Abstract
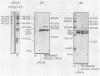
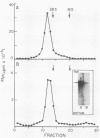
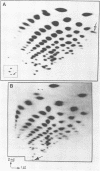
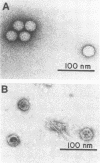
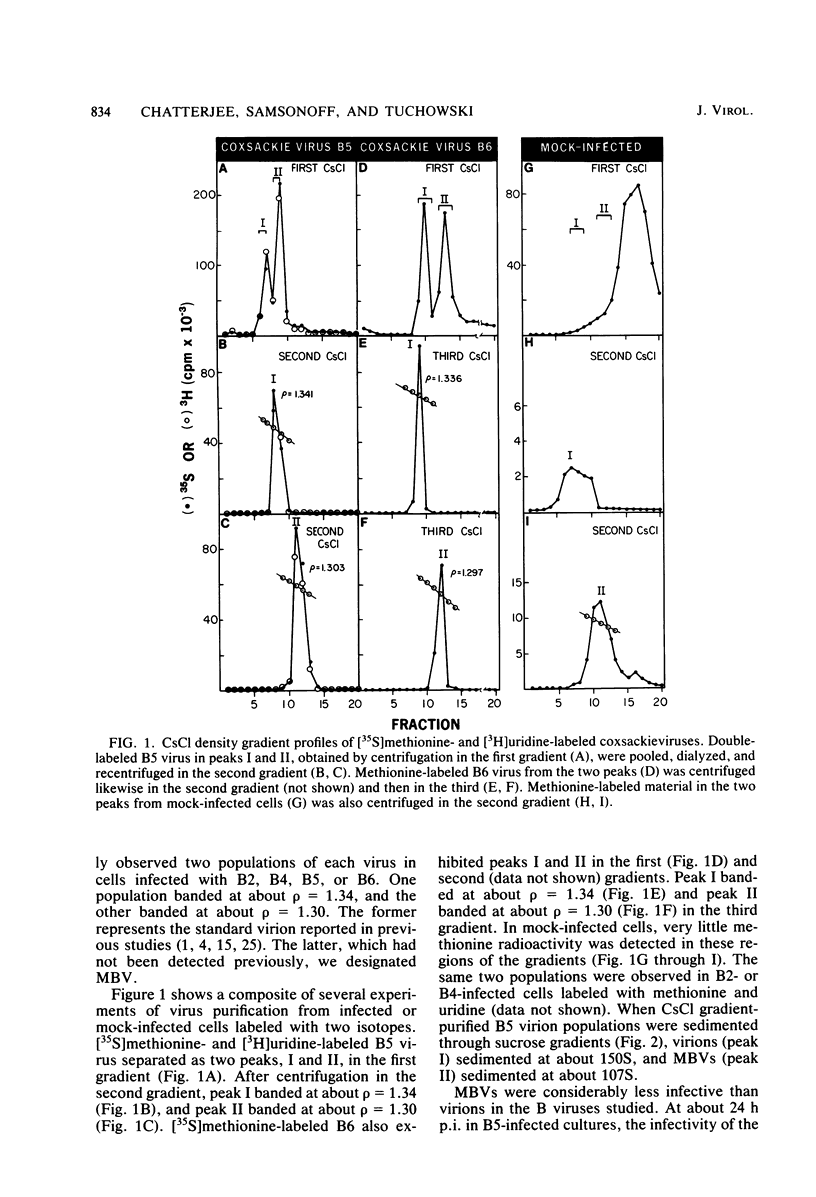
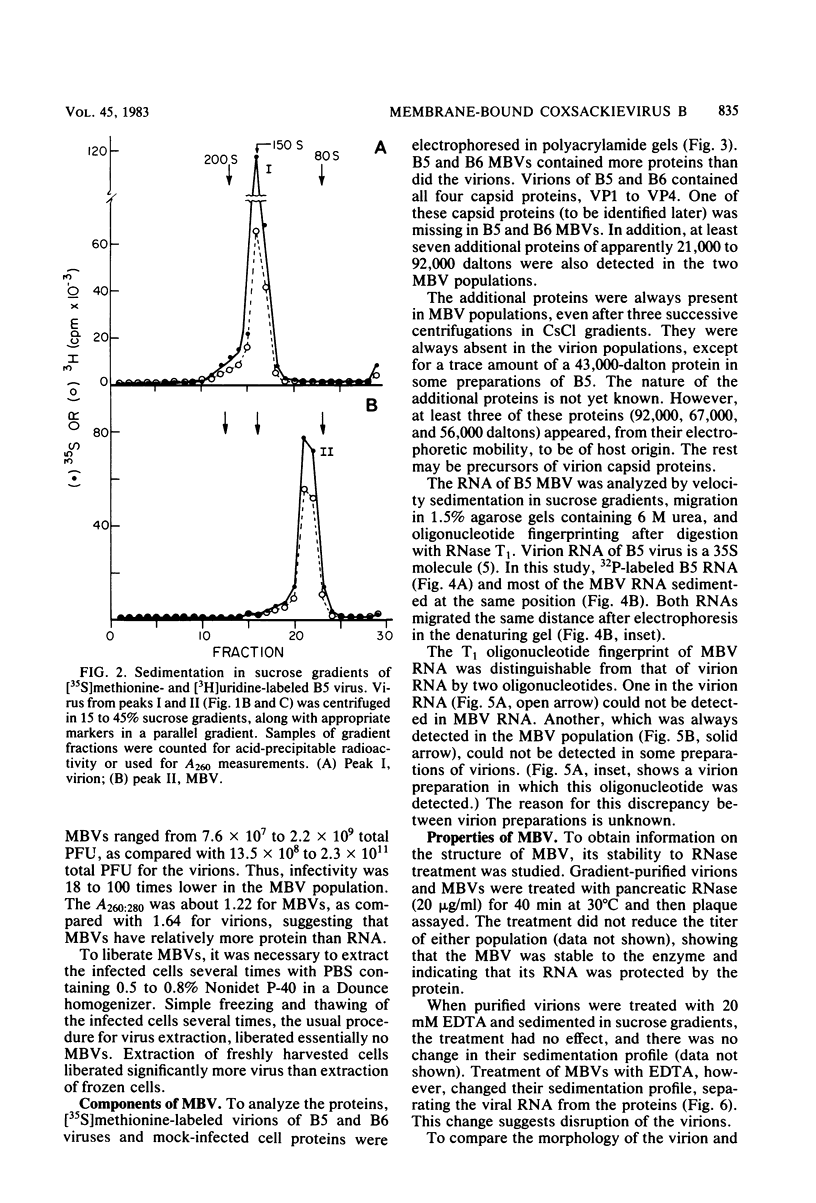
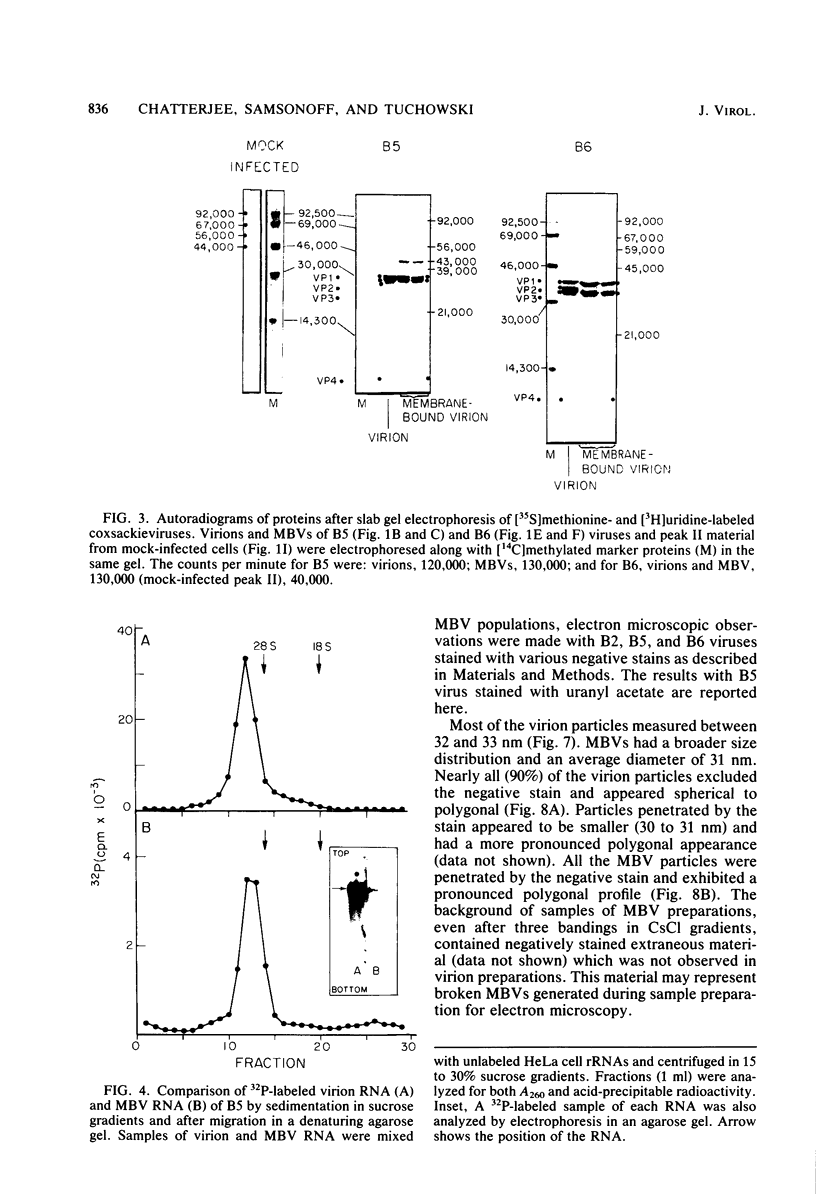
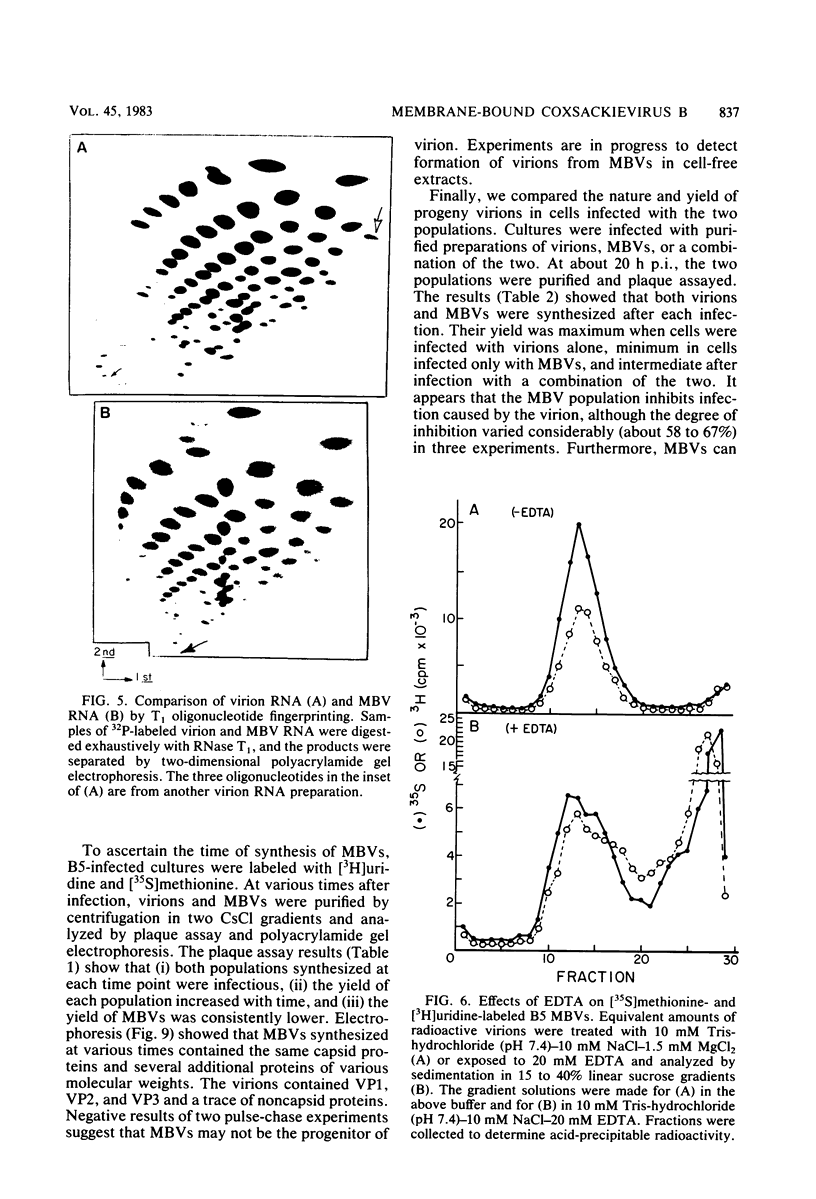
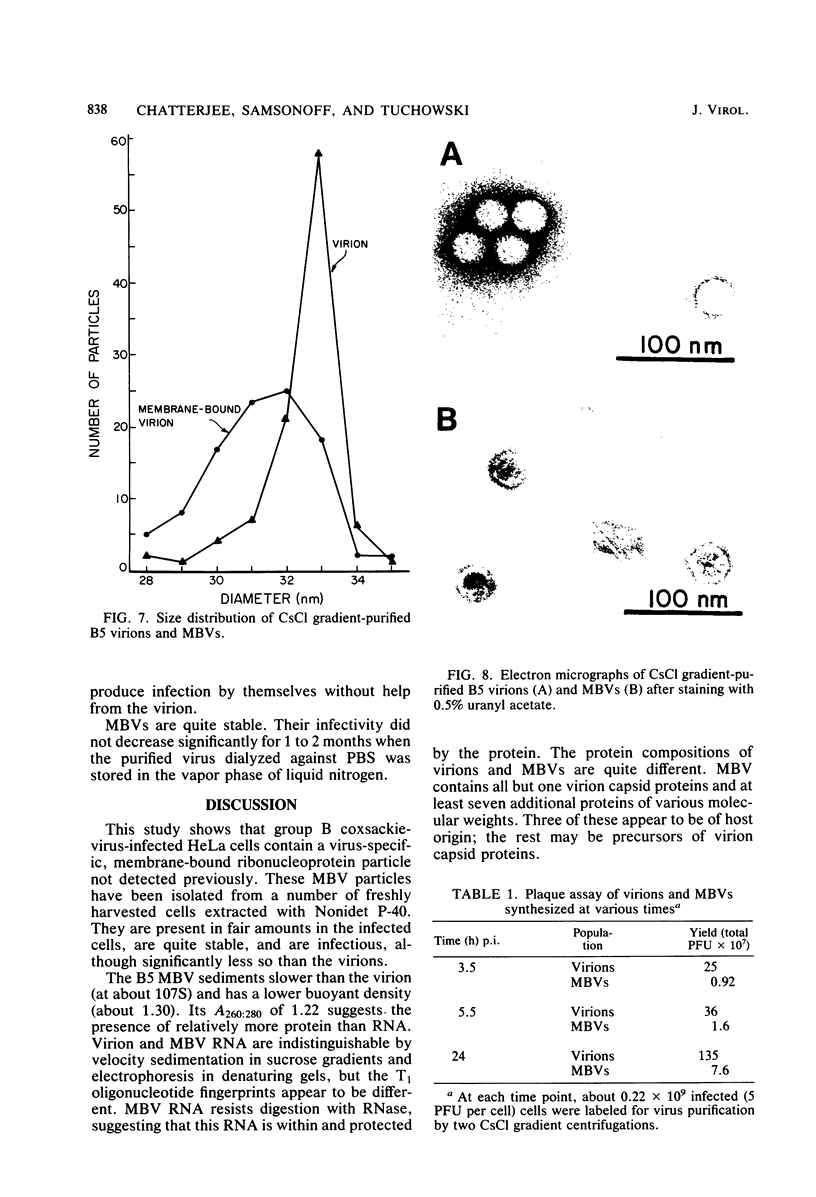
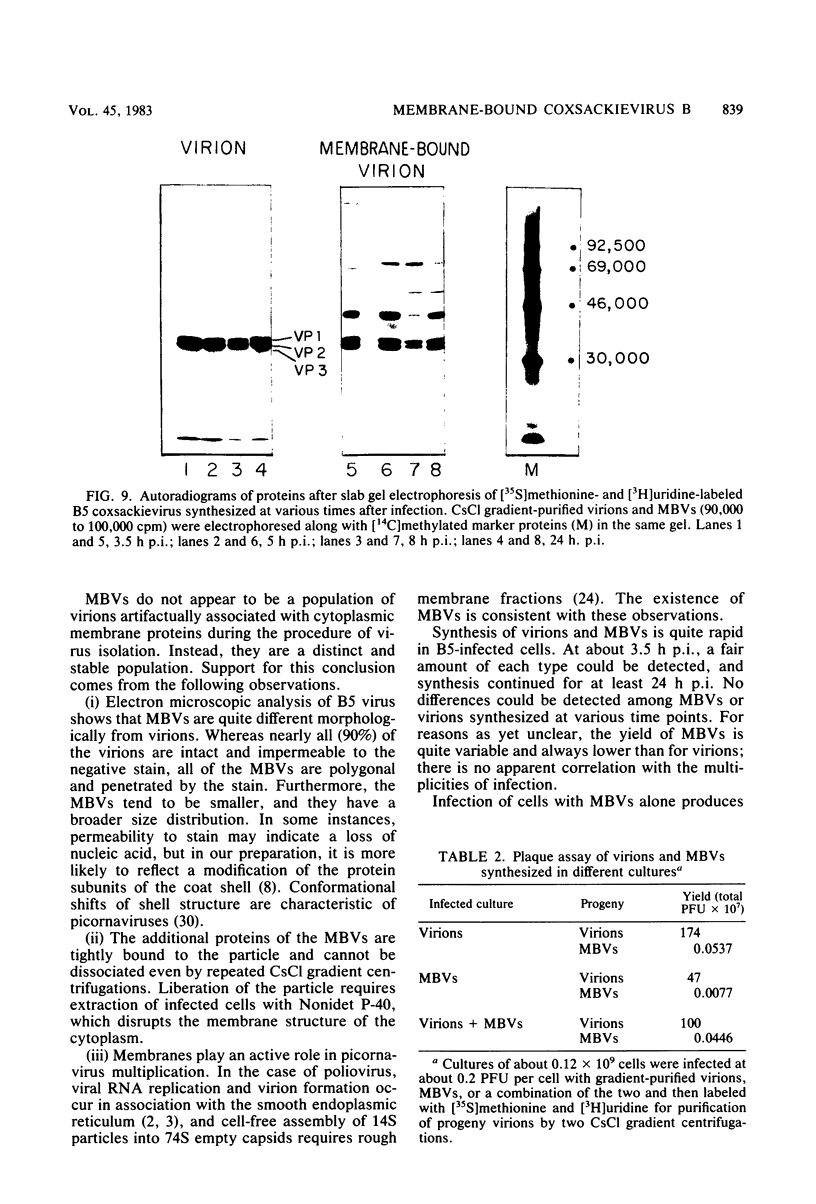
HeLa cells infected with several group B coxsackieviruses contain a previously undetected, virus-specific ribonucleoprotein particle which we designated membrane-bound virion (MBV). MBVs of B5 virus have a pronounced polygonal appearance and are slightly smaller than virions. The particles sediment more slowly (at about 107S) and have a lower buoyant density (about 1.30). They contain 35S virion RNA; only three, and not four, capsid proteins; and at least seven additional proteins with apparent molecular weights of 21,000 to 92,000. Three of the latter proteins appear to be of host origin; the rest may be precursors of virion capsid proteins. The RNA is resistant to digestion by RNase, and EDTA treatment disrupts the particle. MBVs are infectious, although significantly less so than virions. Cells infected with MBVs produce both types of progeny, virions and MBVs. In coinfected cultures, the yield of progeny is lower than in cells infected with virions alone, suggesting interference by MBVs. Synthesis of both types can be detected within 3.5 h after infection, and synthesis continues for 24 h.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown F., Wild T. F., Rowe L. W., Underwood B. O., Harris T. J. Comparison of swine vesicular disease virus and Coxsackie B5 virus by serological and RNA hybridization methods. J Gen Virol. 1976 May;31(2):231–237. doi: 10.1099/0022-1317-31-2-231. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Compans R. W. The formation of poliovirus particles in association with the RNA replication complexes. J Gen Virol. 1973 Oct;21:99–108. doi: 10.1099/0022-1317-21-1-99. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Chatterjee N. K., Tuchowski C. Comparison of capsid polypeptides of group B coxsackie-viruses and polypeptide synthesis in infected cells. Arch Virol. 1981;70(3):255–269. doi: 10.1007/BF01315132. [DOI] [PubMed] [Google Scholar]
- Chatterjee N. K., Tuchowski C. Translation of coxsackievirus B RNAs in a rabbit reticulocyte lysate: characterization of the genome RNA, reaction conditions for translation, and analysis of the products. Arch Virol. 1981;70(3):271–283. doi: 10.1007/BF01315133. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J., Harris W. J. Heterogeneity of apparently empty poliovirus particles stained with phosphotungstic acid after heating of the virus at "low" temperature. Virology. 1967 Apr;31(4):715–719. doi: 10.1016/0042-6822(67)90200-0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Baltimore D. Morphogenesis of poliovirus. II. Demonstration of a new intermediate, the proviron. J Virol. 1973 Nov;12(5):1122–1130. doi: 10.1128/jvi.12.5.1122-1130.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Guttman N., Baltimore D. Morphogenesis of poliovirus 3. Formation of provirion in cell-free extracts. J Virol. 1973 Nov;12(5):1181–1183. doi: 10.1128/jvi.12.5.1181-1183.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Griffith M. M., Sauck J. R., Upson R. H., Carlson E. C. Properties and origins of infectious rhinovirus type 14 particles of different buoyant densities. J Virol. 1975 Nov;16(5):1265–1272. doi: 10.1128/jvi.16.5.1265-1272.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman N., Baltimore D. Morphogenesis of poliovirus. IV. existence of particles sedimenting at 150S and having the properties of provirion. J Virol. 1977 Aug;23(2):363–367. doi: 10.1128/jvi.23.2.363-367.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPEREN S., EGGERS H. J., TAMM I. EVIDENCE FOR UNCOUPLED SYNTHESIS OF VIRAL RNA AND VIRAL CAPSIDS. Virology. 1964 Sep;24:36–46. doi: 10.1016/0042-6822(64)90145-x. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Yin F. H., Noble-Harvey J. Fractionation of biologically active and inactive populations of human rhinovirus type 2. Virology. 1975 Feb;63(2):384–394. doi: 10.1016/0042-6822(75)90311-6. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Wimmer E. "Fingerprinting" high molecular weight RNA by two-dimensional gel electrophoresis: application to poliovirus RNA. Nucleic Acids Res. 1976 Jul;3(7):1647–1658. doi: 10.1093/nar/3.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Holland J. J., Perrault J. Generation of defective interfering particles in picornaviruses. Virology. 1980 Jan 30;100(2):408–418. doi: 10.1016/0042-6822(80)90532-2. [DOI] [PubMed] [Google Scholar]
- Perlin M., Phillips B. A. In vitro assembly of polioviruses. 3. Assembly of 14 S particles into empty capsids by poliovirus-infected HeLa cell membranes. Virology. 1973 May;53(1):107–114. doi: 10.1016/0042-6822(73)90469-8. [DOI] [PubMed] [Google Scholar]
- Philipson L., Beatrice S. T., Crowell R. L. A structural model for picornaviruses as suggested from an analysis of urea-degraded virions and procapsids of coxsackievirus B3. Virology. 1973 Jul;54(1):69–79. doi: 10.1016/0042-6822(73)90115-3. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., MAYER M. M., RAPP H. J. Immunochemical studies of poliovirus. III. Further studies on the immunologic and physical properties of poliovirus particles produced in tissue culture. J Immunol. 1958 Nov;81(5):419–425. [PubMed] [Google Scholar]
- Rowlands D. J., Sangar D. V., Brown F. A comparative chemical and serological study of the full and empty particles of foot-and mouth disease virus. J Gen Virol. 1975 Mar;26(3):227–238. doi: 10.1099/0022-1317-26-3-227. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]