Abstract
Background
Tuberculosis (TB) often coincides with nutritional deficiencies. The effects of micronutrient supplementation on TB treatment outcomes, clinical complications, and mortality are uncertain.
Methods
We conducted a randomized, double-blind, placebo-controlled trial of micronutrients (vitamins A, B complex, C, and E, as well as selenium) in Dar es Salaam, Tanzania. We enrolled 471 human immunodeficiency virus (HIV)–infected and 416 HIV-negative adults with pulmonary TB at the time of initiating chemotherapy and monitored them for a median of 43 months.
Results
Micronutrients decreased the risk of TB recurrence by 45% overall (95% confidence interval [CI], 7% to 67%; P = .02) and by 63% in HIV-infected patients (95% CI, 8% to 85%; P = .02). There were no significant effects on mortality overall; however, we noted a marginally significant 64% reduction of deaths in HIV-negative subjects (95% CI, −14% to 88%; P = .08). Supplementation increased CD3+ and CD4+ cell counts and decreased the incidence of extrapulmonary TB and genital ulcers in HIV-negative patients. Micronutrients reduced the incidence of peripheral neuropathy by 57% (95% CI, 41% to 69%; P < .001), irrespective of HIV status. There were no significant effects on weight gain, body composition, anemia, or HIV load.
Conclusions
Micronutrient supplementation could improve the outcome in patients undergoing TB chemotherapy in Tanzania.
Tuberculosis (TB) remains a major cause of mortality worldwide; in 2005 alone, up to 1.6 million people died of TB [1]. The majority of new cases occur in Asia and sub-Saharan Africa, where the HIV epidemic is in part responsible for the high rates of recurrence, morbidity, and mortality [2, 3] despite the availability of effective anti-TB chemotherapy.
Patients with TB very frequently suffer from deficiencies of nutrients—such as vitamins A, B complex, C, and E, as well as selenium [4]—that are fundamental to the integrity of the immune response [5, 6]. In Dar es Salaam, Tanzania, vitamin A deficiency was found in 27% of patients with TB, compared with 7% in patients without TB [7]. Marginal status of other micronutrients is also prevalent in this setting [8, 9]. It is uncertain whether correction of such deficiencies via micronutrient supplementation leads to improved outcomes in the course of TB. One trial in Indonesia suggested a potential beneficial effect of vitamin A and zinc on early sputum smear conversion [10], whereas a study in Tanzania indicated no effects of multiple micronutrients on culture conversion [11] but a positive effect on weight gain and survival [12]. By contrast, a trial in Malawi found no effect of multimicronutrients on mortality [13]. It is not known whether micronutrients would affect recurrences, immunological parameters, or clinical complications in patients receiving anti-TB treatment.
We hypothesized that micronutrient supplementation in patients with TB would decrease the risk of adverse TB treatment outcomes, mortality, and morbidity and improve nutritional and immunological parameters. We conducted a randomized, placebo-controlled clinical trial among adults with pulmonary TB in Dar es Salaam, Tanzania, to test this hypothesis. Both HIV-negative and HIV-infected patients were included.
METHODS
Between April 2000 and April 2005, we enrolled 887 adults with pulmonary TB to participate in a randomized clinical trial. The primary aims of the study were to examine the effect of micronutrient supplementation on (1) culture negativity at 1 month after initiation of treatment, (2) mortality during at least 24 months of follow-up, and (3) TB recurrences. Secondary outcomes were changes from baseline in (1) viral load among HIV-infected participants, (2) CD4+ cell counts, and (3) body weight.
We first identified all patients with clinically suspected TB who had positive sputum smears for acid-fast bacilli (AFB) at 5 outpatient TB clinics in Dar es Salaam, Tanzania. We evaluated the following inclusion criteria among 7213 patients identified: age between 18 and 65 years, Karnofsky performance score [14] ≥40%, plan to stay in Dar es Salaam for 2 years, not being pregnant, and not having received anti-TB treatment for more than 4 weeks during the previous year. For the 3623 subjects who fulfilled all criteria, we obtained 2 additional early morning sputum samples to confirm the smear results at the Muhimbili National Hospital Tuberculosis Reference Laboratory by use of the Ziehl-Neelsen smear-staining technique. We confirmed smear-positive TB in 3188 people and sought informed consent for HIV-1 testing among them on the day of initiation of anti-TB treatment. We provided pretest counseling to the 2463 patients who consented and obtained a blood sample to determine infection with HIV-1 and hemoglobin concentrations. Trained research assistants explained the general aims of the study to the patients and invited them to return to the clinic within 7 days of the initiation of the anti-TB treatment. HIV-1 infection was assessed using 2 sequential ELISAs (Wellcozyme, Murex Biotech; Enzygnost anti-HIV1+2, Behring); discrepant results were resolved by Western blot test (Genetic Systems). When patients returned for their HIV assay results, the research assistants provided post-test counseling and verified hemoglobin concentrations. They sought consent to participate in the trial among subjects whose hemoglobin concentration was >70 g/L. A sample size of 600 was initially calculated to detect a ≥35% treatment effect on culture negativity at 1 month with 80% statistical power. This sample was later expanded to 887 patients to maximize power for other end points. We deliberately included as many HIV-infected patients as possible; thus, the number of HIV-positive participants was larger (n = 471) than the number of HIV-negative patients (n = 416).
Consenting subjects were randomly assigned in computer-generated permuted blocks of 20, stratified by HIV status, to receive a daily oral dose of 1 of 2 regimens: micronutrients (5000 IU of retinol, 20 mg of vitamin B1, 20 mg of vitamin B2, 25 mg of vitamin B6, 100 mg of niacin, 50 μg of vitamin B12, 500 mg of vitamin C, 200 mg of vitamin E, 0.8 mg of folic acid, and 100 μg of selenium) or placebo. These doses represent between 6 and 10 times the recommended dietary allowance (RDA) and were being tested at the time among HIV-infected adults from this setting [15]. We chose multiples of the RDA because previous observational studies suggested that HIV-infected individuals need higher dietary intakes of micronutrients to achieve normal serum concentrations [16]. Active tablets and placebo were indistinguishable in size, taste, and color. All clinical and research staff were unaware of the subjects’ treatment assignment. All patients received standard anti-TB treatment following the DOTS (directly observed treatment, short course) scheme [17], per the Tanzania National TB and Leprosy Programme prevalent at the time. In brief, patients received a daily combination of rifampicin, isoniazid, pyrazinamide, and ethambutol under direct observation of a health worker during the first 2 months. The patients then self-administered isoniazid and ethambutol daily during the following 6 months. At the time of the study, antiretroviral medications were unavailable in Tanzania for the majority of HIV-infected persons, including those who participated in this trial.
At the randomization visit, research nurses collected information on age, level of education, marital status, and indicators of socioeconomic status. Follow-up was conducted monthly at the study clinics, where research nurses exchanged bottles containing treatment regimen and obtained anthropometric measurements by standardized procedures [18]. They also measured single-frequency bioelectrical impedance (BIA) for body composition analyses, by use of BIA-101Q analyzers (RJL Systems) with tetrapolar lead placement at 50 kHz and 800 μA. Fat mass and fat-free mass were calculated from the BIA measurements by use of Kotler’s equations [19]. We intended to obtain specimens at the first visit; at 1, 2, 5, 8, and 12 months from randomization; and approximately every 6 months thereafter until the end of follow-up. These specimens included sputum for AFB smears and cultures and a blood sample for measurement of hemoglobin and albumin concentrations and of T cell subtypes (CD4+, CD8+, and CD3+ cells) by use of the FACScount and FACSCAN systems (Becton Dickinson). Viral load was quantified in samples from HIV-infected patients by use of the Roche Amplicor assay (version 1.5). Results were available in 1321 samples from 439 (93%) of the total 471 HIV-infected participants. Each patient had a median of 3 viral load measurements (mean, 3.0; SD, 1.5; interquartile range [IQR], 2– 4). Physician visits were scheduled every 3 months. During these visits, study physicians inquired about the health of the subject during the preceding period and performed a complete physical examination. The stage of HIV disease was assessed according to the World Health Organization system [20].
When subjects missed a clinic visit or traveled out of the city, their homes were visited to ask neighbors or relatives about survival status. Because recruitment occurred over a 5-year period, the length of follow-up for survival until the end of the study in August 2005 varied for each patient. The median follow-up time was 43 months (IQR, 28–53 months)—52 months (IQR, 47–57 months) for HIV-negative patients and 30 months (IQR, 15– 41 months) for HIV-infected patients.
Compliance with the study regimen was evaluated at every monthly clinic visit by the research nurse, by counting the tablets remaining in previously dispensed bottles. We calculated the proportion of tablets absent from these bottles from the total number of tablets the subject should have taken. Compliance was high and was independent of treatment arm; 85% during the entire follow-up period (P = .92 for the difference between arms), 90% during the first 8 months (P = .09), and 87% during the first 2 years (P = .52). HIV-infected participants tended to have slightly higher compliance than did those who were HIV negative: 87% versus 83%, respectively, over the duration of follow-up.
We assessed baseline characteristics of the patients for clinically relevant differences by treatment arm. We examined the effects of micronutrient supplements on TB treatment outcome as well as nutritional, immunological, and clinical end points following the intent-to-treat principle. Treatment effects were assessed in the entire cohort and separately by HIV status at baseline. TB-related end points included treatment failure, early recurrence, and late recurrence. Treatment failure by 1 month was defined as positive AFB cultures at 1 month from the initiation of treatment. Early recurrences were deemed to have occurred in patients with positive cultures after 1 month, among those who had become culture negative by 1 month after treatment initiation. Late recurrences were defined as any positive cultures after 8 months from the initiation of treatment. Recurrences included both endogenous reactivation and exogenous reinfection, which could not be distinguished in this study. We calculated the relative risks (RRs) and 95% confidence intervals (CIs) for each of these outcomes by treatment arm.
We used Cox proportional hazards models to assess the effect of the supplements on mortality. Among HIV-infected participants, we investigated the effect of micronutrients on mortality, progression of HIV disease from stage 3 to 4, and mortality or disease progression, whichever occurred first. For these end points, we defined the end of follow-up as the date when HIV stage was last assessed. The proportional hazards assumption was tested by introducing cross-product terms between treatment and time into the Cox models.
We examined the effects of micronutrients on CD4+, CD8+, and CD3+ cell counts, viral load (in HIV-infected participants), indicators of nutritional status (weight, mid-upper arm circumference, body mass index [BMI], fat mass, fat-free mass, and albumin concentrations), and hemoglobin concentrations. We also evaluated the impact of supplementation on individual clinical signs reported during the month before the physician visits or clinical diagnoses made by the study physicians. These included fever, nausea/vomiting, diarrhea, fatigue, skin rashes, oral thrush, difficult or painful swallowing, extrapulmonary TB, peripheral neuropathy, and genital ulcers. We also registered hospital admissions and outpatient visits that occurred outside the study schedule.
Treatment effects on these end points were analyzed by use of generalized estimating equations (GEEs). These models do not require that all patients have the same number of follow-up assessments or that the follow-up measurements be obtained at exactly the same time points (see figure 1 for data on the numbers of subjects with known survival status, culture results, and T cell measurements at various time points). We assumed a standard normal distribution for repeated continuous end points (T cell subsets, log10 viral load, anthropometry, albumin concentration, and hemoglobin concentration) and estimated average differences by treatment arm during follow-up. For recurrent binary outcomes (morbidity), we assumed an underlying binomial distribution and estimated RRs and 95% CIs. We used an exchangeable correlation structure to account for within-subject correlations and adjusted the models for the follow-up time when the measurements had been obtained and for the baseline values. We analyzed the data for the entire period and for the first 8 months, coinciding with the expected end of TB treatment. No adjustments were made for multiple comparisons. All analyses were done by use of SAS software (version 9.1; SAS Institute).
Figure 1.
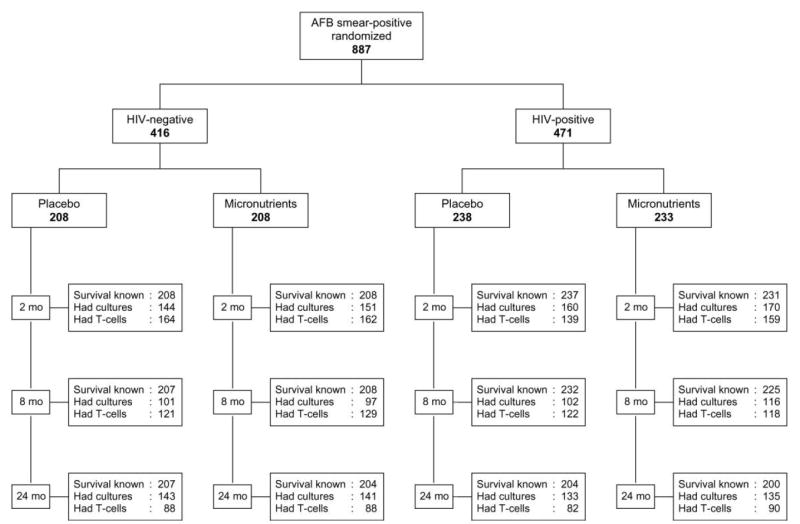
Trial profile. AFB, acid-fast bacilli.
A data and safety monitoring board reviewed the progress of the study and the results of the interim analyses. All subjects provided written informed consent to participate in the study. The institutional review boards of the Muhimbili University College of Health Sciences, the Tanzanian National AIDS Control Program, and the Harvard School of Public Health approved the study protocol. None of the sponsors of the study had any role in the study design, data collection, data analysis, data interpretation, or writing of the report.
RESULTS
Subject characteristics
The majority of participants were male (67%). The average age was 30 years for HIV-negative subjects and 34 for those who were HIV infected. There were no relevant differences in baseline characteristics by treatment arm (table 1).
Table 1.
Characteristics of the study population.
| HIV negative
|
HIV positive
|
|||
|---|---|---|---|---|
| Characteristic | Placebo (n = 208) | Micronutrients (n = 208) | Placebo (n = 238) | Micronutrients (n = 233) |
| Female, % (no.) | 21.6 (45) | 26.0 (54) | 40.8 (97) | 43.4 (101) |
|
| ||||
| Age, years | 30.6 ± 9.0 | 29.4 ± 9.0 | 34.4 ± 8.9 | 33.7 ± 7.9 |
|
| ||||
| Has primary education, % (no.) | 89.8 (187) | 91.4 (190) | 89.6 (213) | 87.1 (203) |
|
| ||||
| Body mass index, kg/m2 | 18.9 ± 2.5 | 18.9 ± 2.5 | 19.6 ± 2.9 | 19.3 ± 2.8 |
|
| ||||
| Hemoglobin concentration, g/L | 111 ± 17 | 110 ± 16 | 98 ± 18 | 98 ± 18 |
|
| ||||
| Culture negative, % (no.)a | 31.3 (47) | 37.4 (55) | 20.8 (20) | 33.3 (30) |
|
| ||||
| CD4+ cell count, cells/mm3 | 699 ± 254 | 709 ± 261 | 339 ± 256 | 305 ± 227 |
|
| ||||
| CD8+ cell count, cells/mm3 | 419 ± 181 | 431 ± 203 | 844 ± 459 | 829 ± 434 |
|
| ||||
| CD3+ cell count, cells/mm3 | 1176 ± 404 | 1198 ± 426 | 1260 ± 627 | 1203 ± 591 |
|
| ||||
| HIV disease clinical stage 4,b % (no.) | … | … | 10.2 (17) | 7.9 (14) |
|
| ||||
| Log10 viral load, copies/mm3 | … | … | 4.6 ± 0.9 | 4.6 ± 1.0 |
NOTE. Data are mean ± SD values, unless otherwise indicated.
Sums may be less than the totals because of missing values.
According to the staging system proposed by the World Health Organization.
TB treatment outcome
Treatment failures by 1 month after initiation of anti-TB treatment were slightly less common, although not significantly so, among patients assigned to the micronutrients arm (table 2). Among patients whose TB cultures had become negative by 1 month after the initiation of treatment, micronutrients significantly decreased the risk of a recurrence during the remaining duration of treatment by 45% (95% CI, 7% to 67%; P = .02). This effect appeared to be larger among HIV-infected patients (63% [95% CI, 8% to 85%]; P = .02). Among HIV- infected patients who were assigned to the micronutrients arm, recurrences 8 months after treatment initiation were 34% less common, albeit nonsignificantly (95% CI, −67% to 31%; P = .23).
Table 2.
Effect of micronutrient supplementation on tuberculosis treatment failure and recurrences.
| % with outcome (no. at risk)
|
||||
|---|---|---|---|---|
| Outcome | Placebo | Micronutrients | RR (95% CI) | Pa |
| Treatment failure by 1 month after treatment initiationb | ||||
|
| ||||
| All subjects | 20.7 (314) | 15.3 (314) | 0.74 (0.53 to 1.04) | .08 |
|
| ||||
| HIV-negative subjects | 24.8 (157) | 18.1 (149) | 0.73 (0.47 to 1.13) | .15 |
|
| ||||
| HIV-positive subjects | 16.6 (157) | 12.7 (165) | 0.77 (0.45 to 1.31) | .33 |
|
| ||||
| Recurrence between 1 and 8 months after treatment initiationc | ||||
|
| ||||
| All subjects | 15.3 (216) | 8.4 (238) | 0.55 (0.33 to 0.93) | .02 |
|
| ||||
| HIV-negative subjects | 18.1 (105) | 13.0 (108) | 0.72 (0.38 to 1.35) | .30 |
|
| ||||
| HIV-positive subjects | 12.6 (111) | 4.6 (130) | 0.37 (0.15 to 0.92) | .02 |
NOTE. CI, confidence interval; RR, relative risk.
χ2 test.
Positive culture at 1 month after treatment initiation.
At least 1 positive culture between 1 and 8 months among patients with negative cultures at 1 month after treatment initiation.
Mortality and HIV disease progression
There were 155 deaths over 2880 patient-years of follow-up, 77 in the placebo arm and 78 in the micronutrients arm (hazard ratio [HR], 1.03 [95% CI, 0.75 to 1.40]; P = .88). The majority of deaths (140) occurred in the HIV-positive group, with a cumulative incidence of 29.7% during a median 2.5 years. In this group, micronutrient supplements had no effect on mortality (66 deaths in the placebo arm vs. 74 deaths in the micronutrients arm; HR, 1.16 [95% CI, 0.83 to 1.61]; P = .39). Similarly, there was no effect on HIV disease progression to stage 4 among 313 patients who were at stage 3 at baseline (HR, 1.08 [95% CI, 0.72 to 1.61]; P = .73). Among HIV-negative participants, there were 11 deaths in the placebo arm and 4 in the micronutrients arm, representing a non–statistically significant 64% reduction in mortality (HR, 0.36 [95% CI, 0.12 to 1.14]; P = .08).
Clinical complications
We examined the effect of micronutrient supplementation on the incidence of signs and symptoms during follow-up. Supplementation resulted in a significant 57% reduction of risk for peripheral neuropathy (95% CI, 41% to 69%; P < .001), irrespective of HIV status. Micronutrients were also related to a 73% decreased risk for clinical diagnoses of extrapulmonary TB (95% CI, 25% to 90%; P = .01), particularly among HIV-negative patients (RR, 0.24 [95% CI, 0.06 to 0.97]; P = .04). Also in this subgroup, the supplements appeared to significantly reduce the incidence of genital ulcers (RR, 0.10 [95% CI, 0.01 to 0.80]; P = .03).
T cell subsets, HIV load, and nutritional indicators
Micronutrients significantly increased CD3+ and CD4+ cell counts among HIV-negative subjects (table 3). In this group, a significant increase in CD8+ cell counts was also apparent during the first 8 months in the study. There were no significant effects of micronutrient supplements on T cell subsets or viral load among HIV-infected participants.
Table 3.
Effect of micronutrient supplementation on T cell counts (cells/mm3) and viral load (copies/mm3).
| All patients
|
HIV negative
|
HIV positive
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Placebo, meana | Micronutrients, mean difference (95% CI)b | P | Placebo, meana | Micronutrients, mean difference (95% CI)b | P | Placebo Meana | Micronutrients, mean difference (95% CI)b | P |
| Entire period | |||||||||
|
| |||||||||
| CD4+ cell count | 537 ± 306 | 28 (−2 to 59) | .07 | 740 ± 224 | 46 (6 to 86) | .02 | 340 ± 240 | −5 (−37 to 26) | .74 |
|
| |||||||||
| CD8+ cell count | 712 ± 379 | 33 (−10 to 75) | .13 | 490 ± 180 | 27 (−6 to 61) | .11 | 929 ± 397 | 58 (−12 to 127) | .10 |
|
| |||||||||
| CD3+ cell count | 1331 ± 473 | 71 (17 to 125) | .01 | 1300 ± 360 | 87 (25 to 148) | .006 | 1361 ± 561 | 60 (−30 to 151) | .19 |
|
| |||||||||
| Log10 viral load | … | … | … | … | … | … | 4.74 ± 0.83 | −0.08 (−0.22 to 0.05) | .23 |
|
| |||||||||
| First 8 months | |||||||||
|
| |||||||||
| CD4+ cell count | 543 ± 301 | 27 (−7 to 61) | .13 | 693 ± 236 | 55 (9 to 101) | .02 | 373 ± 275 | −4 (−46 to 38) | .86 |
|
| |||||||||
| CD8+ cell count | 652 ± 409 | 59 (8 to 110) | .02 | 429 ± 174 | 45 (8 to 81) | .02 | 906 ± 449 | 76 (−12 to 163) | .09 |
|
| |||||||||
| CD3+ cell count | 1275 ± 538 | 102 (30 to 175) | .006 | 1184 ± 372 | 117 (46 to 188) | .001 | 1378 ± 665 | 86 (−43 to 216) | .19 |
|
| |||||||||
| Log10 viral load | … | … | … | … | … | … | 4.72 ± 0.96 | −0.09 (−0.25 to 0.08) | .29 |
Data are the means ± SD of the average measurement during follow-up for each subject.
Data are the mean difference between the micronutrient group and the placebo group. The mean differences, 95% confidence intervals (CIs), and corresponding P values were estimated from generalized estimating equations, after adjustment for baseline measurements and follow-up time.
The supplements had no effects on a number of nutritional parameters, including body weight, BMI, mid-upper arm circumference, fat mass or fat-free mass, and albumin concentrations (table 4). Average hemoglobin concentrations during follow-up increased in both HIV-infected and HIV-negative patients, irrespective of treatment assignment. Among HIV-negative persons, this increase appeared to be higher in those assigned to micronutrients, but the treatment effect was not statistically significant. Similarly, the risk of anemia during follow-up was 58% lower in HIV-negative persons assigned to the micronutrients arm, compared with those assigned to the placebo arm (95% CI, −84% to 10%; P = .08).
Table 4.
Effect of micronutrient supplementation on nutritional parameters.
| All patients
|
HIV negative
|
HIV positive
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Placebo, meana | Micronutrients, mean difference (95% CI)b | P | Placebo, meana | Micronutrients, mean difference (95% CI)b | P | Placebo, meana | Micronutrients, mean difference (95% CI)b | P |
| Entire period | |||||||||
|
| |||||||||
| Body mass index, kg/m2 | 21.3 ± 3.1 | −0.1 (−0.4 to 0.1) | .15 | 21.3 ± 2.9 | −0.2 (−0.5 to 0.1) | .22 | 21.2 ± 3.3 | −0.1 (−0.4 to 0.1) | .37 |
|
| |||||||||
| Albumin concentration, g/dL | 3.4 ± 0.7 | 0.0 (−0.1 to 0.1) | .99 | 3.6 ± 0.6 | 0.1 (0.0 to 0.2) | .13 | 3.2 ± 0.8 | −0.1 (−0.2 to 0.0) | .13 |
|
| |||||||||
| Hemoglobin concentration, g/L | 123 ± 18 | 1.0 (−1.3 to 2.8) | .46 | 131 ± 16 | 2.2 (−0.4 to 4.9) | .09 | 115 ± 17 | −0.8 (−3.6 to 2.1) | .60 |
|
| |||||||||
| First 8 months | |||||||||
|
| |||||||||
| Body mass index, kg/m2 | 20.9 ± 2.9 | 0.0 (−0.2 to 0.2) | .91 | 20.8 ± 2.6 | −0.1 (−0.3 to 0.2) | .58 | 21.0 ± 3.2 | 0.0 (−0.2 to 0.3) | .74 |
|
| |||||||||
| Albumin concentration, g/dL | 3.3 ± 1.1 | −0.1 (−0.2 to 0.1) | .29 | 3.5 ± 1.1 | −0.1 (−0.3 to 0.2) | .52 | 3.2 ± 1.0 | −0.1 (−0.3 to 0.1) | .27 |
|
| |||||||||
| Hemoglobin concentration, g/L | 118 ± 20 | 1.4 (−1.0 to 3.8) | .24 | 124 ± 18 | 3.6 (0.3 to 6.9) | .03 | 112 ± 20 | −0.3 (−3.4 to 2.8) | .86 |
Data are the means ± SD of the average measurement during follow-up for each subject.
Data are the mean difference between the micronutrient group and the placebo group. The mean differences, 95% confidence intervals (CIs), and corresponding P values were estimated from generalized estimating equations, after adjustment for baseline measurements and follow-up time.
DISCUSSION
In this randomized clinical trial, micronutrient supplements appeared to decrease the risk of early TB recurrences among HIV-infected patients. Supplementation resulted in greater CD4+ and CD3+ lymphocyte counts, especially among HIV-negative subjects. Also in this group there were significant reductions attributable to the supplements in the incidence of extrapulmonary TB and genital ulcers. Micronutrients significantly decreased the incidence of peripheral neuropathy, irrespective of HIV status.
Two previous randomized clinical trials had examined the effect of micronutrients on TB treatment outcomes. In a study of 80 Indonesians with pulmonary TB, Karyadi et al. [10] reported earlier negative conversion of smears by 8 weeks among those who had received 5000 IU of vitamin A and 15 mg of zinc during anti-TB treatment for 6 months relative to those who had received placebo. In another trial in Mwanza, Tanzania, among 499 patients, Range et al. [11] found no effect of multimicronutrients or zinc on the proportion of patients with negative cultures by 8 weeks after the initiation of treatment. None of these trials, however, reported the effect of micronutrients on TB recurrences after culture or smear conversion to negative. Our finding of a protective effect of micronutrients against TB recurrences is relevant considering that TB reactivation is very common among HIV-infected persons [3]. Should this finding be confirmed in other settings, routine micronutrient supplementation might be considered a part of standard anti-TB therapy.
We noted significantly greater CD3+ and CD4+ cell counts in HIV-negative patients assigned to the micronutrients arm than in those assigned to the placebo arm. This increase could represent an enhancement of cellular immunity, which might have mediated the apparent effects of micronutrients on extrapulmonary TB and genital ulcers. Although the effect of micronutrients on T cell subsets among patients with TB has not been investigated previously, one recent trial in HIV-negative pregnant women from Tanzania also indicated a positive effect of micronutrient supplementation on CD3+ and CD4+ cell counts [21].
Micronutrients had no statistically significant effects on all-cause mortality or weight gain in our trial. This contrasts with results from the Mwanza study, in which multimicronutrient supplementation including zinc resulted in a significant 71% reduction in mortality by 8 months among HIV-infected patients and in a 2.4-kg effect on weight irrespective of HIV status [12]. One major difference between the Mwanza study and ours was the supplement composition, since we did not include zinc, copper, or vitamin D. In addition, the amount of selenium in our supplement was half that in the Mwanza preparation, whereas niacin, vitamin C, and vitamin E were 2.5–3.3 times higher. Zinc plays a fundamental role in immunity [22], but results from observational studies have raised some concerns about the safety of zinc supplementation in HIV-infected adults, because higher dietary intake of zinc appeared to be related to decreased survival [23]. Further studies are warranted to confirm the beneficial role of multimicronutrient supplementation including zinc in HIV-infected patients with TB.
Peripheral neuropathy is a relatively frequent complication of TB treatment, especially among HIV-coinfected patients [24]. It could be the result of isoniazid interference with vitamin B6 (pyridoxine) metabolism. Prophylactic supplementation with small doses of pyridoxine had been advocated in malnourished adult patients receiving isoniazid to prevent peripheral neuropathy [25]. Our finding of a protective effect of micronutrient supplementation including B6 on peripheral neuropathy appears to support this recommendation. Vitamin E deficiency has also been associated with peripheral neuropathy [26]. Although it is unknown whether vitamin E deficiency is highly prevalent in this population, the supplements could have contributed to the prevention of peripheral neuropathy through improved vitamin E status.
In conclusion, supplementation with micronutrients at multiples of the RDA reduced the incidence of TB recurrences among HIV-infected adults with TB who were not receiving antiretroviral treatment. Among HIV-negative adults, micronutrients appeared to increase T cell counts and reduced the incidence of complications. The impact of micronutrient supplementation on TB-related outcomes needs to be ascertained among HIV-infected patients receiving antiretroviral therapy. Should the potential benefits of micronutrient supplementation in HIV-uninfected patients with TB be confirmed in other settings, it could become a useful and relatively inexpensive element of TB treatment regimens.
Acknowledgments
We thank the patients, whose participation made this study possible. We are grateful to the study coordinator, Dorothy Mallya, and to the study physicians, research nurses, and laboratory and administrative staff at Muhimbili University of Health and Allied Sciences in Dar es Salaam, Tanzania, for their contributions to the project.
Financial support: National Institute of Allergy and Infectious Diseases (grant U01AI045441 to support the study); US Department of Agriculture (cooperative agreement 58-1950-7-707 with S.N.M.).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.World Health Organization. Global tuberculosis control: surveillance, planning, financing. Geneva: World Health Organization; 2007. [Google Scholar]
- 2.Harries AD, Hargreaves NJ, Kemp J, et al. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet. 2001;357:1519–23. doi: 10.1016/S0140-6736(00)04639-0. [DOI] [PubMed] [Google Scholar]
- 3.Korenromp EL, Scano F, Williams BG, Dye C, Nunn P. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis. 2003;37:101–12. doi: 10.1086/375220. [DOI] [PubMed] [Google Scholar]
- 4.van Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev. 2003;61:81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 5.Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–64. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb AL, Villamor E. Update: effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutr Rev. 2007;65:181–217. doi: 10.1111/j.1753-4887.2007.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 7.Mugusi FM, Rusizoka O, Habib N, Fawzi W. Vitamin A status of patients presenting with pulmonary tuberculosis and asymptomatic HIV-infected individuals, Dar es Salaam, Tanzania. Int J Tuberc Lung Dis. 2003;7:804–7. [PubMed] [Google Scholar]
- 8.Baylin A, Villamor E, Rifai N, Msamanga G, Fawzi WW. Effect of vitamin supplementation to HIV-infected pregnant women on the micronutrient status of their infants. Eur J Clin Nutr. 2005;59:960–8. doi: 10.1038/sj.ejcn.1602201. [DOI] [PubMed] [Google Scholar]
- 9.Kupka R, Msamanga GI, Spiegelman D, et al. Selenium status is associated with accelerated HIV disease progression among HIV-1-infected pregnant women in Tanzania. J Nutr. 2004;134:2556–60. doi: 10.1093/jn/134.10.2556. [DOI] [PubMed] [Google Scholar]
- 10.Karyadi E, West CE, Schultink W, et al. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr. 2002;75:720–7. doi: 10.1093/ajcn/75.4.720. [DOI] [PubMed] [Google Scholar]
- 11.Range N, Andersen AB, Magnussen P, Mugomela A, Friis H. The effect of micronutrient supplementation on treatment outcome in patients with pulmonary tuberculosis: a randomized controlled trial in Mwanza, Tanzania. Trop Med Int Health. 2005;10:826–32. doi: 10.1111/j.1365-3156.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 12.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr. 2006;95:762–70. doi: 10.1079/bjn20051684. [DOI] [PubMed] [Google Scholar]
- 13.Semba RD, Kumwenda J, Zijlstra E, et al. Micronutrient supplements and mortality of HIV-infected adults with pulmonary TB: a controlled clinical trial. Int J Tuberc Lung Dis. 2007;11:854–9. [PubMed] [Google Scholar]
- 14.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of nitrogen mustards in the palliative treatment of cancer. Cancer. 1948;1:634–56. [Google Scholar]
- 15.Fawzi WW, Msamanga GI, Spiegelman D, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 16.Baum M, Cassetti L, Bonvehi P, Shor-Posner G, Lu Y, Sauberlich H. Inadequate dietary intake and altered nutrition status in early HIV-1 infection. Nutrition. 1994;10:16–20. [PubMed] [Google Scholar]
- 17.World Health Organization. Treatment of tuberculosis: guidelines for national programmes. 3. Geneva: World Health Organization; 2003. [Google Scholar]
- 18.Lohman TG, Roche AF, Martorell Re . Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 19.Kotler DP, Burastero S, Wang J, Pierson RN., Jr Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr. 1996;64:489S–97S. doi: 10.1093/ajcn/64.3.489S. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Wkly Epidemiol Rec. Vol. 65. 1990. Interim proposal for a WHO staging system for HIV infection and disease; pp. 221–4. [PubMed] [Google Scholar]
- 21.Fawzi WW, Msamanga GI, Urassa W, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356:1423–31. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 22.Prasad AS. Zinc: mechanisms of host defense. J Nutr. 2007;137:1345–9. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- 23.Tang AM, Graham NM, Saah AJ. Effects of micronutrient intake on survival in human immunodeficiency virus type 1 infection. Am J Epidemiol. 1996;143:1244–56. doi: 10.1093/oxfordjournals.aje.a008712. [DOI] [PubMed] [Google Scholar]
- 24.Breen RA, Miller RF, Gorsuch T, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax. 2006;61:791–4. doi: 10.1136/thx.2006.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snider DE., Jr Pyridoxine supplementation during isoniazid therapy. Tubercle. 1980;61:191–6. doi: 10.1016/0041-3879(80)90038-0. [DOI] [PubMed] [Google Scholar]
- 26.Traber MG, Sokol RJ, Ringel SP, Neville HE, Thellman CA, Kayden HJ. Lack of tocopherol in peripheral nerves of vitamin E-deficient patients with peripheral neuropathy. N Engl J Med. 1987;317:262–5. doi: 10.1056/NEJM198707303170502. [DOI] [PubMed] [Google Scholar]


