells) of very small gamma-ray contributions to the total radiation dose from neutron irradiation. Micronucleus (MN) data from previous experimental studies of Rithidech (Rithidech et al., 1990) using neutron sources of different energies and varying gamma-ray contributions to the dose are reevaluated here in the context of gamma-radiation hormesis.
We also looked for evidence for photon radiation hormesis related to low-dose X- and gamma-ray suppression of cells with spontaneous chromosomal damage (presumably via apoptotic removal). The radiations studied were: (a) photons, i.e., 662-keV gamma rays, 70-kVp X rays, and 250-kVp X rays; and (b) monoenergetic neutrons, i.e., 0.22-, 0.44-, 1.5-, 5.9-, and 13.7- MeV. For the monoenegetic neutrons, the gamma-ray contributions to the total radiation dose were 1 %, 1%, 2%, 6%, and 6%, respectively (Table 1). Corresponding contributions from other components to the dose (protons, neutrons, other ions) were 99%, 99%, 98%, 94%, and 94% respectively. Cells with residual chromosomal damage (assayed by the presence of MN in binucleated human lymphocytes following irradiation in vitro) were used as a biological endpoint for determining hormetic effects of low doses of radiation. Two types of dosimetric variables were used: (a) absorbed radiation dose, and (b) average radiation microdose to nuclear-size volumes (NSV) due to single events. The indicated microdose is indicated here by <z1> and has been used by others in microdosimetric modeling of radiation hormesis (Feinendegen 2005). The symbol z represents specific energy (stochastic microscopic dose). The subscript 1 is used to specify that the microdose of interest relates to a single hit to each biological micromass of interest (e.g., cell, cell nucleus, etc.). The average value for the microscopic dose z equals the macroscopic absorbed dose D. The average number of hits to NSV is given by the ratio D/<z1>.
TABLE 1.
Typical RARAF neutron energy and secondary gamma-ray characteristicsa
| Neutron energy (MeV) and spread (± %) | γ-ray dose (%) | Production reaction |
|---|---|---|
| 0.22 (25) | 1 | T(p,n)3He |
| 0.44 (14) | 1 | T(p,n)3He |
| 1.5 (10) | 2 | T(p,n)3He |
| 5.9(6) | 6 | D(d,n)3He |
| 13.7 (1) | 6 | T(d,n)4He |
T, tritium; D, deteriuim; p, proton; n, neutron; d, deuteron
It has been well recognized that an analysis of chromosomal damage in metaphase cells prepared from peripheral blood lymphocytes stimulated to proliferate in vitro is one of the most reliable assays for detecting genomic damage from either low- or high-LET radiation, under various conditions (Lloyd and Edwards 1983; Bender et al. 1988). However, measuring chromosomal damage in metaphase cells is expensive and labor intensive; therefore, the MN assay is used as an alternative because of its simplicity and the speed by which cells can be scored. Scoring of a larger number of cells results in increased statistical power to detect even slight yet important differences among groups because the greater number of observations facilitates an enhanced statistical analysis. The MN assay is also possibly applicable to automation analysis (Tates et al. 1990). Development of the cytokinesis-block micronucleus (CBMN) assay (Fenech and Morley, 1985) has greatly improved the usefulness of the MN method. In this assay, cytochalasin B (Cyt-B) disrupts cytokinesis preventing daughter cells from separating. This disruption results in the formation of binucleated cells. With this method, the scoring of MN can be confined to cells that have undergone one cell division following the exposure, i.e. binucleated cells. Based upon these reasons, the MN assay was selected as a biological endpoint for evaluating the hormetic effects of low dose radiation.
It is known that MN can arise from acentric fragments (kinetochore-negative MN) or lagging whole chromosomes (kinetochore-positive MN) which are not incorporated into daughter nuclei at the time of cell division. Thus, MN reflect not only clastogenicity, but also spindle fiber disruption by genotoxic agents. Some existing evidence demonstrates that radiation-induced MN are mainly derived from acentric fragments (Heddle and Carrano 1977; Littlefield et al. 1989). On the other hand, substantial data show that radiation can cause kinetochore-positive MN (Eastmond and Tucker 1989; Fenech and Morley 1989; Cornforth and Goodwin 1991). The intercellular distribution of MN has been reported to be Poisson (Ramalho et al. 1988; Littlefield et al. 1989) or hyper-Poisson (Prosser et al. 1988), or over-dispersed (Hoffmann et al. 1993; Vral et al. 1994), depending on the radiation dose. While the frequencies of MN and acentric fragments increasingly diverge at doses of sparsely ionizing radiation above 1 Gy, it appears that at low doses of this type of radiation virtually all acentric fragments can be recovered as MN (Ramalho et al. 1988; Littlefield et al. 1989; Vral et al. 1994).
Using the CBMN method, a number of studies have been conducted in human lymphocytes to investigate the induction of MN by radiation and to compare MN data with data on metaphase chromosome-aberration induction (Almassy et al. 1987; Mitchell and Norman 1987; Kormos and Koteles 1988; Prosser et al. 1988; Littlefield et al. 1989; Balasem and Ali 1991; Vral et al. 1998). Although the induction of chromosome aberrations (determined by metaphase assay) in human peripheral blood lymphocytes by high-LET radiation (particularly neutrons) has been established, not much information exists on MN induction among exposed human lymphocytes. Published data demonstrate a linear dose-response for MN induction in human lymphocytes by high doses of fission neutrons (250 to 1500 mGy) (Ban et al. 1991) and for fast neutron irradiation (60 to 1000 mGy) of mouse zygotes (Pampfer et al. 1992). Our study is the first to focus on lower doses (10 to 100 mGy) where hormetic effects related to the gamma-ray contribution to the dose may occur. We are not aware of any previous publication where hormesis has been reported in relationship to neutron irradiation of cells in culture. This paper provides the first such reporting and demonstrates hormesis in relation to the gamma-ray component of the dose.
2. MATERIALS AND METHODS
Blood Sample Collection
Peripheral blood samples (approximately 5 mL) were collected by venipuncture into heparinized syringes from five nonsmoking healthy male volunteers (age ranging from 40–45 years old) using established blood-borne pathogen/biohazard safety protocols. These volunteers had no known history of previous exposure to clastogenic agents. This study was conducted under the approval of the Institutional Review Board of Brookhaven National Laboratory. After collection, blood samples were kept at room temperature until irradiation. Whole blood samples were irradiated before culture initiation.
Irradiation Procedures
X-ray exposures of blood samples were done at the Medical Department, Brookhaven National Laboratory (BNL), Upton, New York, using a dose rate of 0.3 Gy/min. Gamma-ray exposures were done at the Controlled Environment Radiation Facility of the Biology Department, BNL, using a dose rate of 0.5 Gy/min. For exposures to “monoenergetic” fast neutrons (high-LET radiation), whole blood samples were transported at room temperature (in a temperature controlled vehicle) to the Radiological Accelerator Facility (RARAF) of Columbia University, New York. The device produces essentially monoenergetic neutrons of various mean energies. Those selected were: 0.22-, 0.44-, 1.5-, 5.9-, and 13.7-MeV. Dose rates ranged from 0.05 to 0.6 Gy/hr, as described previously (Bond et al. 1997). Table 1 shows typical RARAF neutron energies and secondary gamma-ray characteristics. Irradiation was done at room temperature in a controlled area for both types of radiation. Due to the time needed for transportation of blood samples to and from RARAF, the irradiation of whole blood samples was performed at 4 hours after blood drawing. To be consistent, this schedule was also applied to X- and gamma-irradiation at BNL. Subsequently, irradiated whole blood samples were kept at room temperature for another 4 hours before the initiation of cultures. Overall, culture initiation of all blood samples was done within 8 hours after blood drawing.
Culture Methodology
The condition of blood lymphocyte cultures was similar to that routinely used in our laboratory (Rithidech et al. 2005), except that whole blood, not isolated lymphocytes, was used in this study. To do this, 1 mL of whole blood was added to a 15-mL conical tube containing 9 mL of RPMI 1640 medium, supplemented with 15% heat-inactivated fetal bovine serum, 1% glutamine, 1% pennicillin/streptomycin, and 5μg/mL phytohemagglutinin (PHA). Cells were incubated at 37°C in humidified 5% CO2 atmosphere. Two samples from each volunteer were irradiated at each dose and the experiments were duplicated. In each experiment, there were 4 culture tubes per dose for all treatments. The protocol for the CBMN developed by Fenech and Morley (Fenech and Morley 1985) was followed. A detailed protocol of the CBMN assay has been presented elsewhere (Fenech 2007). Briefly, at 44 hours after culture initiation, Cytochalasin-B (Cyt-B) was added to each culture tube, resulting in a final concentration of 3 μg/mL, to block cytokinesis (which normally occurs in the telophase phase of the cell cycle). Cells were harvested 28 hours after the addition of Cyt-B. The total culture time was 72 hours which resulted in the formation of many first division binucleated cells (35–60% or more binucleated cells as a proportion of viable cells, i.e. all cells excluding necrotic and apoptotic cells) that were scored for the induction of MN (Albertini et al. 2000; Fenech 2000). Although the MN assay can also provide information on apoptotic cells [i.e. cells with chromatin condensation within the nucleus and intact cytoplasmic and nuclear boundaries; and cells exhibiting nuclear fragmentation into smaller nuclear bodies within an intact cytoplasmic membrane (Fenech et al. 2003)], the scope of this study was to score the occurrence of MN in binucleated cells only.
Micronucleus Analysis
Basically, the method routinely used in our laboratory (Rithidech et al. 2005) was applied. Briefly, at the harvest time, after centrifugation at 1,500 rpm for 5 min, cell pellets were treated with 5 ml of a solution containing 1:1 v/v MilliQ water:RPMI 1640 medium (mild hypotonic solution) for 3 minutes. After another centrifugation, cells were washed twice with the fixative solution (3:1 v/v methanol:acetic acid). Fixed cells were dropped gently on clean microscope slides, air-dried and stained with 10% Gurr Giemsa (BDH, Santa Monica, CA) for 8 minutes. The slides were coded before scoring. Only binucleated cells with well-preserved cytoplasm were scored for MN (under a light microscope with a 40 × 10 magnification). The criteria for selection of binucleated cells and identification of MN given in the HUMN project website [http://HUMN.org] were followed. In brief, cells having two distinct nuclei of approximately equal size, which may be attached by a fine nucleoplasmic bridge or overlap slightly or touch each other at the edges, were selected. The number of MN (with the size varying from 1/16 to 1/3 of the mean diameter of the main nuclei) located within the cytoplasm of a binucleated cell was scored and recorded. The numbers of binucleated cells with one, two, three or more MN were then tabulated.
Evaluating Group Differences in the Number of Micronucleated Cells
Significant differences between the numbers of micronucleated cells (one or more MN) between a selected reference group and another comparison (irradiated) group were evaluated based on a 1 degree of freedom (df) chi-square test. Both an unirradiated control and an irradiated group were used as references but for different analyses. Expected numbers of micronucleated cells for the comparison group (irradiated group in each case) were evaluated based on the frequency of micronucleated cells among the reference group. In some cases the study-matched controls were used as reference. In other cases study-specific control data were combined to increase sample size. This was done to increase power for demonstrating a radiation-related reduction in the MN frequency to below the spontaneous level (which was quite small and fluctuated between studies).
Data for low-energy neutrons were also used as a reference when looking for a protective effect of the very small, gamma-ray component to the neutron dose. For these analyses data for 0.22-MeV and 0.44-MeV neutrons which have similar interaction characteristics (i.e., similar micro-doses to NSV and the same gamma-ray contribution [approximately 1%] to the total dose) and similar relative biological effectiveness [RBE; (Miller et al. 1999)] were combined to comprise a single reference group.
Confidence Interval for the Proportion of Micronucleated Cells
The binomial parameter φ as used here represents the true proportion of micronucleated cells among a large population of n surviving irradiated human lymphocytes. We use an unbiased point estimate of φ given by ρ = M/N, where M is the presumably binomially distributed observed number of residual micronucleated cells among samples of N cells scored. Our 95% interval estimate of φ is based on the normal distribution approximation to the binomial distribution (Fleiss 1981; Rosner 2000) which yields the approximate 100% × (1 − α) confidence interval ρ ± Z1–α/2[ρ(1 − ρ)/N]1/2, where Z is the standardized normal distribution and α = 0.05. The normal approximation is considered valid so long as Nρ(1 − ρ) ≥ 5 (Rosner 2000), which is the case for all of the data used in this paper.
Significant Differences Between ρ for Controls and Irradiated Groups
Significant increases and reduction in ρ after radiation exposure relative to a reference level ρ* (based on the group-specific proportion for pooled controls) were evaluated as follows:
When there is an apparent increase, i.e. ρ > ρ*:
When there is an apparent decrease, i.e. ρ < ρ*:
In the above two equations, Φ is the normal probability density function evaluated at proportion x when the distribution has mean proportion ρ and variance ρ(1 − ρ)/N. These relationships arise based on the normal distribution approximation to the binomial distribution for M, conditional on N.
Estimation of the Gamma-Ray Protection Factor
When evaluating the possible suppression of neutron-induced residual damage via gamma-ray hormesis it is beneficial to evaluate the protection factor [PROFAC; (Scott and Di Palma 2006)] which here represents the expected proportion of micronucleated cells prevented due to protective processes associated with radiation hormesis. These protective processes are thought to include induced high fidelity repair of DNA damage and selective apoptosis that removes damaged cells (Scott and Di Palma 2006; Portess et al. 2007). The PROFAC was evaluated as 1 −[(observed micronucleated cells)/(expected micronucleated cells)]. The expected frequency of micronucleated cells was evaluated based on a reference group comprised of data for 0.22- and 0.44-MeV neutrons. This frequency was multiplied by the number of cells scored in the comparison group (1.5- or 5.9- or 13.7-MeV neutrons).
A dose-response relationship for the PROFAC was constructed using <z1> as the independent variable. We show later that as <z1> increases, PROFAC decreases. The decrease may relate to deleterious bystander effects of nuclear hits by high-LET events that suppress protective signaling associated with hormesis (Scott and Di Palma 2006). For demonstrating suppression of protection against residual biological damage (i.e., decreases in PROFAC), we therefore have used <z1> evaluated based on NSV rather than cell-size volumes. However, for low-LET irradiation, protective bystander effects are also important and may involve volumes much larger than that for a single cell since ultra low X-ray doses that hit only a small number of cells at risk (but more than one cell) can induce protective signaling (Day et al. 2006, 2007). Thus, for developing a complete theory of radiobiological effects, two levels of dosimetry may be required, microdosimetry (for suppression of protective signaling) and macrodosimetry (for stimulation of protective signaling). We speculate that the suppressive microdosimetric effect may relate to deficient cell nucleus to mitochondria signaling, leading to a loss of one or more key pathways associated with protective apoptosis (Bauer 2007; Portess et al. 2007) that eliminates aberrant cells. In circumstances where mainly protective bystander effects occur, absorbed dose would therefore be expected to be more relevant than average hits to NSV.
Estimation of <z1> for Different Radiations of Interest
The dose reflects the corresponding 1-hit dose to the cell nucleus. However, it also reflects the 1-hit dose to any other volume of the same size within the cell (e.g., equivalent volume within the cytoplasm). The indicated average hit depends on the volume of the biological micro-masses considered (here all volumes in the irradiated target are the size of the cell nucleus). When estimated for a given radiation source and biological micromass, <z1> can then be scaled by LET (Leonard, 2007) or by values obtained for 1 μm size spherical biological micromasses as are reported in the BEIR IV Report (NAS/NRC 1988). This is the approach we used to obtain results presented in Table 2 with 250-kVp X rays being used as reference. Leonard (2007) reported the value <z1> = 2 mGy/hit for 250-kVp X irradiation of human lymphocytes based on data from Pohl-Rüling et al. (Pohl-Ruling et al. 1983). Values of LET used in scaling were obtained from NCRP Report 104 (NCRP 1990).
TABLE 2.
The value of <z1> for each type of radiation
| Radiation | <z1> | Scaling Based On |
|---|---|---|
| 137Cs gamma rays | 0.64 mGy/hit | 1-μm sphere |
| 70-kVp X rays | 3.6 mGy/hit | 1-μm sphere |
| 250-kVp X rays | 2.0 mGy/hit | No scaling |
| 13.7-MeV neutrons | 20.0 mGy/hit | 1-μm sphere |
| 5.9-MeV neutrons | 54.0 mGy/hit | LET |
| 1.5-MeV neutrons | 64.0 mGy/hit | LET |
| 0.44-MeV neutrons | 80.0 mGy/hit | 1-μm sphere |
| 0.22-MeV neutrons | 94.0 mGy/hit | LET |
When the probability of damage occurrence is expressed as a function of z1 (the actual hit size), the resultant dose-response curve is called a hit-size effectiveness function (Bond et al. 1995; Sondhaus et al. 1996). Such functions in theory could be used to explain the different effectiveness of different radiation in producing biological damage to cells. Whether or not this is feasible can be evaluated on the basis of biological damage correlating with <z1>. Later we use the relative frequency of micronucleated cells (for one or more MN per cell) evaluated at a fixed low dose (10 mGy) and evaluated relative to 250-kVp X rays to test for a significant correlation of biological damage with <z1>.
3. RESULTS AND DISCUSSION
The frequency and distribution of MN in human lymphocytes induced by various doses of low- and high-LET radiation are shown in Table 3. Table 4 shows results of comparing irradiated groups relative to study-matched controls to see if there is any evidence for hormesis or for an increase in risk. All doses above 10 mGy demonstrated significant increases in the frequency of micronucleated cells. None of the 10-mGy data showed a significant hormetic effect. The control data were then combined (total cells = 79,950) to look for a possible significant suppression in the frequency of micronucleated cells after irradiation. Only the 10-mGy data for gamma-ray exposure was found to be significantly suppressed (p = 1.73 × 10−3).
TABLE 3.
Frequency and distribution of micronuclei in binucleated human lymphocytes according to radiation type and dose (* Numbers in parentheses are nuclei per cell)
| Radiation
|
Energy
|
Dose (mGy)
|
Total cells scored
|
Total micronuclei frequency
|
Mean micronuclei per 1000 cells ± S.E.
|
Micronuclei distribution/cell
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | >3* | ||||||
| Neutron | 0.22-MeV | 0 | 12,019 | 153 | 13.52 ± 4.53 | 11,876 | 131 | 11 | 0 | 1(4) |
| 10 | 8,882 | 150 | 16.27 ± 4.57 | 8,757 | 107 | 16 | 1 | 1(7) | ||
| 50 | 8,658 | 185 | 23.09 ± 5.29 | 8,491 | 149 | 15 | 2 | 1(4) | ||
| 100 | 11,744 | 386 | 30.75 ± 6.63 | 11,564 | 275 | 56 | 3 | 0 | ||
| Neutron | 0.44-MeV | 0 | 13,680 | 74 | 6.25 ± 1.44 | 13,606 | 74 | 0 | 0 | 0 |
| 10 | 12,027 | 93 | 9.01 ± 4.02 | 11,838 | 82 | 4 | 1 | 0 | ||
| 50 | 11,964 | 182 | 16.75 ± 2.84 | 11,802 | 160 | 2 | 0 | 0 | ||
| 100 | 9,625 | 278 | 33.50 ± 5.95 | 9,376 | 227 | 21 | 3 | 0 | ||
| Neutron | 1.5-MeV | 0 | 9,060 | 42 | 4.45 ± 1.20 | 9,018 | 42 | 0 | 0 | 0 |
| 10 | 9,769 | 64 | 6.24 ± 1.05 | 9,709 | 56 | 4 | 0 | 0 | ||
| 50 | 9,827 | 116 | 11.21 ± 2.11 | 9,727 | 84 | 15 | 0 | 0 | ||
| 100 | 9,873 | 176 | 16.71 ± 2.43 | 9,717 | 149 | 12 | 1 | 0 | ||
| Neutron | 5.9-MeV | 0 | 10,942 | 48 | 4.35 ± 2.16 | 10,896 | 44 | 2 | 0 | 0 |
| 10 | 13,730 | 105 | 6.24 ± 2.36 | 13,631 | 93 | 6 | 0 | 0 | ||
| 50 | 12,787 | 151 | 9.64 ± 2.57 | 12,649 | 125 | 13 | 0 | 1(8) | ||
| 100 | 10,526 | 178 | 13.80 ± 3.15 | 10,364 | 144 | 18 | 0 | 0 | ||
| Neutron | 13.7-MeV | 0 | 11,025 | 26 | 1.18 ± 0.76 | 11,001 | 22 | 2 | 0 | 0 |
| 10 | 9,833 | 40 | 3.98 ± 0.97 | 9,794 | 38 | 1 | 0 | 0 | ||
| 50 | 8,150 | 44 | 5.53 ± 0.75 | 8,110 | 36 | 4 | 0 | 0 | ||
| 100 | 9,997 | 102 | 10.31 ± 1.18 | 9,904 | 84 | 9 | 0 | 0 | ||
| X rays | 70-kVp | 0 | 9,256 | 57 | 5.51 ± 1.97 | 9,201 | 53 | 2 | 0 | 0 |
| 10 | 7,549 | 44 | 5.57 ± 1.74 | 7,505 | 44 | 0 | 0 | 0 | ||
| 50 | 8,826 | 85 | 9.17 ± 1.24 | 8,747 | 72 | 5 | 0 | 0 | ||
| 100 | 6,683 | 102 | 12.75 ± 0.39 | 6,585 | 94 | 4 | 0 | 0 | ||
| X rays | 250-kVp | 0 | 6,069 | 57 | 9.35 ± 0.06 | 6,046 | 44 | 5 | 1 | 0 |
| 10 | 10,263 | 86 | 8.43 ± 0.20 | 10,184 | 72 | 7 | 0 | 0 | ||
| 50 | 7,915 | 85 | 10.72 ± 1.24 | 7,837 | 74 | 1 | 3 | 0 | ||
| 100 | 5,080 | 77 | 15.09 ± 0.44 | 5,007 | 70 | 2 | 1 | 0 | ||
| γ rays | 662-keV | 0 | 7,899 | 19 | 2.39 ± 0.17 | 7,881 | 17 | 1 | 0 | 0 |
| 10 | 7,716 | 23 | 2.96 ± 0.55 | 7,695 | 19 | 2 | 0 | 0 | ||
| 50 | 6,047 | 28 | 4.22 ± 1.18 | 6,020 | 26 | 1 | 0 | 0 | ||
| 100 | 6,705 | 42 | 6.25 ± 0.11 | 6,664 | 40 | 1 | 0 | 0 | ||
TABLE 4.
Test for significant differences between study-specific controls and irradiated groups for the number of micronucleated cells among human lymphocytes evaluated by radiation type and dose level
| Dose mGy | Total Cells | Cells Without Micronuclei | Observed Micronucleated Cells | Expected Micronucleated Cells | Chi Square | p-value | Significant Hormetic Effect |
|---|---|---|---|---|---|---|---|
| Neutron, 0.22-MeV | |||||||
| 0 | 79950 | 79525 | 425 | 425 | |||
| 10 | 8882 | 8757 | 125 | 47.2 | 128.8 | 7.38E–30 | no |
| 50 | 8658 | 8491 | 167 | 46.0 | 319.7 | 1.70E–71 | no |
| 100 | 11744 | 11564 | 180 | 62.4 | 222.6 | 2.45E–50 | no |
| Neutron, 0.44-MeV | |||||||
| 0 | 79950 | 79525 | 425 | 425.0 | |||
| 10 | 12027 | 11838 | 189 | 63.9 | 246 | 1.97E–55 | no |
| 50 | 11964 | 11802 | 162 | 63.6 | 153 | 3.71E–35 | no |
| 100 | 9625 | 9376 | 249 | 51.2 | 769 | 2.9E–169 | no |
| Neutron, 1.5-MeV | |||||||
| 0 | 79950 | 79525 | 425 | 425.0 | |||
| 10 | 9769 | 9709 | 60 | 51.9 | 1.3 | 2.62E–01 | no |
| 50 | 9827 | 9727 | 100 | 52.2 | 43.9 | 3.45E–11 | no |
| 100 | 9873 | 9717 | 156 | 52.5 | 205 | 1.48E–46 | no |
| Neutron, 5.9-MeV | |||||||
| 0 | 79950 | 79525 | 425 | 425.0 | |||
| 10 | 13730 | 13631 | 99 | 73.0 | 9.3 | 0.002265 | no |
| 50 | 12787 | 12649 | 138 | 68.0 | 73 | 1.65E–17 | no |
| 100 | 10526 | 10364 | 162 | 56.0 | 202 | 7.44E–46 | no |
| Neutron, 13.7-MeV | |||||||
| 0 | 79950 | 79525 | 425 | 425.0 | |||
| 10 | 9833 | 9794 | 39 | 52.3 | 3.4 | 6.57E–02 | suggested |
| 50 | 8150 | 8110 | 40 | 43.3 | 0.3 | 0.612613 | no |
| 100 | 9997 | 9904 | 93 | 53.1 | 30 | 4.2E–08 | no |
| X Rays, 70-kVp | |||||||
| 0 | 79950 | 79525 | 425 | 425.0 | |||
| 10 | 7549 | 7505 | 44 | 40.1 | 0.375 | 5.40E–01 | no |
| 50 | 8826 | 8747 | 79 | 46.9 | 22.1 | 2.65E–06 | no |
| 100 | 6683 | 6585 | 98 | 35.5 | 110.5 | 7.8E–26 | no |
| X rays, 250-kVp | |||||||
| 0 | 79950 | 79525 | 425 | 425.0 | |||
| 10 | 10263 | 10184 | 79 | 54.6 | 11.0 | 0.000906 | no |
| 50 | 7915 | 7837 | 78 | 42.1 | 30.8 | 2.8E–08 | no |
| 100 | 5080 | 5007 | 73 | 27.0 | 79 | 7.01E–19 | no |
| γ rays, 662-keV | |||||||
| 0 | 79950 | 79525 | 425 | 425.0 | |||
| 10 | 7716 | 7695 | 21 | 41.0 | 9.82 | 1.73E–03 | yes |
| 50 | 6047 | 6020 | 27 | 32.1 | 0.8 | 3.63E–01 | no |
| 100 | 6705 | 6664 | 41 | 35.6 | 0.8 | 0.368247 | no |
Dose-Response Curves
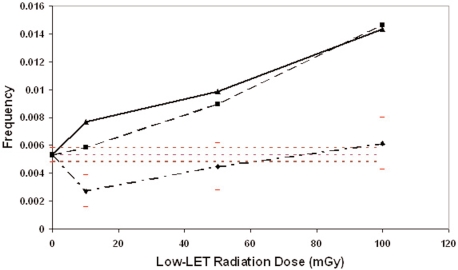
Figure 1 shows the resulting photon dose-response curves for the biological damage (frequency of one or more MN per cell) as a function of the absorbed radiation dose for 70-kVp X rays, 250-kVp X rays, and 662-keV gamma rays. The horizontal dashed lines comprise the 95% confidence band for the combined control dataset. Ninety-five percent confidence intervals for the data are also presented for the gamma-ray data but are not presented for the X-ray data to avoid a cluttered presentation of the results. The gamma-ray curve in Fig. 1 is J-shaped, which is characteristic of hormetic responses (Calabrese and Baldwin 2000). We speculate that low doses can cause a mild stress response that leads to the elimination of aberrant cells via protective apoptosis, as has been reported elsewhere (Scott and Di Palma 2006; Portess et al. 2007). We further speculate that the critical volume for initiating the protective apoptosis by gamma irradiation is larger than the size of the cell as has been implicated by results of studies conducted by Day et al. (Day et al. 2006, 2007) where doses for which most cells were not hit led to protective signaling that apparently eliminated aberrant (mutant) cells from the population. The absence of hormetic responses for 70- and 250-kVp X rays was not expected, given that such responses have been demonstrated for neoplastic transformation for similar photon radiation energies (Scott 2004; Scott 2005). However, a key variable in addition to photon radiation energy is the absorbed dose rate. High rates can inhibit protective signaling while low-rate extended exposure can efficiently stimulate protective signaling and expand the dose range over which protective effects are observed (Elmore et al. 2006; Scott and Di Palma 2006).
FIGURE 1.
Dose-response curves for the frequency of micronucleated cells after exposure of human lymphocytes to photon radiations with different energy characteristics: Gamma rays (diamonds); 70-kVp X rays (squares); 250-kVp X rays (triangles). Horizontal dashed lines indicated the 95% confidence region for the pooled control data. Error bars for data points are 95% confidence intervals.
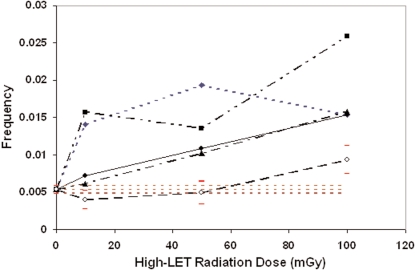
Corresponding results for monoenergetic neutrons are presented in Figure 2. No significant reduction (relative to controls) in the frequency of micronucleated cells was found (p > 0.05) based on the chi-square test related to observed and expected micronucleated cells. However, a significant reduction was found for 13.7-MeV neutrons related to the combined controls when based on a normal distribution with mean ρ and variance ρ(1 − ρ)/N as explained in the Methods section. The horizontal dashed lines in Figure 2 comprise the 95% confidence band for the combined control data. Ninety-five percent confidence intervals are also shown for the 13.7-MeV data. Confidence intervals for the other neutron data were excluded to avoid a cluttered presentation of the results.
FIGURE 2.
Dose-response curves for the frequency of micronucleated cells after exposure of human lymphocytes to monoenergetic neutrons: 13.7-MeV (open circles); 1.5-MeV (triangles); 5.9-Mev (closed circles); 0.44-MeV (squares); 0.22-MeV (diamonds). Horizontal dashed lines indicated the 95% confidence region for the pooled control data. Error bars for data points are 95% confidence intervals.
Demonstration of Gamma-Ray Protection from Neutron-Induced Harm
Table 5 shows results obtained when the 0.22-MeV and 0.44-MeV neutron data were combined and used as reference to see if there is evidence for protection associated with the very small gamma-ray component to the dose. The gamma-ray dose by itself is too small (< 10 mGy) to be expected to cause any significant harm (see gamma-ray dose-response in Figure 1). However, doses in the indicated range have been demonstrated to suppress alpha radiation-induced lung cancer (Sanders 2007). It can be seen from Table 5 that adding a gamma-ray dose as low as 0.1 to 0.5 mGy, when the total radiation dose is 10 mGy, leads to PROFAC estimates of 0.52 to 0.74 when evaluated relative to the low energy neutrons (0.22- and 0.44-MeV data combined). This means that 52% to 74% of the expected micronucleated cells did not occur as a result of gamma-ray induced protection (gamma-ray hormesis). The average and standard deviation for these estimates are 0.62 ± 0.11 and is clearly greater than zero. For a total dose of 50 mGy, the corresponding PROFACs are 0.36, 0.32, and 0.69 respectively. The average and standard deviation for these estimates are 0.46 ± 0.20. For a total dose of 100 mGy, the corresponding PROFACs are 0.21, 0.23, and 0.54. The average and standard deviation for these estimates are 0.33 ± 0.19.
TABLE 5.
Gamma-ray protection factors for suppressing neutron-induced micronuclei among human lymphocytes irradiated in vitro
| Neutrons | Radiation Dose mGy | Gamma-Ray Contribution to the Dose mGy | Total Cells | Cells Without Micro-nuclei | Observed Micro-nucleated Cells | Expected Micro-nucleated Cells | Chi Square | p-value | Protective (Hormetic) Effect of Gamma Rays Implicated | Gamma- Ray Protection Factor PROFAC |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | 10 | 0.1 | 20909 | 20595 | 314 | 314 | ||||
| 1.5-MeV | 10 | 0.2 | 9769 | 9709 | 60 | 147 | 52 | 5.478E–13 | yes | 0.59 |
| 5.9-MeV | 10 | 0.6 | 13730 | 13631 | 99 | 206 | 57 | 5.415E–14 | yes | 0.52 |
| 13.7-MeV | 10 | 0.6 | 9833 | 9794 | 39 | 148 | 81 | 2.054E–19 | yes | 0.74 |
| Reference | 50 | 0.5 | 20622 | 20293 | 329 | 329 | ||||
| 1.5-MeV | 50 | 1 | 9827 | 9727 | 100 | 157 | 21 | 4.85E–06 | yes | 0.36 |
| 5.9-MeV | 50 | 3 | 12787 | 12649 | 138 | 204 | 22 | 3.188E–06 | yes | 0.32 |
| 13.7-MeV | 50 | 3 | 8150 | 8110 | 40 | 130 | 63 | 1.74E–15 | yes | 0.69 |
| Reference | 100 | 1 | 21369 | 20940 | 429 | 429 | ||||
| 1.5-MeV | 100 | 2 | 9873 | 9717 | 156 | 198 | 9.2 | 0.0024569 | yes | 0.21 |
| 5.9-MeV | 100 | 6 | 10526 | 10364 | 162 | 211 | 12 | 0.0006098 | yes | 0.23 |
| 13.7-MeV | 100 | 6 | 9997 | 9904 | 93 | 201 | 59 | 1.596E–14 | yes | 0.54 |
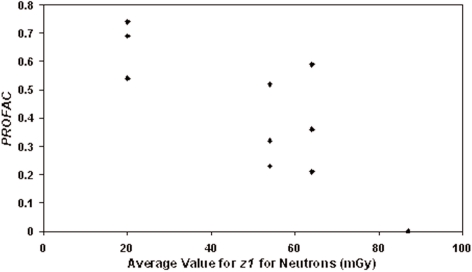
Figure 3 shows the PROFAC decreases as <z1> increases. Three values for the reference neutrons (0.22- and 0.44-MeV combined data set) are plotted at PROFAC = 0 and <z1> given by the average of the values for these radiations (i.e., 87 mGy/hit). However, the three values appear as a single point. At the dose of 10 mGy, the average hit to NSV would be 10/87 = 0.11. Thus, about 1 in 10 cell nuclei are expected to be hit. The correlation coefficient for the data in Figure 3 is R = −0.87 ± 0.15 (R2 = 0.76, t = 5.63, degrees of freedom =10, p = 0.00011). Thus, the negative correlation is highly significant and is suggestive of an inhibitory function of high values for <z1> (relative to gamma rays where <z1> = 0.64 mGy/hit) with respect to the protective processes that contribute to gamma-ray hormesis. The data are in fact consistent with the existence of a hit effectiveness function with respect to the suppression of protective cell signaling associated hormesis. The fact that the results in Figure 3 show an increased PROFAC in association with a 10-mGy macroscopic absorbed neutron dose when the NSV hit size (mGy/hit) decreases relative to the reference neutrons with their particularly high <z1> values (80–94 mGy/hit), implicates a robust protective cellular-community response to DNA damage stimulated by low-dose gamma radiation. The protective response is thought to involve selective removal of aberrant cells via protective apoptosis (Bauer 2007; Portess et al. 2007).
FIGURE 3.
Gamma-ray protection factors against neutron-induced micronucleated cells plotted as a function of <z1>. PROFAC = 0 for the reference group (0.22- and 0.44-MeV neutrons combined) which is plotted (three superimposing data points) at the middle of the range 80–94 mGy/hit for <z1>.
Checking for Additional Evidence for a Hit Size Effectiveness Function
Table 6 shows data for the relative frequency of micronucleated cells (for one or more MN per cell) evaluated at a fixed 10-mGy absorbed dose and evaluated relative to 250-kVp X rays. The data are arranged according to increasing values for <z1> which is a measure of the size of the radiation hit to NSV. The relative frequency is correlated with <z1> (correlation coefficient R = 0.78; p < 0.02) supporting the existence of a hit-size effective function for induced chromosomal damage as has been predicted by Bond et al (Bond et al. 1995; Sondhaus et al. 1996).
TABLE 6.
Absolute and relative frequencies for one or more micronuclei per cell for different values of <z1> and a fixed dose of 10 mGy
| Type of Radiation | <z1> in mGy/hit | Absolute Frequency | Relative Frequencya |
|---|---|---|---|
| Gamma rays (662 keV) | 0.64 | 2.72e–3 | 0.35 |
| 250-kVp X rays | 2 | 7.70e–3 | 1.0 |
| 70-kVp X rays | 3.6 | 5.83e–3 | 0.76 |
| 13.7-MeV neutrons | 20 | 3.97e–3 | 0.51 |
| 5.9-MeV neutrons | 54 | 7.21e–3 | 0.94 |
| 1.5-MeV neutrons | 64 | 6.14e–3 | 0.80 |
| 0.44-MeV neutrons | 80 | 1.57e–2 | 2.0 |
| 0.22-MeV neutrons | 94 | 1.41e–2 | 1.8 |
Relative to 250-kVp X rays
Impact of Reducing the Proton, Neutron, and Ions Contribution to Dose
Skeptics may claim that the reduction in risk associated with increasing the gamma-ray contribution to dose could be explained based on the corresponding reduction in the contribution to dose from protons, neutrons, and other ions. An increase in the gamma-ray dose of only 0.1 mGy was found to be protective for 1.5-MeV neutrons when compared to a reference group comprised of 0.22- and 0.44-MeV neutrons. For the reference group the excess frequency of micronucleated cells per unit dose after 10-mGy exposure was [(314/20,900) – (217/25699)]/10 mGy = 6.58 ×10−4/mGy. Reducing the proton, neutron, and other ions contribution to the dose by 0.1 mGy would be expected to decrease the micronucleated cell frequcncy by 0.1*6.58 × 10−4/mGy = 6.58 × 10−5. The expected reduction in micronucleated cells among 9,769 irradiated cells would be 9,769*6.58 × 10−5 = 0.64 cells when for the 1.5-MeV neutron exposure only 60 micronucleated cells were observed as compared to 147 expected (Table 5). Thus, a 0.1-mGy reduction in the proton, neutron, and ions contribution to the dose could account for a loss of no more than 0.64 or approximately 1 micronucleated cell. In contrast, gamma-ray-induced protective signaling appears to have eliminated essentially all of the missing (147 – 60 = 87) micronucleated cells.
Research Implications for Low-Dose Biodosimetry and Cancer Risk Assessment
Our results have important implications for neutron biological dosimetry and for low-dose risk assessment. For large mammals such as humans, the gamma-ray contribution to the dose varies over the body. Thus, a biological-dosimetry-based calibration curve for chromosomal damage to lymphocytes derived using a fixed gamma-ray contribution to the dose may be inappropriate for neutron biological dosimetry for humans. A calibration curve based on averaging dose-responses over the varying gamma-ray contribution to the dose over the body would seem more appropriate. This average, however, likely varies for different individuals due to differences in body mass. Exposure geometry differences can also be important. Body-mass and exposure-geometry-specific averages would therefore be needed.
For cancer risk assessment for monoenergetic fast neutrons, the gamma-ray component to the dose appears to be quite important so far as the occurrence of gamma-ray hormesis. Indeed, differences in the low-dose RBE for neutrons of differing energies and different energy spectra may largely relate to differences in the protective gamma-ray component to the dose (a novel concept). Gamma-ray protection can also arise via stimulation of immunity against cancer (Liu 2007). The existence of gamma-ray hormesis during low-dose neutron irradiation does not support the linear-no-threshold (LNT) hypothesis of radiation-induced deleterious stochastic radiobiological effects. For varying body sizes and a fixed total radiation dose, larger body sizes may be associated with reduced stochastic effects such as cancer, due to greater gamma-ray protection than for a smaller body size.
In summary, our research has demonstrated for the first time gamma-ray hormesis during low-dose neutron irradiation. This protective effect may largely be responsible for variation in the RBE of neutrons of different energies and for neutrons with different energy spectra when the total radiation dose is ≤ 100 mGy. The protective effect is thought to relate to biology (gamma-ray activation of high-fidelity DNA repair and stimulating of apoptosis of aberrant cells). Thus, the RBE for neutron-induced stochastic radiobiological effects may not depend only on physics (e.g., LET and lineal energy spectra) but also on biology (DNA repair and apoptosis). Stimulation of immune system functioning by low doses of gamma rays could also impact the low-dose neutron RBE for in vivo radiobiological effects such as cancer.
Acknowledgment
This study was supported in part by DOE Grant # DE-AC02-76CH00016 (V.P. Bond), DOE Grant #DE-FG 02-02ER63311 (K. Noy Rithidech) and DOE Grant #DE-FG02-03ER63657 and Lovelace Respiratory Research Institute (B.R. Scott). Also, the support from the Office of the Vice President for Research and Office of Scientific Affairs, School of Medicine of Stony Brook University (to K. Noy Rithidech) is greatly appreciated.
REFERENCES
- Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DEG, Tice R, Waters MD, Aitio A. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Resh. 2000;463:111–172. doi: 10.1016/s1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Almassy Z, Krepinsky AB, Bianco A, Koteles GJ. The present state and perspectives of micronucleus assay in radiation protection. A review. Int J Rad Appl Instrum [A] 1987;38:241–249. doi: 10.1016/0883-2889(87)90033-5. [DOI] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Raaphorst GP, Mitchel RE. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- Balasem AN, Ali AS. Establishment of dose-response relationships between doses of Cs-137 gamma-rays and frequencies of micronuclei in human peripheral blood lymphocytes. Mutat Res. 1991;259:133–138. doi: 10.1016/0165-1218(91)90047-p. [DOI] [PubMed] [Google Scholar]
- Ban S, Donovan MP, Cologne JB, Sawada S. Gamma-ray- and fission neutron-induced micronuclei in PHA stimulated and unstimulated human lymphocytes. J Radiat Res (Tokyo) 1991;32:13–22. doi: 10.1269/jrr.32.13. [DOI] [PubMed] [Google Scholar]
- Bauer G. Low dose radiation and intercellular induction of apoptosis: potential implications for the control of oncogenesis. Int J Radiat Biol. 2007;83:873–888. doi: 10.1080/09553000701727523. [DOI] [PubMed] [Google Scholar]
- Bender MA, Awa AA, Brooks AL, Evans HJ, Groer PG, Littlefield LG, Pereira C, Preston RJ, Wachholz BW. Current status of cytogenetic procedures to detect and quantify previous exposures to radiation. Mutat Res. 1988;196:103–159. doi: 10.1016/0165-1110(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Bond VP, Benary V, Sondhaus CA. A different perception of the linear, nonthreshold hypothesis for low-dose irradiation. Proc Natl Acad Sci U S A. 1991;88:8666–8670. doi: 10.1073/pnas.88.19.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond VP, Varma M, Feinendegen LE, Wuu CS, Zaider M. Application of the HSEF to assessing radiation risks in the practice of radiation protection. Health Phys. 1995;68:627–631. doi: 10.1097/00004032-199505000-00001. [DOI] [PubMed] [Google Scholar]
- Bond V, Cronkite EP, Bullis JE, Wu CS, Zadier M. An HSEF for murine leukemia. In: Goodhead DT, Menzel HG, editors. Microdosimetry, An Interdisciplinary Approach. The Royal Society of Chemistry; Cambridge, U.K.: 1997. 1997. pp. 228–231. [Google Scholar]
- Calabrese EJ, Baldwin LA. Radiation hormesis: the demise of a legitimate hypothesis. Hum Exp Toxicol. 2000;19:76–84. doi: 10.1191/096032700678815611. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Test of the linear-no threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995;68:157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Cornforth MN, Goodwin EH. The dose-dependent fragmentation of chromatin in human fibroblasts by 3.5-MeV alpha particles from 238Pu: experimental and theoretical considerations pertaining to single-track effects. Radiat Res. 1991;127:64–74. [PubMed] [Google Scholar]
- Day TK, Zeng G, Hooker AM, Bhat M, Scott BR, Turner DR, Sykes PJ. Extremely low priming doses of X radiation induce an adaptive response for chromosomal inversions in pKZ1 mouse prostate. Radiat Res. 2006;166:757–766. doi: 10.1667/RR0689.1. [DOI] [PubMed] [Google Scholar]
- Day TK, Zeng G, Hooker AM, Bhat M, Scott BR, Turner DR, Sykes PJ. Adaptive response for chromosomal inversions in pKZ1 mouse prostate induced by low doses of x-radiation delivered after a high dose. Radiat Res. 2007;167:682–692. doi: 10.1667/RR0764.1. [DOI] [PubMed] [Google Scholar]
- Eastmond DA, Tucker JD. Kinetochore localization in micronucleated cytokinesis-blocked Chinese hamster ovary cells: a new and rapid assay for identifying aneuploidy-inducing agents. Mutat Res. 1989;224:517–525. doi: 10.1016/0165-1218(89)90079-7. [DOI] [PubMed] [Google Scholar]
- Elmore E, Lao XY, Ko M, Rightnar S, Nelson G, Redpath J. Neoplastic transformation in vitro induced by low doses of 232 MeV protons. Inter Journal of Radiat Biol. 2005;81:291–297. doi: 10.1080/09553000500140324. [DOI] [PubMed] [Google Scholar]
- Elmore E, Lao XY, Kapadia R, Redpath JL. The effect of dose rate on radiation-induced neoplastic transformation in vitro by low doses of low-LET radiation. Radiat Res. 2006;166:832–838. doi: 10.1667/RR0682.1. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78:3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Pollycove M, Neumann RD. Whole-body responses to low-level radiation exposure: New concepts in mammalian radiobiology. Exp Hematol. 2007;35:37–46. doi: 10.1016/j.exphem.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protocols. 2007;2:1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley AA. Kinetochore detection in micronuclei: an alternative method for measuring chromosome loss. Mutagenesis. 1989;4:98–104. doi: 10.1093/mutage/4.2.98. [DOI] [PubMed] [Google Scholar]
- Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res. 2003;534:65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- Fleiss J. Statistical methods for rates and proportions. New York – Chichester – Brisbane – Toronto – Singapore: John Wiley & Sons; 1981. [Google Scholar]
- Heddle JA, Carrano AV. The DNA content of micronuclei induced in mouse bone marrow by γ-irradiation: Evidence that micronuclei arise from acentric chromosomal fragments. Mutat Res. 1977;44:63–69. [Google Scholar]
- Hoffmann GR, Colyer SP, Littlefield LG. Induction of micronuclei by bleomycin in G0 human lymphocytes: I. Dose-response and distribution. Environ Mol Mutagen. 1993;21:130–135. doi: 10.1002/em.2850210206. [DOI] [PubMed] [Google Scholar]
- Hooker AM, Bhat M, Day TK, Lane JM, Swinburne SJ, Morley AA, Sykes PJ. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat Res. 2004;162:447–452. doi: 10.1667/rr3228. [DOI] [PubMed] [Google Scholar]
- Kormos C, Koteles GJ. Micronuclei in X-irradiated human lymphocytes. Mutat Res. 1988;199:31–35. doi: 10.1016/0027-5107(88)90227-8. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Adaptive response and human benefit: Part I. A microdosimetry dose-dependent model. Int J Radiat Biol. 2007;83:115–131. doi: 10.1080/09553000601123047. [DOI] [PubMed] [Google Scholar]
- Littlefield LG, Sayer AM, Frome EL. Comparisons of dose-response parameters for radiation-induced acentric fragments and micronuclei observed in cytokinesis-arrested lymphocytes. Mutagenesis. 1989;4:265–270. doi: 10.1093/mutage/4.4.265. [DOI] [PubMed] [Google Scholar]
- Liu SZ. Cancer control related to stimulation of immunity by low-dose radiation. Dose-Response. 2007;5:39–47. doi: 10.2203/dose-response.06-108.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DC, Edwards AA. Chromosome aberrations in human lymphocytes: Effect of radiation quality, dose and dose rate. New York: Alan R. Liss; 1983. [Google Scholar]
- Luckey TD. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982;43:771–789. doi: 10.1097/00004032-198212000-00001. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Radiation Hormesis. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Miller RC, Marino SA, Martin SG, Komatsu K, Geard CR, Brenner DJ, Hall EJ. Neutron-energy-dependent cell survival and oncogenic transformation. J Radiat Res (Tokyo) 1999;40(Suppl):53–59. doi: 10.1269/jrr.40.s53. [DOI] [PubMed] [Google Scholar]
- Mitchel RE, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- Mitchell JC, Norman A. The induction of micronuclei in human lymphocytes by low doses of radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;52:527–535. doi: 10.1080/09553008714552031. [DOI] [PubMed] [Google Scholar]
- NAS/NRC. National Academy Press; Washington, DC: 1988. National Academy of Sciences/National Research Council. The Effects on Populations of Exposures to Low Levels of Ionizing Radiation, BEIR III, Committee on the Biological Effects of Ionizing Radiations. [Google Scholar]
- NCRP. The relative biological effectiveness of radiations of different quality. Bethesda, MD: National Council on Radiation Protection and Measurements; 1990. [Google Scholar]
- Pampfer S, Muller WU, Streffer C. Preimplantation growth delay and micronucleus formation after in vivo exposure of mouse zygotes to fast neutrons. Radiat Res. 1992;129:88–95. [PubMed] [Google Scholar]
- Pohl-Ruling J, Fischer P, Haas O, Obe G, Natarajan AT, van Buul PP, Buckton KE, Bianchi NO, Larramendy M, Kucerova M, Polikova Z, Leonard A, Fabry L, Palitti F, Sharma T, Binder W, Mukherjee RN, Mukherjee U. Effect of low-dose acute X-irradiation on the frequencies of chromosomal aberrations in human peripheral lymphocytes in vitro. Mutat Res. 1983;110:71–82. doi: 10.1016/0027-5107(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Portess DI, Bauer G, Hill MA, O’Neill P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67:1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- Prosser JS, Moquet JE, Lloyd DC, Edwards AA. Radiation induction of micronuclei in human lymphocytes. Mutat Res. 1988;199:37–45. doi: 10.1016/0027-5107(88)90228-x. [DOI] [PubMed] [Google Scholar]
- Ramalho A, Sunjevaric I, Natarajan AT. Use of the frequencies of micronuclei as quantitative indicators of X-ray-induced chromosomal aberrations in human peripheral blood lymphocytes: comparison of two methods. Mutation Research. 1988;207:141–146. doi: 10.1016/0165-7992(88)90078-4. [DOI] [PubMed] [Google Scholar]
- Rithidech K, Tice RR, Bond VP. Induction of micronuclei in human lymphocytes after in vitro exposure to low doses of neutrons. Env Mol Mutagen. 1990;15:50. [Google Scholar]
- Rithidech KN, Tungjai M, Whorton EB. Protective effect of apigenin on radiation-induced chromosomal damage in human lymphocytes. Mutat Res. 2005;585:96–104. doi: 10.1016/j.mrgentox.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. Fifth Edition. Australia, Canada, Mexico, Singapore, Spain, United Kingdom, United States: Duxbury Press; 2000. [Google Scholar]
- Sanders CL. Inhibition of 239Pu alpha radiation-induced pulmonary carcinogenesis by low dose 169Yb gamma radiation. J of the Nuclear Society of Thailand. 2008 In Press. [Google Scholar]
- Sanders CL, Scott BR. Smoking and hormesis as confounding factors in radiation pulmonary carcinogenesis. Dose-Response. 2008;6:53–79. doi: 10.2203/dose-response.06-003.Sanders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. A biological-based model that links genomic instability, bystander effects, and adaptive response. Mutat Res. 2004;568:129–143. doi: 10.1016/j.mrfmmm.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Scott BR. Stochastic thresholds: A novel explanation of nonlinear dose-response relationships. Dose-Response. 2005;3:547–567. doi: 10.2203/dose-response.003.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. Low-dose-radiation stimulated natural chemical and biological protection against lung cancer. Dose-Response. 2008a doi: 10.2203/dose-response.07-025.Scott. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RB. It’s time for new low-dose-radiation risk assessment paradigm — one that acknowledges hormesis. Dose-Response. Dose-Response. 2008b doi: 10.2203/dose-response.07-005.Scott. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Di Palma J. Sparsely ionizing diagnostic and natural background radiation are likely preventing cancer and other genomic-instability associated diseases. Dose Response. 2006;5:230–255. doi: 10.2203/dose-response.06-002.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Sanders CL, Mitchel REJ, Boreham DR. CT scans may reduce rather than increase the risk of cancer. J Am Physicians Surg. 2008;13(1):8–11. [Google Scholar]
- Shadley JD, Wolff S. Very low doses of X-rays can cause human lymphocytes to become less susceptible to ionizing radiation. Mutagenesis. 1987;2:95–96. doi: 10.1093/mutage/2.2.95. [DOI] [PubMed] [Google Scholar]
- Sondhaus CA, Bond VP, Feinendegen LE. The use of cell-oriented factors and the hit size effectiveness function in radiation protection. Health Physics. 1996;70:868–876. doi: 10.1097/00004032-199606000-00013. [DOI] [PubMed] [Google Scholar]
- Tates AD, van Welie MT, Ploem JS. The present state of the automated micronucleus test for lymphocytes. Int J Radiat Biol. 1990;58:813–825. doi: 10.1080/09553009014552191. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Masse R. The debate on the use of linear no threshold for assessing the effects of low doses. J Radiol Prot. 2006;26:317–324. doi: 10.1088/0952-4746/26/3/N01. [DOI] [PubMed] [Google Scholar]
- Vral A, Verhaegen F, Thierens H, De Ridder L. Micronuclei induced by fast neutrons versus 60Co gamma-rays in human peripheral blood lymphocytes. Int J Radiat Biol. 1994;65:321–328. doi: 10.1080/09553009414550381. [DOI] [PubMed] [Google Scholar]
- Vral A, Louagie H, Thierens H, Philippe J, Cornelissen M, de Ridder L. Micronucleus frequencies in cytokinesis-blocked human B lymphocytes after low dose gamma-irradiation. Int J Radiat Biol. 1998;73:549–555. doi: 10.1080/095530098142103. [DOI] [PubMed] [Google Scholar]
- Wilson JW, Townsend LW. Radiation safety in commercial air traffic: a need for further study. Health Physics. 1988;55:1001–1003. [PubMed] [Google Scholar]