Abstract
Study Objectives:
Brief arousals have been systematically scored during sleep for more than 20 years. Despite significant knowledge concerning the importance of arousals for the sleep process in normal subjects and patients, comprehensive age norms have not been published.
Methods:
Seventy-six normal subjects (40 men) without sleep apnea or periodic limb movements of sleep, aged 18 to 70 years, slept in the sleep laboratory for 1 or more nights. Sleep and arousal data were scored by the same scorer for the first night (comparable to clinical polysomnograms) and summarized by age decade.
Results:
There were no statistically significant differences for sex or interaction of sex by age (p > .5 for both). The mean arousal index increased as a function of age. Newman-Keuls comparisons (.05) showed arousal index in the 18- to 20-year and 21- to 30-year age groups to be significantly less than the arousal index in the other 4 age groups. Arousal index in the 31-to 40-year and 41-to 50-year groups was significantly less than the arousal index in the older groups. The arousal index was significantly negatively correlated with total sleep time and all sleep stages (positive correlation with stage 1 and wake).
Conclusions:
Brief arousals are an integral component of the sleep process. They increase with other electroencephalographic markers as a function of age. They are highly correlated with traditional sleep-stage amounts and are related to major demographic variables. Age-related norms may make identification of pathologic arousal easier.
Citations:
Bonnet M; Arand D. EEG Arousal Norms by Age. J Clin Sleep Med 2007;3(3):271–274
Keywords: EEG arousal, arousal, ontogeny, sleep stages
Despite the fact that brief arousals have been scored as a part of evaluation of both research and clinical sleep studies for more than 20 years, comprehensive norms for arousals as a function of age or pathology have not been published. Brief arousal counts are currently published in most sleep research studies and tabulated in most clinical patient reports. The American Sleep Disorders Association published guidelines for the scoring of arousals in sleep tests in 1992.1 A few studies have reported arousal index (AI) based upon the American Sleep Disorders Association criteria in various age groups. Wong et al2 reported an AI of 7.6 (SEM 1.11) in normal 6-year-old children. Mathur and Douglas3 reported a significant (r = .6) correlation between age and AI in a population of 55 normal subjects with an age range of 20 to 70 years. Gosselin et al4 reported AI in groups of young (20–35 years) and older (50–65 years) normal sleepers. They did not find a significant difference as a function of age. The AI was 10.3 (SEM 1.0) in the younger group and 11.7 (SEM 1.12) in the older group. Finally, Boselli et al5 reported arousal data from a group of 40 subjects with an age range of 10 to 80 years and showed a high correlation between AI and age (r = .85). However, parametric data were only presented from broad age bands. The American Academy of Sleep Medicine has recently produced an evidence-based review of arousals and arousal scoring that summarizes scoring reliability data and provides empiric support for the importance of scoring arousals, as defined in 19926 without normative data. Although 2 of 3 studies have shown age effects, useful decade-specific norms are currently not available for arousals. This paper provides those norms by decade.
Data from a number of previous studies of normal sleepers from a broad age range7–14 were rescored by a single trained scorer with careful attention to the American Sleep Disorders Association guidelines and reliability to produce sufficient data for each decade of adult life through the sixth decade.
METHOD
Subjects
Subjects were selected from previous studies of normal sleepers with the goal of identifying at least 12 subjects in each decade of life from teenaged through 60-year-old subjects. Recruitment and general requirements for participation in research by normal individuals have been consistent over the time period examined. All subjects had originally been selected from ads in the local papers (including local university papers) for participants in sleep research. Individuals considered further had completed a screening questionnaire that indicated that they did not consume more than 250 mg of caffeine daily. Selected subjects denied problems with their sleep. Specifically, they reported that their sleep latency was less than 30 minutes and that they were not bothered by frequent awakenings or early morning awakening. They reported that they usually did not take naps on weekdays. They reported that their usual time in bed on weekdays was between 7 and 9 hours. Potential subjects who had histories strongly suggestive of circadian desynchrony (eg, shift workers), sleep apnea, or periodic leg movements were excluded. Subjects with a history of psychiatric care or use of psychoactive medication were excluded.
All subjects were assigned their own bedroom with a desk and chair. Subjects participated in the study in groups of 1 to 2 individuals. The bedroom windows were blocked to eliminate daylight, and subjects performed all study procedures alone in their assigned room. Time cues were not available in the subject rooms, but no attempt was made to limit access to time cues in the other areas of the lab.
Sleep recordings (LE-A2, RE-A2, C3-A2, OZ-A1, V5-right clavicle, airflow, chest movements and leg electromyogram) were made during night. Subjects found to have sleep apnea or periodic leg movements were excluded from the study. All sleep recordings were scored in 30-second epochs using Rechtschaffen and Kales15 criteria. The electroencephalographic (EEG) montage was chosen to maximize identification of alpha, as recommended by the American Sleep Disorders Association.1
Design
The initial night in the sleep laboratory was selected for analysis. This night was chosen both because it was most available and because it corresponds to the night that is most commonly analyzed in patient evaluations. As a result, data should be comparable with those from most clinical polysomnograms. However, it has also been shown that a significant first-night effect does not exist for arousals16 so that the data should also be comparable with data from other laboratory nights. Subjects arrived at the laboratory 1 to 2 hours prior to their normal bed time to allow for placement of recording devices. Subjects had an undisturbed night of sleep and were allowed to leave in the morning after recording apparatus had been removed.
Scoring
A single trained scorer achieved 90% sleep-stage and arousal scoring reliability with laboratory gold-standard recordings. The scorer then rescored all of the records for sleep stages, EEG arousals, respiration, and limb movements to ensure consistent and contemporaneous scoring using Rechtschaffen and Kales15 criteria and American Sleep Disorders Association arousal criteria.1 A laboratory gold-standard recording was scored after each 10 records to demonstrate continuing reliability against lab standards. Recordings from the data set were also rescored periodically. The median arousal scoring reliability from 7 observations was 89%.
Results
Demographic data for the 76 subjects by age group are presented in Table 1. Sleep-stage and arousal data are presented by age group in Table 2. It can be seen that there was a significant increase in both total arousals and AI as a function of age. For AI, the index was similar in 18- and 20-year age groups and significantly lower than in the 30- and 40-year age groups, which were in turn significantly lower than in 50- and 60-year age groups. There were no statistically significant differences for sex or interaction of sex by age (p > .5 for both).
Table 1.
Demographic Data
| Age Group, y | Subjects, no. | Men, no. | Weight, lb | BMI, kg/m2 |
|---|---|---|---|---|
| 18–20 | 12 | 3 | 153 ± 6.3 | 23.2 ± 0.58 |
| 21–30 | 13 | 7 | 157 ± 6.1 | 23.5 ± 0.80 |
| 31–40 | 13 | 7 | 163 ± 6.1 | 25.4 ± 0.81 |
| 41–50 | 10 | 6 | 164 ± 7.3 | 25.4 ± 0.86 |
| 51–60 | 14 | 12 | 184 ± 6.1 | 27.6 ± 1.35 |
| 61–70 | 14 | 12 | 187 ± 5.9 | 27.8 ± 0.61 |
Weight and body mass index (BMI) are expressed as mean ± SEM.
Table 2.
Mean Total ASDA Arousals, Arousal Index, and Arousals Corrected for Arousals Associated With Limb Movements and Apnea
| Parameter | Age Group, y | F value | Difference | |||||
|---|---|---|---|---|---|---|---|---|
| 18–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | |||
| EEG Arousals | 80 ± 27 | 83 ± 33 | 116 ± 44 | 109 ± 29 | 128 ± 40 | 130 ± 42 | 4.43* | e |
| AI | 10.6 ± 4.0 | 10.8 ± 4.6 | 16.8 ± 6.2 | 16.5 ± 5.6 | 21.9 ± 8.9 | 21.9 ± 6.8 | 8.15* | a |
| TST, min | 439 | 446 | 403 | 395 | 358 | 350 | 13.4* | a |
| Sleep stage, % of TST | ||||||||
| 1 | 7 | 9 | 12 | 14 | 16 | 16 | 3.98* | b |
| 2 | 52 | 54 | 52 | 50 | 45 | 46 | 2.35* | g |
| SWS | 19 | 13 | 7 | 7 | 3 | 3 | 11.6* | c |
| REM | 18 | 19 | 18 | 14 | 17 | 15 | 1.76 | |
| Awakenings, no. | 21.5 | 22.9 | 29.8 | 34.7 | 43.3 | 42 | 6.88* | f |
| TIB, min | 480 | 480 | 480 | 480 | 465 | 456 | 2.48* | None differ |
| SE, % | 95.1 | 94.6 | 88.0 | 85.3 | 79.2 | 80.7 | 16.4* | a |
| REM latency | 108 | 125 | 114 | 103 | 84.5 | 114 | 1.13 | |
| Leg Index | 4 | 3 | 7 | 7 | 4 | 2 | 0.68 | |
| Leg AI | 0.8 | 0.8 | 1.4 | 1.8 | 0.4 | 1.6 | 1.69 | |
| Apnea Index | 0 | 0 | 0 | 0.7 | 1.3 | 1.8 | 0.76 | |
| Apnea AI | 0 | 0 | 0 | 0.3 | 0.5 | 0.2 | NS | |
| Final AI | 9.8 | 10.1 | 16.0 | 14.9 | 16.2 | 21.2 | 6.06* | d |
ASDA refers to American Sleep Disorders Association; EEG, electroencephalogram; AI, arousal index; TST, total sleep time; SWS, slow-wave sleep; TIB, time in bed; SE, sleep efficiency; REM, rapid eye movement sleep;
a: 18 = 20 < 30 = 40 < 50 = 60;
b: 18 < 40 = 50 = 60; 20 < 60;
c: 18 > 20 > 30 = 40 = 50 = 60
d: 18 = 20 < 30 = 40 = 50 < 60;
e: 18 = 20 < 50 = 60;
f: 18 = 20 = 30 < 50 = 60
g: 20 > 50
Expected differences were found in sleep-stage amounts as a function of age. There were significant decreases in total sleep time, slow-wave sleep, and sleep efficiency, with significant increases in stage 1 sleep and awakenings.
The subject population was controlled to eliminate patients with sleep apnea or periodic limb movements. However, apneas and limb movements were found in all age groups. These are also summarized in Table 2, along with arousals associated with these events so that the level of arousals associated with these events could be calculated as a function of age. Significant age-related effects were not found for any apnea or limb-movement variable. However, a final AI, with arousals associated with apnea and limb movements subtracted from the AI was also calculated. These “corrected” data are still consistent with a general increase in arousals as a function of age.
Because similar changes in arousals and sleep variables were found as a function of age, AI was correlated with a set of sleep and demographic variables. These data are presented in Table 3. Significant correlations are marked in the table. A significance level of p < .01 was chosen to control for the possibility of chance findings related to the large number of correlations. It can be seen that AI was significantly correlated with all of the sleep variables presented except for rapid eye movement (REM) latency and time in bed. Increased arousals correlated positively with stage 1, wake time, awakenings, and stage changes, as expected from known relationships in disturbed sleep.17 Significant negative correlations were found for total sleep time, stage 2, slow-wave sleep, REM, and sleep efficiency. AI was also significantly and positively correlated with both age and body mass index. The significant correlation between AI and total sleep time remained significant even when age was partialled out (AI × TST.age r = −.47), but the correlation of AI with age became nonsignificant when the effect of total sleep time was eliminated (AI × AGE.tst r = .21). The significant correlation between AI and body mass index disappeared when age was partialled out (AI × BMI.age r = .15).
Table 3.
Arousal Correlations with Sleep and Demographic Variables
| Arousal Index | Total sleep time | Stage 1a | SWSa | REMa | |
|---|---|---|---|---|---|
| Total sleep time | −.67* | ||||
| Stage 1a | .50* | −.49* | |||
| Stage 2a | −.41* | .57* | −.54* | ||
| SWSa | −.45* | .49* | −.55* | ||
| REMa | −.43* | .39* | −.32* | .02 | |
| Wake | .69* | −.82* | .44* | −.53* | −.38* |
| Stage changes | .39* | −.03 | −.11 | .37* | −.31* |
| Awakenings | .64* | −.49* | .37* | −.46* | −.30* |
| Time in bed | −.24 | .59* | −.22 | .19 | .10 |
| Sleep efficiency | −.71* | .89* | −.47* | .54* | .40* |
| REM latency | .19 | .07 | .10 | .17 | −.44* |
| Sexb | −.28 | .41* | −.41* | .25 | .07 |
| BMI | .42* | −.40* | .22 | −.36* | −.30* |
| Age | .58* | −.70* | .46* | −.62* | −.20 |
Sleep stage as a percentage of the total sleep time. SWS refers to slow-wave sleep; REM, rapid eye movement sleep; BMI, body mass index.
Men coded as 1 and women as 2.
p < .01
DISCUSSION
Brief arousals have been noted as a normal component of sleep for many years.18 As sleep disorders medicine has evolved, the role of arousals in sleep disorders and daytime sleepiness has become apparent.19 However, determination of a pathologic number of arousals in a patient under baseline or treatment conditions has been difficult without knowing the expected numbers of EEG arousals as a function of age.
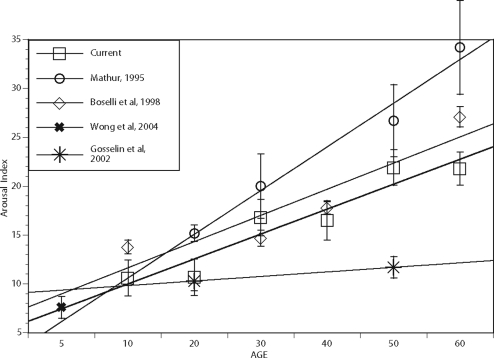
Data from the current study and the other studies reviewed in the introduction are all plotted with standard errors and straight-line regression line in Figure 1. As can be seen from Figure 1, the straight-line fit from the current data is almost a perfect parallel with the Boselli et al5 data shifted lower by 1 arousal per hour. The best-fit line from the current data also intersects with the mean from the Wong et al2 data for 5-year-old children, implying a useful extrapolation to lower age ranges. In agreement with both the Boselli et al5 and Mathur and Douglas3 studies, a significant correlation between age and AI was found in the current data. The correlation was somewhat lower than that reported in the earlier studies, but this could be related to the significantly larger sample size in the current study. The current data do not support the extremely large increase in AI found by Mathur and Douglas3 in older subjects. It is not clear if the Mathur and Douglas3 results represent the relatively small sample size of older subjects, underlying sleep disorders that may not have been well screened, or perhaps the difficulty in scoring arousals in older subjects who have increased high-frequency EEG and increased incidence of alpha intrusion.
Figure 1.
Arousal index and linear regression line from the current data (AI) and other referenced arousal studies. Standard error for each mean is marked.
The strong correlation of AI with other sleep variables validates brief arousals as a normal component of sleep. Increasing arousals with age were very strongly related to increases in wake time and number of awakenings, long recognized as core components of aging sleep.20 The correlation between AI and total sleep time remained statistically significant when the effect of aging was partialled out, but the correlation between AI and age became nonsignificant when total sleep time was controlled. This suggests that the relationship between these EEG parameters also extends beyond a simple age relationship. Similarly, the significant correlation between AI and body mass index was lost when age was controlled.
Subject groups in this study were relatively small (10–14 subjects). Small groups limit the likelihood of finding age-related differences, but the group size in this study is similar to the group size in the major reference for EEG sleep norms by age.20 However, the small number of women in the older groups coupled with a majority of women in the youngest group (that nonetheless did not differ from the 21- to 30-year-old sample) could explain the inability to find sex-related changes in arousal. Additional research will be required to more clearly delineate sex differences in EEG arousals.
Footnotes
Disclosure Statement
This is not an industry supported study. Drs. Bonnet and Arand have indicated no financial conflicts of interest.
REFERENCES
- 1.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 2.Wong TK, Galster P, Lau TS, et al. Reliability of scoring arousals in normal children and children with obstructive sleep apnea syndrome. Sleep. 2004;27:1139–45. doi: 10.1093/sleep/27.6.1139. [DOI] [PubMed] [Google Scholar]
- 3.Mathur R, Douglas NJ. Frequency of EEG arousals from nocturnal sleep in normal subjects. Sleep. 1995;18:330–3. doi: 10.1093/sleep/18.5.330. [DOI] [PubMed] [Google Scholar]
- 4.Gosselin N, Michaud M, Carrier J, et al. Age difference in heart rate changes associated with micro-arousals in humans. Clin Neurophysiol. 2002;113:1517. doi: 10.1016/s1388-2457(02)00189-x. [DOI] [PubMed] [Google Scholar]
- 5.Boselli M, Parrino L, Smerieri A, et al. Effect of age on EEG arousals in normal sleep. Sleep. 1998;21:351–7. [PubMed] [Google Scholar]
- 6.Bonnet MH, Doghramji K, Roehrs T, et al. The Scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med, 2007;3:133–145. [PubMed] [Google Scholar]
- 7.Bonnet MH. Effect of 64 hours of sleep deprivation upon sleep in geriatric normals and insomniacs. Neurobiol Aging. 1986;7:89–96. doi: 10.1016/0197-4580(86)90145-4. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet MH, Arand DL. Metabolic rate and the restorative function of sleep. Physiol Behav. 1996;59:777–82. doi: 10.1016/0031-9384(95)02093-4. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal effects. Electroencephalogr Clin Neurophysiol. 1997;102:390–6. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet MH, Arand DL. Level of arousal and the ability to maintain wakefulness. J Sleep Res. 1999;8:247–54. doi: 10.1046/j.1365-2869.1999.00168.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet MH, Arand DL. Arousal components which differentiate the MWT from the MSLT. Sleep. 2001;24:441–50. doi: 10.1093/sleep/24.4.441. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet MH, Rosa RR. Sleep and performance in young adults and older insomniacs and normals during acute sleep loss and recovery. Biol Psychol. 1987;25:153–72. doi: 10.1016/0301-0511(87)90035-4. [DOI] [PubMed] [Google Scholar]
- 15.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems for sleep stages of human subjects. Washington, DC: Public Health Service, U.S. Government Printing Office; 1968. [Google Scholar]
- 16.Bonnet JP, Arand DL. Do arousals demonstrate a first night effect? Sleep. 2006;29:A340. [Google Scholar]
- 17.Yang Q, Phillips C, Melehan K, et al. Effects of short-term CPAP withdrawal on neurobehavioral performance in patients with obstructive sleep apnea. Sleep. 2006;29:545–52. doi: 10.1093/sleep/29.4.545. [DOI] [PubMed] [Google Scholar]
- 18.Halasz P, Kundra O, Rajna P, et al. Micro-arousals during nocturnal sleep. Acta Physiol Acad Sci Hung. 1979;54:1–12. [PubMed] [Google Scholar]
- 19.Carskadon MA, Dement WC. Nocturnal determinants of daytime sleepiness. Sleep. 1982;5(Suppl 2):S73–81. doi: 10.1093/sleep/5.s2.s73. [DOI] [PubMed] [Google Scholar]
- 20.Williams L, Karacan I, Hursch C. Electroencephalography of Human Sleep: Clinical Applications. New York: John Wiley & Sons; 1974. pp. 1–169. [Google Scholar]