Abstract
Evidence that brain glutamatergic activity is pathologically elevated in bipolar disorder suggests that mood stabilizers are therapeutic in the disease in part by downregulating glutamatergic activity. Such activity can involve the second messenger, arachidonic acid (AA, 20:4n-6). We tested this hypothesis with regard to valproic acid (VPA), when stimulating glutamatergic N-methyl-D-aspartate (NMDA) receptors in rat brain and measuring AA and related responses. An acute subconvulsant dose of NMDA (25 mg/kg i.p.) or saline was administered to unanesthetized rats that had been treated i.p. daily with VPA (200 mg/kg) or vehicle for 30 days. Quantitative autoradiography following intravenous [1-14C]AA infusion was used to image regional brain AA incorporation coefficients k*, markers of AA signaling. In chronic vehicle-pretreated rats, NMDA compared with saline significantly increased k* in 41 of 82 examined brain regions, many of which have high NMDA receptor densities, and also increased brain concentrations of the AA metabolites, prostaglandin E2 (PGE2) and thromboxane B2 (TXB2). VPA pretreatment reduced baseline concentrations of PGE2 and TXB2, and blocked the NMDA induced increases in k* and in eicosanoid concentrations. These results, taken with evidence that carbamazepine and lithium also block k* responses to NMDA in rat brain, suggest that mood stabilizers act in bipolar disorder in part by downregulating glutamatergic signaling involving AA.
Keywords: valproic acid, NMDA, arachidonic acid, bipolar disorder, phospholipase A2, prostaglandin E2
Introduction
Valproic acid (VPA, 2-propylpentaenoic acid) is approved as a mood stabilizer for treating bipolar disorder, particularly its manic phase [17, 39, 62]. Although inhibition of GABAergic neurotransmission is considered its major pharmacological action, VPA has many other central effects [43]. It can inhibit histone deacetylase [45] and brain microsomal long-chain fatty acyl-CoA synthethase [12], increase brain levels of the neuroprotective proteins bcl-2 and brain derived neurotrophic factor [23, 36], and alter transcription in brain of many genes [13].
VPA also has been reported to block excitatory responses induced by N-methyl-D-aspartate (NMDA) in vivo and in vitro, NMDA-induced convulsions in vivo [37, 38, 47, 57, 91, 96], and other aspects of brain glutamatergic activity [50, 78, 89, 92]. In view of evidence of upregulated or otherwise disturbed brain glutamatergic neurotransmission in patients with bipolar disorder [41, 50, 59, 90], we thought it of interest to see whether VPA could interfere with NMDA receptor initiated signaling involving the second messenger, arachidonic acid (AA, 20:4n-6). AA and its metabolites have multiple effects, including regulation of neuronal activity, gene transcription, apoptosis, sleep and cerebral blood flow [49, 58, 81].
Binding of glutamate or of NMDA to NMDA receptors will increase intracellular Ca2+, thereby activating Ca2+-dependent enzymes including AA-selective cytosolic phospholipase A2 (cPLA2). cPLA2 activation releases unesterified AA from the stereospecifically numbered-2 position of membrane phospholipid, which leads to increased formation via cyclooxygenase (COX) enzymes of eicosanoids such as prostaglandin E2 (PGE2) and thromboxane B2 (TXB2) [33, 46, 52, 79, 87, 93]. We have developed an in vivo method to image this activation process, and decided to use the method to image the effect of chronic VPA in rats on the NMDA receptor-mediated AA signal [5]. Prior reports indicate that other mood-stabilizers, lithium and carbamazepine, when given chronically to rats block this NMDA-mediated AA signal [5, 9].
The method to measure the AA signal involves infusing radiolabeled [1-14C]AA intravenously following administration of drug or vehicle, imaging regional brain radioactivity after 15 min with quantitative autoradiography, and converting these images into regional AA incorporation coefficients k*. k* represents the AA that has been released during the signal and metabolized to eicosanoids and other products and is independent of changes in cerebral blood flow [28, 74–76].
In this study, we measured k* for AA in 82 brain regions of unanesthetized rats that had been injected with VPA (200 mg/kg i.p.) or vehicle (saline) daily for 30 days as described [22]. These rats were injected i.p. acutely with a subconvulsant dose (25 mg/kg) of NMDA [5, 65] or with saline. Whole brain concentrations of PGE2 and TXB2 also were measured.
Experimental Procedures
Animals and Diets
Experiments were conducted following the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health Publication No. 86-23) and were approved by the Animal Care and Use Committee of the National Institute of Child Health and Development. Two-month-old male Fischer CDF (F-344)/CrlBR rats (Charles River Laboratories, Wilmington, MA, USA) were acclimatized for 1 week in an animal facility with regulated temperature, humidity and light cycle, and had ad libitum access to food (NIH-31 diet, Zeigler, Gardners, PA, USA) and water. The diet contained (as percent of total fatty acids) 20.1% saturated, 22.5% monounsaturated, 47.9% linoleic, 5.1% α-linolenic, 0.02% AA, 2.0% eicosapentaenoic, and 2.3% docosahexaenoic acid.
Drugs and tracers
[1-14C]AA in ethanol (53 mCi/mmol, >98% pure, Moravek Biochemicals, Brea, CA, USA) was evaporated and resuspended in HEPES buffer, pH 7.4, containing 50 mg/ml fatty acid-free bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA). NMDA (25 mg/kg, Sigma-Aldrich) or saline was administered i.p.. The NMDA dose has been reported to produce paroxysmal spikes and spike trains but not status epilepticus in rats [65] and to significantly increase k* for AA [5, 9]. VPA-treated rats received 200 mg/kg i.p. VPA (sodium salt; Sigma-Aldrich) in saline once daily for 30 days, as previously described [12, 14, 22, 73]. Three hours after the last VPA injection, the plasma VPA concentration equals 31 ± 6 (mean ± SD) μg/ml [22], slightly below the therapeutic concentration range (45–150 μg/ml) recommended for bipolar disorder [35]. A control group received the same volume of saline (vehicle) under parallel conditions.
Surgical Procedures and Tracer Infusion
On the morning following the 30th VPA or vehicle injection, a rat was anesthetized with 2–3% halothane in O2, and PE 50 polyethylene catheters were inserted into the right femoral artery and vein as described previously [5]. The wound was closed with surgical clips and the rat was wrapped loosely, with its upper body remaining free, in a fast-setting plaster cast (DePuy Inc., Raynam, MA, USA) that was taped to a wooden block. Surgery lasted 20–25 min. The rat was allowed to recover from anesthesia for 3 h in a quiet environment maintained at 25°C. Body temperature was maintained at 36.4–37.1°C using a feedback heating device and rectal thermometer. Arterial blood pressure and heart rate were measured with a blood pressure recorder (CyQ 103/302; Cybersense, Inc., Nicholasville, KY, USA). Arterial blood pH, pO2 and pCO2 were measured with a blood gas analyzer (Rapidlab 248, Bayer Health Care Diagnostics Division, Norwood MA, USA).
Ten minutes after injecting NMDA or saline, 2 ml [1-14C]AA (170 pCi/kg) was infused into the femoral vein for 5 min at a rate of 400 pl/min using an infusion pump (Harvard Apparatus Model 22, Natick, MA, USA). Twenty minutes after starting the infusion, the rat was euthanized with an overdose of Nembutal® (90 mg/kg, i.v.) and decapitated. The brain was removed in less than 30 s, frozen in 2-methylbutane maintained at −40°C with dry ice, and stored at −80°C until sectioned. Thus, brains in the present study were sampled within 4 hours after the last daily VPA injection.
Chemical Analysis
Thirteen arterial blood samples collected before, during and after [1-14C]AA infusion were centrifuged immediately (30 s at 18,000 g). Total lipids were extracted from 30 pl of plasma with 3 ml chloroform:methanol (2:1, by vol) and 1.5 ml 0.1 M KCl using Folch procedure [34]. Radioactivity was determined in 100 pl of the lower organic phase by liquid scintillation counting. As reported [26], greater than 97% of plasma radioactivity at 5 min following [1-14C]AA infusion was radiolabeled AA, and brain phospholipids accounted for greater than 81% of brain lipid radioactivity over 2 hours, whereas aqueous metabolites of AA account for 10% at 5 min and decrease with time.
Quantitative Autoradiography
Frozen brains were cut in serial 20-pm thick coronal sections in a cryostat at −20°C. The sections were placed for 5 weeks together with calibrated [14C]methylmethacrylate standards on Kodak Ektascan C/RA film (Eastman Kodak Company, Rochester, NY, USA). Brain regions from autoradiographs were identified from a stereotaxic rat brain atlas [67], and were sampled in both hemispheres. The average of bilateral measurements for each region from three consecutive brain sections was used to calculate regional radioactivity (nCi/g of brain) by digital quantitative densitometry, using a Macintosh computer and the public domain NIH Image program 1.62 (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Regional incorporation coefficients k* (ml plasma/s/g brain) of AA were calculated as [76],
| (Eq. 1) |
equals plasma radioactivity determined by scintillation counting (nCi/ml), equals brain radioactivity (nCi/g brain), and t equals time after starting [1-14C]AA infusion.
Brain prostaglandin E2 and thromboxane B2 concentrations
In separate experiments, 3 h and 45 min after the last of 30 daily injections of VPA or vehicle, a rat was injected i.p. with NMDA (25 mg/kg) or saline. Ten minutes later, it was anesthetized with Nembutal® (45 mg/kg, i.p.) and immediately subjected to head-focused microwave irradiation (5.5 kW, 3.8 s; Cober Electronics, Stamford, CT, USA) to stop brain lipid metabolism [15, 30]. Half-brains were weighed, homogenized with 18 volumes of hexane:isopropanol (3:2, by vol) using a glass Tenbroeck homogenizer and the homogenate was centrifuged for 5 min at 800 g. Tissue residues were rinsed with 3 × 2 volumes of the same solvent. The resultant lipid extract was concentrated to dryness under nitrogen and resuspended in the enzyme immunoassay buffer provided with the polyclonal PGE2 and TXB2 kits (Oxford Biochemical Research, Oxford, MI, USA).
Statistical analyses
An unpaired two-tailed t-test was used to compare mean physiological parameters in chronic VPA- and vehicle-treated rats, using GraphPad Prism version 4.0b (GraphPad Software, San Diego, CA, USA, www.graphpad.com). A standard two-way analysis of variance (ANOVA) was performed with SPSS 11.0 (SPSS Inc., Chicago, IL, USA, http://www.spss.com), to compare chronic VPA-versus-vehicle with acute NMDA-versus-saline with regard to arterial plasma radioactivity input functions, brain PGE2 and TXB2 concentrations and regional values of k*. Where interactions between VPA and NMDA were statistically insignificant, probabilities of effects of VPA and NMDA were reported. Where the interactions were significant, probabilities of main effects of VPA and NMDA were not reported [85]. Instead, unpaired two-tailed t-tests were used to compare NMDA and saline responses between chronic VPA- and vehicle-treated rats as well as saline responses in VPA- compared with vehicle-treated rats. Other comparisons were not considered relevant. A post-hoc test was not used to avoid a correction for multiple comparisons. Data are reported as the mean ± SD, with statistical significance taken as p ≤ 0.05.
Results
Physiology, behavior and arterial plasma radioactivity
Rats injected daily with VPA for 30 days weighed significantly less than vehicle-treated rats (Table 1). In chronic vehicle-treated rats, acute NMDA (25 mg/kg) produced repeated cycles of activity (head weaving and body movements) lasting on average 4 s, following by a “calm” period averaging 9 s, with the net cycling period lasting a mean of 95 s (Table 1). The mean durations of the behavioral parameters were not significantly different in chronic VPA-treated rats. Furthermore, compared with acute saline, NMDA did not significantly affect arterial pH, pCO2, pO2 or blood pressure, but significantly decreased heart rate by 21–23% in both chronic vehicle and VPA-treated groups (Table 1), as previously reported [5].
Table 1.
Effects of chronic valproate and acute NMDA on physiological parameters
| Chronic vehicle
|
Chronic valproate
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline | NMDA | Saline | NMDA | |||||
| Before | After | Before | After | Before | After | Before | After | |
| Body weight (g) | 276 ± 15 | - | 265 ± 10 | - | 248 ± 19* | - | 249 ± 16* | - |
| Body temperature (°C) | 36.4 ± 0.2 | 36.6 ± 0.3 | 36.4 ± 0.3 | 36.5 ± 0.2 | 36.6 ± 0.2 | 36.7 ± 0.3 | 36.4 ± 0.3 | 36.6 ± 0.3 |
| Heart rate (beats/min) | 413 ± 31 | 427 ± 39 | 443 ± 32 | 342 ± 35*** | 448 ± 23 | 451 ± 22 | 458 ± 21 | 361 ± 12*** |
| Arterial blood pressure (mmHg) | ||||||||
| Systolic | 145 ± 18 | 144 ± 20 | 150 ± 15 | 154 ± 18 | 132 ± 19 | 128 ± 19 | 134 ± 16 | 134 ± 15 |
| Diastolic | 102 ± 7 | 106 ± 9 | 105 ± 10 | 103 ± 8 | 100 ± 6 | 102 ± 7 | 98 ± 7 | 99 ± 8 |
| pH | 7.442 ± 0.029 | 7.466 ± 0.019 | 7.446 ± 0.024 | 7.472 ± 0.023 | 7.447 ± 0.029 | 7.447 ± 0.012 | 7.462 ± 0.026 | 7.474 ± 0.008 |
| pO2 (mmHg) | 94.9 ± 7.5 | 97.3 ± 17.5 | 95.9 ± 6.9 | 93.8 ± 8.3 | 98.4 ± 6.3 | 97.7 ± 10.9 | 97.4 ± 6.5 | 98.8 ± 17.5 |
| pCO2 (mmHg) | 33.2 ± 2.5 | 35.6 ± 7.4 | 33.2 ± 2.8 | 35.6 ± 1.9 | 38.9 ± 2.3 | 37.8 ± 3.8 | 31.9 ± 0.7 | 34.1 ± 1.6 |
| Behavior duration (s) | ||||||||
| Cycle | ||||||||
| Activity | 4 ± 1 | 5 ± 1 | ||||||
| Calm | 9 ± 2 | 10 ± 1 | ||||||
| Net cycling | 95 ± 15 | 105 ± 10 | ||||||
Values are the means ± SD (n = 4–6) measured before [1- C]AA infusion.
p < 0.001 effect of 25 mg/kg, i.p. NMDA in chronic vehicle- or valproate-treated rats; -, not measured
Neither chronic VPA nor acute NMDA modified the time-course of arterial plasma radioactivity (Eq. 1) following intravenous [1-14C]AA infusion. The mean integral of radioactivity in the plasma organic fraction, (nCi × s)/ml (n = 6), did not differ significantly between groups: chronic vehicle plus saline, 180,783 ± 24,145; chronic vehicle plus NMDA, 170,249 ± 16,855; VPA plus saline, 162,752 ± 18,879; VPA plus NMDA, 149,555 ± 21,089.
Regional brain AA incorporation coefficients, k*
Figure 1 presents coronal autoradiographs of brains from rats given saline or NMDA after chronic vehicle or VPA. Values of k* for AA, calculated by Eq. 1, are color-coded. The figure shows no apparent difference in regional values of k* in response to saline between the animals treated chronically with VPA compared with vehicle (both given acute saline). Acute NMDA increased k* in many gray matter regions of the chronic vehicle-treated rat, but had no evident effect on k* in the VPA-treated rat. Data obtained from such autoradiographs are summarized in Table 2 and Fig. 2.
Fig 1. Coronal autoradiographs showing effects of NMDA and valproic acid on brain regional AA incorporation coefficients k* in rats.
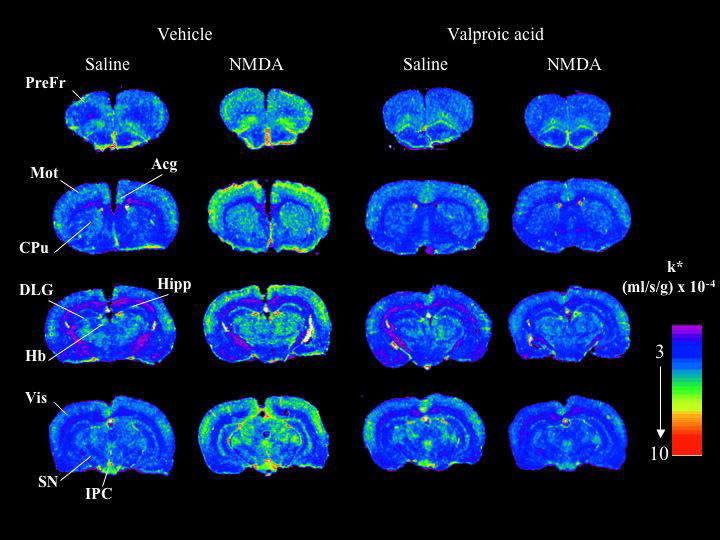
Values of k* (ml/s/g brain × 10−4) are given on a color scale. Abbreviations: Acg, anterior cingulate cortex; CPu, caudate-putamen; DLG, dorsal lateral geniculate nucleus; Hb, habenular nucleus; Hipp, hippocampus; IPC, interpeduncular nucleus; Mot, motor cortex; PreFr, prefrontal cortex; SN, substantia nigra; Vis, visual cortex.
Table 2.
Effect of chronic valproic acid on NMDA-induced regional AA incorporation coefficients, k* in rat brain
| Chronic Saline | Chronic Valproate | Valproate × NMDA interaction | Valproate effect | NMDA effect | |||
|---|---|---|---|---|---|---|---|
| Brain region | Saline (n = 5) | NMDA (n = 6) | Saline (n = 6) | NMDA (n = 6) | P-value | P-value | P-value |
| Prefrontal cortex layer I | 4.03 ± 0.20 | 5.55 ± 0.61*** | 4.39 ± 0.43 | 4.24 ± 0.41 | < 0.001 | ||
| Prefrontal cortex layer IV | 4.46 ± 0.37 | 6.31 ± 0.82** | 4.90 ± 0.54 | 4.55 ± 0.36 | < 0.001 | ||
| Primary olfactory cortex | 3.87 ± 0.58 | 4.96 ± 0.68* | 3.92 ± 0.59 | 3.89 ± 0.50 | 0.003 | ||
| Frontal cortex (10) | |||||||
| Layer I | 4.17 ± 0.45 | 5.57 ± 0.53** | 4.56 ± 0.31 | 4.12 ± 0.66 | < 0.001 | ||
| Layer IV | 4.49 ± 0.52 | 6.49 ± 0.92** | 5.20 ± 0.49* | 4.57 ± 0.21a | < 0.001 | ||
| Frontal cortex (8) | |||||||
| Layer I | 4.21 ± 0.40 | 6.07 ± 1.02** | 4.36 ± 0.25 | 4.46 ± 0.44 | < 0.001 | ||
| Layer IV | 4.83 ± 0.62 | 6.63 ± 0.97** | 4.56 ± 0.45 | 4.76 ± 0.51 | 0.011 | ||
| Pyriform cortex | 3.39 ± 0.55 | 3.88 ± 0.55 | 3.61 ± 0.53 | 3.53 ± 0.54 | 0.219 | 0.777 | 0.387 |
| Anterior cingulate cortex | 4.68 ± 0.47 | 7.53 ± 1.5** | 4.78 ± 0.54 | 5.09 ± 0.31 | 0.002 | ||
| Motor cortex | |||||||
| Layer I | 4.23 ± 0.54 | 6.07 ± 0.89** | 4.60 ± 0.59 | 4.62 ± 0.57 | 0.004 | ||
| Layer II –III | 4.40 ± 0.65 | 6.12 ± 1.02* | 4.78 ± 0.51 | 4.57 ± 0.71 | 0.006 | ||
| Layer IV | 4.67 ± 0.71 | 6.31 ± 0.92** | 5.25 ± 0.39 | 5.18 ± 0.25 | 0.004 | ||
| Layer V | 3.47 ± 0.35 | 5.05 ± 0.73** | 4.14 ± 0.26** | 3.75 ± 0.56 | < 0.001 | ||
| Layer VI | 3.35 ± 0.42 | 4.82 ± 0.84** | 3.68 ± 0.54 | 3.84 ± 0.56 | 0.010 | ||
| Somatosensory cortex | |||||||
| Layer I | 4.23 ± 0.49 | 5.62 ± 0.55** | 4.66 ± 0.42 | 4.25 ± 0.53 | < 0.001 | ||
| Layer II – III | 4.39 ± 0.52 | 5.89 ± 0.67** | 5.09 ± 0.22 | 4.34 ± 0.54 | < 0.001 | ||
| Layer IV | 4.81 ± 0.55 | 6.51 ± 1.08* | 5.27 ± 0.37 | 4.31 ± 0.48 | < 0.001 | ||
| Layer V | 4.25 ± 0.37 | 5.65 ± 0.67** | 4.47 ± 0.36 | 4.50 ± 0.41 | 0.003 | ||
| Layer VI | 4.19 ± 0.47 | 5.47 ± 0.69** | 4.28 ± 0.35 | 4.38 ± 0.46 | 0.012 | ||
| Auditory cortex | |||||||
| Layer I | 5.37 ± 2.03 | 7.50 ± 1.00* | 5.35 ± 0.66 | 4.88 ± 0.92 | 0.019 | ||
| Layer IV | 6.12 ± 1.48 | 7.54 ± 1.55 | 4.98 ± 0.54 | 5.29 ± 0.50 | 0.373 | 0.011 | 0.168 |
| Layer VI | 5.21 ± 1.68 | 6.53 ± 1.17 | 4.71 ± 0.38 | 4.78 ± 1.16 | 0.214 | 0.032 | 0.171 |
| Visual cortex | |||||||
| Layer I | 4.16 ± 0.51 | 6.43 ± 0.33*** | 4.52± 0.25 | 4.24 ± 0.30 | < 0.001 | ||
| Layer IV | 4.47 ± 0.38 | 6.26 ± 0.94*** | 4.54 ± 0.22 | 4.83 ± 1.04 | 0.001 | ||
| Layer VI | 4.29 ± 0.62 | 6.37 ± 0.64*** | 4.49 ± 0.29 | 4.64 ± 0.34 | 0.025 | ||
| Preoptic area (LPO/MPO) | 3.54 ± 0.45 | 3.96 ± 0.35 | 3.81 ± 0.60 | 3.82 ± 0.49 | 0.326 | 0.762 | 0.297 |
| Suprachiasmatic nu | 3.29 ± 0.62 | 3.96 ± 0.61 | 3.72 ± 0.67 | 3.78 ± 0.72 | 0.283 | 0.656 | 0.208 |
| Globus pallidus | 3.37 ± 0.39 | 3.65 ± 0.22 | 3.34 ± 0.24 | 3.52 ± 0.39 | 0.728 | 0.546 | 0.093 |
| Bed nu stria terminalis | 3.46 ± 0.60 | 4.00 ± 0.63 | 3.46 ± 0.22 | 3.25 ± 0.22 | 0.060 | 0.064 | 0.392 |
| Olfactory tubercle | 4.33 ± 0.44 | 4.91 ± 0.57 | 3.60 ± 0.38 | 3.59 ± 0.38 | 0.133 | < 0.001 | 0.144 |
| Diagonal band Dorsal | 4.02 ± 0.32 | 4.21 ± 0.56 | 3.72 ± 0.45 | 3.89 ± 0.52 | 0.949 | 0.138 | 0.377 |
| Ventral | 3.79 ± 0.35 | 4.19 ± 0.38 | 3.76 ± 0.19 | 3.71 ± 0.21 | 0.079 | 0.051 | 0.179 |
| Amygdala basolateral/medial | 4.19 ± 0.64 | 4.60 ± 0.82 | 3.67 ± 0.31 | 3.87 ± 0.14 | 0.653 | 0.013 | 0.199 |
| Hippocampus | |||||||
| CA1 | 3.66 ± 0.35 | 4.37 ± 0.56** | 3.47 ± 0.34 | 3.45 ± 0.35 | 0.048 | ||
| CA2 | 3.99 ± 0.67 | 4.85 ± 0.30* | 3.76 ± 0.25 | 3.61 ± 0.27 | 0.006 | ||
| CA3 | 3.99 ± 0.65 | 5.10 ± 0.64* | 3.70 ± 0.20 | 3.74 ± 0.44 | 0.022 | ||
| Dentate gyrus | 3.63 ± 0.31 | 5.01 ± 0.67** | 3.79 ± 0.27 | 3.56 ± 0.20 | < 0.001 | ||
| SLM | 4.03 ± 0.53 | 5.02 ± 0.69* | 4.02 ± 0.14 | 4.13 ± 0.39 | 0.040 | ||
| Accumbens nucleus | 3.94 ± 0.55 | 4.79 ± 0.50* | 4.07 ± 0.37 | 4.00 ± 0.17 | 0.016 | ||
| Caudate putamen | |||||||
| Dorsal | 3.98 ± 0.35 | 5.14 ± 0.18*** | 3.96 ± 0.34 | 4.10 ± 0.40 | 0.002 | ||
| Ventral | 4.00 ± 0.36 | 5.02 ± 0.30*** | 3.97 ± 0.42 | 3.74 ± 0.17 | 0.001 | ||
| Lateral | 3.96 ± 0.39 | 5.30 ± 0.49*** | 4.11 ± 0.53 | 3.80 ± 0.27 | < 0.001 | ||
| Medial | 3.93 ± 0.36 | 5.06 ± 0.42** | 3.35 ± 0.33 | 3.55 ± 0.48 | 0.013 | ||
| Septal nu lateral | 3.43 ± 0.49 | 4.17 ± 0.18 | 3.35 ± 0.23 | 3.54 ± 0.48 | 0.107 | 0.061 | 0.069 |
| Septal nu medial | 3.97 ± 0.45 | 4.10 ± 0.50 | 3.33 ± 0.33 | 3.35 ± 0.34 | 0.436 | 0.070 | 0.081 |
| Habenular nu lateral | 6.21 ± 1.12 | 7.07 ± 0.19 | 6.51 ± 1.19 | 6.51 ± 0.94 | 0.290 | 0.746 | 0.290 |
| Habenular nu medial | 6.46 ± 1.33 | 7.16 ± 0.30 | 6.13 ± 0.23 | 6.27 ± 0.92 | 0.405 | 0.083 | 0.218 |
| Lateral geniculate nu dorsal | 4.49 ± 0.46 | 5.72 ± 0.42** | 4.59 ± 0.50 | 4.58 ± 0.54 | 0.007 | ||
| Medial geniculate nu | 5.01 ± 0.88 | 6.09 ± 0.64 | 4.88 ± 0.33 | 4.89 ± 0.57 | 0.053 | 0.019 | 0.074 |
| Thalamus | |||||||
| Ventroposterior lateral nu | 4.11 ± 0.33 | 4.48 ± 0.56 | 4.18 ± 0.37 | 4.44 ± 0.56 | 0.798 | 0.921 | 0.126 |
| Ventroposterior medial nu | 4.20 ± 0.39 | 4.64 ± 0.59 | 4.25 ± 0.33 | 4.38 ± 0.31 | 0.685 | 0.829 | 0.142 |
| Paratenial nu | 4.14 ± 0.26 | 5.17 ± 0.57** | 4.08 ± 0.37 | 4.26 ± 0.32 | 0.022 | ||
| Anteroventral nu | 5.66 ± 0.67 | 7.15 ± 0.70** | 5.77 ± 0.62 | 5.54 ± 0.61 | 0.005 | ||
| Anteromedial nu | 4.51 ± 0.37 | 4.98 ± 0.58 | 4.50 ± 0.39 | 4.58 ± 0.37 | 0.301 | 0.277 | 0.153 |
| Reticular nu | 4.42 ± 0.39 | 5.50 ± 0.45 | 4.18 ± 0.41 | 4.30 ± 0.38 | 0.072 | 0.245 | 0.125 |
| Paraventricular nu | 4.28 ± 0.47 | 4.25 ± 0.35 | 4.06 ± 0.45 | 4.38 ± 0.49 | 0.093 | 0.745 | 0.158 |
| Parafascicular nu | 4.17 ± 0.76 | 5.73 ± 0.51** | 4.19 ± 0.28 | 4.12 ± 0.52 | 0.002 | ||
| Subthalamic nu | 5.05 ± 0.83 | 5.91 ± 0.37 | 5.23 ± 0.38 | 5.30 ± 0.37 | 0.078 | 0.332 | 0.061 |
| Hypothalamus | |||||||
| Supraoptic nu | 4.27 ± 0.81 | 3.30 ± 0.57 | 4.00 ± 0.22 | 3.80 ± 0.79 | 0.178 | 0.689 | 0.189 |
| Lateral | 3.36 ± 0.61 | 3.98 ± .22 | 3.54 ± 0.47 | 3.75 ± 0.40 | 0.274 | 0.888 | 0.088 |
| Anterior | 3.68 ± 0.35 | 3.82 ± 0.49 | 3.04 ± 0.35 | 3.72 ± 0.78 | 0.238 | 0.116 | 0.079 |
| Periventricular | 3.01 ± 0.43 | 3.12 ± 0.32 | 3.09 ± 0.35 | 3.39 ± 0.54 | 0.587 | 0.335 | 0.260 |
| Arcuate | 3.51 ± 0.67 | 3.38 ± 0.47 | 3.26 ± 0.41 | 3.19 ± 0.48 | 0.875 | 0.307 | 0.630 |
| Ventromedial | 3.87 ± 0.38 | 3.77 ± 0.60 | 3.72 ± 0.16 | 3.65 ± 0.76 | 0.941 | 0.547 | 0.689 |
| Posterior | 3.37 ± 0.19 | 3.32 ± 0.20 | 3.67 ± 0.25 | 3.81 ± 0.63 | 0.135 | 0.452 | 0.226 |
| Mammillary nu | 3.74 ± 0.89 | 3.57 ± 0.42 | 3.76 ± 0.14 | 3.52 ± 0.31 | 0.870 | 0.919 | 0.335 |
| Interpeduncular nu | 6.30 ± 0.19 | 8.06 ± 0.66*** | 5.33 ± 0.33*** | 5.70 ± 0.59 | 0.003 | ||
| Substantia nigra | 3.97 ± 0.16 | 5.54 ± 1.02** | 4.17 ± 0.39 | 4.17 ± 0.43 | 0.006 | ||
| Pretectal area | 4.13 ± 0.55 | 4.02 ± 0.36 | 3.89 ± 0.27 | 3.71 ± 0.59 | 0.324 | 0.075 | 0.126 |
| Grey layer Superior colliculus | 4.00 ± 0.43 | 4.44 ± 0.56 | 4.00 ± 0.27 | 0.92 ± 0.32 | 0.144 | 0.160 | 0.314 |
| Superior colliculus | 4.14 ± 0.33 | 4.93 ± 0.90 | 3.89 ± 0.24 | 4.26 ± 0.88 | 0.335 | 0.051 | 0.074 |
| Inferior colliculus | 5.91 ± 1.20 | 7.24 ± 0.55* | 6.25 ± 0.84 | 5.80 ± 1.10 | 0.036 | ||
| Flocculus | 4.52 ± 0.68 | 4.63 ± 0.70 | 4.45 ± 0.48 | 4.75 ± 0.18 | 0.696 | 0.907 | 0.374 |
| Cerebellar gray matter | 4.33 ± 0.34 | 5.64 ± 0.84* | 4.25 ± 0.34 | 4.29 ± 0.39 | 0.010 | ||
| Molecular layer cerebellar gray | 6.02 ± 0.76 | 7.41 ± 0.90* | 6.17 ± 0.53 | 6.04 ± 0.68 | 0.022 | ||
| White matter | |||||||
| Corpus callosum | 3.34 ± 0.60 | 3.68 ± 0.60 | 3.20 ± 0.36 | 3.13 ± 0.33 | 0.320 | 0.101 | 0.506 |
| Zona incerta | 3.47 ± 0.27 | 3.57 ± 0.38 | 3.40 ± 0.33 | 3.39 ± 0.31 | 0.726 | 0.414 | 0.726 |
| Internal capsule | 2.83 ± 0.51 | 2.85 ± 0.39 | 2.84 ± 0.47 | 2.89 ± 0.51 | 0.947 | 0.894 | 0.858 |
| Cerebellar white matter | 3.32 ± 0.35 | 3.06 ± 0.53 | 3.30 ± 0.29 | 2.93 ± 0.35 | 0.725 | 0.651 | 0.071 |
| Non-blood-brain barrier regions | |||||||
| Subfornical organ | 4.50 ± 0.93 | 3.75 ± 0.72 | 3.90 ± 0.99 | 4.06 ± 0.99 | 0.249 | 0.706 | 0.453 |
| Median eminence | 3.58 ± 0.57 | 3.75 ± 0.31 | 3.76 ± 0.46 | 3.56 ± 0.50 | 0.347 | 0.988 | 0.958 |
| Choroid plexus | 21.5 ± 4.44 | 22.1 ± 3.09 | 22.4 ± 2.52 | 23.8 ± 1.15 | 0.737 | 0.309 | 0.417 |
Abbreviations: nu, nucleus; k* = (ml/s/g) × 10−4, each k* value is a mean ± S.D
Main effects are not reported if statistically significant VPA × NMDA interaction when unpaired t-tests were performed.
p < 0.05;
p < 0.01;
p < 0.001; Chronic saline plus NMDA versus chronic saline plus saline, valproate plus saline versus chronic saline plus saline, and valproate plus NMDA versus valproate plus saline.
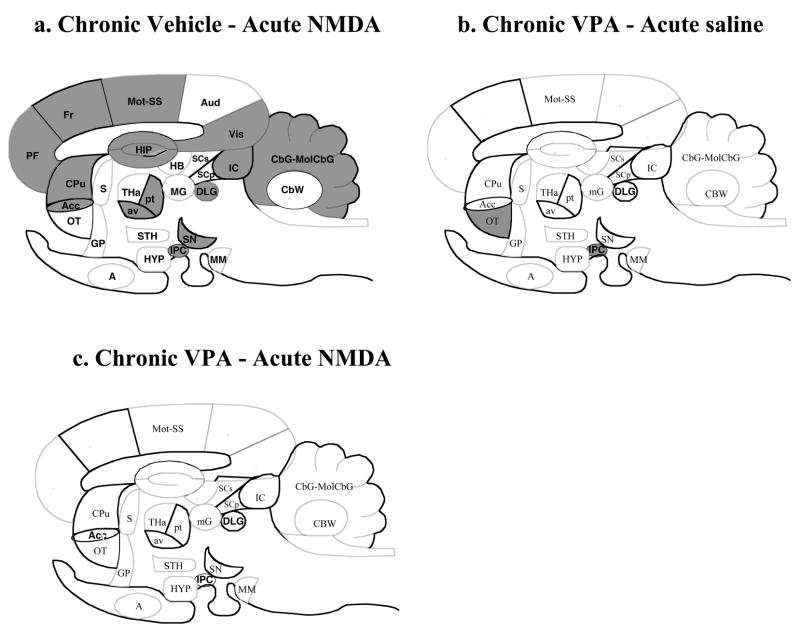
Fig 2. Difference patterns of k* responses to NMDA and valproic acid in sagittal representation of rat brain.
Regions in which k* was increased significantly (p < 0.05) are solid black, regions in which k* was decreased significantly are hatched. List of regions: A, amygdala; Acc, nucleus accumbens; Aud, auditory cortex; av, anteroventral thalamic nucleus; CbG, cerebellar gray matter; CBW, cerebellar white matter; CPu, caudate putamen; DLG, dorsal lateral geniculate nucleus; Fr, frontal cortex; GP, globus pallidus; HB, habenular complex; HIP, hippocampus; HYP, hypothalamus; IC, inferior colliculus; IPC, interpeduncular nucleus; MM, mammillary nucleus; mG, medial geniculate nucleus; MolCBG, molecular layer of cerebellar gray matter; Mot, motor cortex; OT, olfactory tubercle; PF, prefrontal cortex; pt, paratenial thalamic nucleus; SN, substantia nigra; S, septum; SS, somatosensory cortex; SCp, deep layer of superior colliculus; SCs, superficial layer of superior colliculus; STH, subthalamic nucleus; THa, thalamus; Vis, visual cortex.
Effects of NMDA in chronic vehicle-treated rats
Mean values of k* in each of 82 brain regions were subjected to a two-way ANOVA, as illustrated in Table 2. Statistically significant interactions between VPA and NMDA were found in 41, in which unpaired t-tests then showed that NMDA compared with saline significantly increased k* by 19–61% in chronic vehicle-treated rats. Affected regions included prefrontal (38–41%), frontal (34–45%), primary olfactory (28%), anterior cingulate (61%), motor (35–46%), somatosensory (31–35%), auditory layer I (40%) and visual cortical areas (40–55%), hippocampus [CA1, CA2, CA3, dentate gyrus, stratum lacunosum-molecular] (19–38%), nucleus accumbens (22%), caudate-putamen (26–34%), lateral geniculate nucleus dorsal (27%), thalamus [paratenial, anteroventral and parafascicular nuclei] (25–37%), interpeduncular nucleus (28%), substantia nigra (40%), inferior colliculus (23%), and cerebellar gray matter (23–30%). The overall pattern of differences due to NMDA compared with saline in chronic vehicle-treated rats is illustrated in Fig. 2a.
Effects of chronic valproic acid at baseline
In the 41 regions in which VPA × NMDA interactions were statistically significant, chronic VPA compared with chronic vehicle significantly changed mean baseline (post-saline) k* in 3 of them (Table 2) -- frontal cortex (10) layer IV (16%), motor cortex layer V (19%) and interpeduncular nucleus (-15%). In the other 41 regions in which VPA × NMDA interactions were statistically insignificant, chronic VPA had a main effect in 5 of them, but reduced k* only in the olfactory tubercle. Thus, chronic VPA altered baseline k* in 4 of the 82 brain regions studied. The overall pattern of differences due to chronic VPA is illustrated in Fig. 2b.
Effects of acute NMDA in valproic acid-treated rats
NMDA compared with saline changed k* significantly (-12%) in only one of the 41 regions in which VPA × NMDA interactions were statistically significant, frontal cortex (10) layer IV (Table 2). Acute NMDA did not significantly affect k* in any of the 41 regions in which VPA × NMDA interactions were statistically insignificant. In none of these latter regions did NMDA have a main effect on k*. The complete lack of a significant NMDA effect in animals pretreated with VPA is illustrated in Fig. 2c.
Brain PGE2 and TXB2 concentrations
A two-way ANOVA demonstrated statistically significant interactions between VPA and NMDA with regard to brain PGE2 and TXB2 concentrations (Table 3). Consequent t-tests showed that chronic VPA alone significantly decreased basal concentrations of PGE2 by 66% and of TXB2 by 45%. Acute NMDA increased PGE2 and TXB2 concentrations in chronic vehicle-treated rats, but did not significantly affect either concentration in chronic VPA-treated rats.
Table 3.
Effect of NMDA on brain PGE2 and TXB2 concentrations in chronic vehicle- and valproate-treated rats
| Chronic vehicle | Chronic valproate | Valproate × NMDA interaction | |||
|---|---|---|---|---|---|
| Saline | NMDA | Saline | NMDA | P-value | |
| PGE2 (ng/g brain) | 7.6 ± 1.5 | 16.4 ± 4.4** | 2.6 ± 0.4*** | 2.8 ± 0.7 | 0.003 |
| TXB2 (pg/g brain) | 41.4 ± 2.8 | 88.5 ± 8.9*** | 22.8 ± 3.7*** | 23.1 ± 0.7 | < 0.001 |
Each value is a mean ± SD (n = 4).
P < 0.01,
P < 0.001; vehicle plus NMDA versus vehicle plus saline, valproate plus saline versus vehicle plus saline, valproate plus NMDA versus valproate plus saline
Discussion
Consistent with reports that VPA interferes with glutamatergic function and NMDA receptor signaling (see “Introduction”), daily administration of VPA to rats for 30 days, at a dose that produces a plasma VPA concentration relevant to bipolar disorder, prevented the statistically significant increases in AA incorporation coefficients k*, and in whole brain PGE2 and TXB2 concentrations, that were caused by a subconvulsant acute dose of NMDA in chronic vehicle-treated rats. To the extent that glutamatergic signaling via NMDA receptors is pathologically upregulated in bipolar disorder patients, for which evidence exists (see “Introduction”) [41, 50, 59, 90], these results suggest that VPA’s efficacy in the disease is due in part to its ability to dampen upregulated NMDA signaling involving AA and its downstream metabolites. Chronic administration to rats of lithium or carbamazepine, resulting in therapeutic relevant plasma concentrations, also dampen NMDA-induced elevations in k* for AA and in brain eicosanoid [5, 9]. Thus, reduced NMDA signaling involving AA and its metabolites may be common to the therapeutic action of mood-stabilizers in bipolar disorder.
Evidence that cholinomimetics [19] as well as drugs that interfere with dopaminergic [24, 60, 68, 71] or glutamatergic [2, 61, 88] signaling ameliorate bipolar disorder symptoms, and of defective serotonergic signaling [56], has suggested that bipolar symptoms reflect reduced cholinergic, altered serotonergic, and increased dopaminergic and glutamatergic neurotransmission. Our studies in rats now suggest that chronic VPA, lithium and carbamazepine as a group can correct this imbalance, and that the imbalance involves AA as a second messenger [4–9, 21, 77].
Our values of k* in this study agree with published values [5, 9]. NMDA increased k* significantly in 41 of 82 brain structures with high densities of NMDA receptors [66], including the cerebral cortex, caudate-putamen, globus pallidus, hippocampus, thalamus, hypothalamus, colliculus, substantia nigra. Our measured PGE2 and TXB2 concentrations agree with other studies showing elevated brain concentrations of these eicosanoids following acute NMDA [9, 64, 69] and reduced concentrations following chronic VPA [14, 84]. Concentrations of PGD2 and PGF2α also are increased after NMDA [51, 54] but are decreased by chronic VPA [84].
VPA’s ability to suppress NMDA-induced increases in k* for AA and to reduce PGE2 and TXB2 concentrations in rat brain could have been due to its ability to reduce COX-1 and COX-2 expression or interfere directly with the NMDA receptor [14, 73]. When COX enzymes are pharmacologically inhibited or knocked out in rodent brain, k* responses to drugs acting at cPLA2-coupled neuroreceptors are reduced or lost, as are the increases in brain PGE2 and/or TXB2 concentrations [10, 11]. VPA can inhibit cyclic AMP-dependent protein kinases A and C by VPA, both of which can phosphorylate the receptor [32, 53, 94]. VPA also can reduce expression of two NMDA receptor-interacting proteins in rat brain, postsynaptic density protein PSD-95, which is altered in bipolar disorder [90], and type II Ca2+/calmodulin-dependent protein kinase beta subunit [13]. It inhibits histone deacetylase, which acetylates the NMDA receptor transcription factor, specificity protein-1 (Sp1) [3, 70], and can reduce methylation of the reelin gene, which encodes a protein that regulates NMDA receptor surface trafficking and synaptic subunit composition [29, 31, 40]. VPA blocks induction of Fos and of activator protein-1 DNA binding activity, both of which modulate transcription of the NMDA receptor subunit, NR2B [72, 80]. It regulates expression and traffic of NMDA receptors in hippocampal neurons [20, 36], and decreases basal glutamate release and increases glutamate uptake in brain [42, 89, 92]. VPA also may modulate neurotransmission involving cPLA2 and AA coupled to glutamatergic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors [25, 32, 38, 48, 93].
The behavioral effects of NMDA (Table 1) were not altered qualitatively by chronic VPA, lithium [5] or carbamazepine [9]. Thus, AA signaling via NMDA receptors likely does not contribute to these effects. In agreement, neither VPA, lithium or carbamazepine modified the seizure threshold to NMDA in rodents [65, 82], and VPA did not reduce NMDA-induced running/jumping fits, clonic-tonic seizure or mortality in rats [44]. NMDA can promote also synaptic release of acetylcholine, adenosine, serotonin and γ-aminobutyric acid [27, 86, 93, 95].
Since mood-stabilization of bipolar patients appear only after 10 days of oral VPA [16], we as have others [5, 9, 12, 13, 18, 22, 42, 55] studied effects only of chronic VPA in rats. An acute injection of VPA (300 mg/kg) in rats did not alter basal or stimulated extracellular glutamate in the hippocampus, whereas chronic VPA decreased whole brain glutamate concentration [1, 63]. Chronic but not acute VPA administration changed corticotropin releasing factor [83] and AMPA glutamate receptors [32] in rat brain.
In conclusion, chronic VPA pretreatment prevented the statistically significant increases in k* for AA and in PGE2 and TXB2 concentrations that were observed in response to NMDA in chronic vehicle-treated rats. These and observations in rats administered chronic lithium or carbamazepine support the hypothesis that mood stabilizers commonly downregulate brain AA signaling via NMDA receptors.
Acknowledgments
This research was supported (in part) by the Intramural Research program of the National Institutes of Health, National Institute on Aging. None of the authors has a financial or other conflict of interest related to this work.
Abbreviations
- AA
arachidonic acid (20,4n-6)
- PLA2
phospholipase A2
- cPLA2
cytosolic PLA2
- NMDA
N-methyl-D-aspartic acid
- PGE2
prostaglandin E2
- TXB2
thromboxane B2
- VPA
valproic acid
- COX
cyclooxygenase
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
References
- 1.Ahmad S, Fowler LJ, Whitton PS. Effects of combined lamotrigine and valproate on basal and stimulated extracellular amino acids and monoamines in the hippocampus of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:1–8. doi: 10.1007/s00210-004-1008-4. [DOI] [PubMed] [Google Scholar]
- 2.Anand A, Charney DS, Oren DA, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Archives of General Psychiatry. 2000;57:270–6. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- 3.Bai G, Kusiak JW. Functional analysis of the proximal 5′-flanking region of the N-methyl-D-aspartate receptor subunit gene, NMDAR1. J Biol Chem. 1995;270:7737–44. doi: 10.1074/jbc.270.13.7737. [DOI] [PubMed] [Google Scholar]
- 4.Basselin M, Chang L, Bell JM, et al. Chronic lithium chloride administration to unanesthetized rats attenuates brain dopamine D2-like receptor-initiated signaling via arachidonic acid. Neuropsychopharmacology. 2005;30:1064–1075. doi: 10.1038/sj.npp.1300671. [DOI] [PubMed] [Google Scholar]
- 5.Basselin M, Chang L, Bell JM, et al. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–74. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- 6.Basselin M, Chang L, Chen M, et al. Chronic carbamazepine administration attenuates dopamine D2-like receptor-initiated signaling via arachidonic acid in rat brain. Neurochem Res. 2008 doi: 10.1007/s11064-008-9595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basselin M, Chang L, Seemann R, et al. Chronic lithium administration potentiates brain arachidonic acid signaling at rest and during cholinergic activation in awake rats. J Neurochem. 2003;85:1553–62. doi: 10.1046/j.1471-4159.2003.01811.x. [DOI] [PubMed] [Google Scholar]
- 8.Basselin M, Chang L, Seemann R, et al. Chronic lithium administration to rats selectively modifies 5-HT2A/2C receptor-mediated brain signaling via arachidonic acid. Neuropsychopharmacology. 2005;30:461–72. doi: 10.1038/sj.npp.1300611. [DOI] [PubMed] [Google Scholar]
- 9.Basselin M, Villacreses NE, Chen M, et al. Chronic carbamazepine administration reduces N-methyl-D-aspartate receptor-initiated signaling via arachidonic acid in rat brain. Biol Psychiatry. 2007;62:934–43. doi: 10.1016/j.biopsych.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basselin M, Villacreses NE, Langenbach R, et al. Resting and arecoline-stimulated brain metabolism and signaling involving arachidonic acid are altered in the cyclooxygenase-2 knockout mouse. J Neurochem. 2006;96:669–79. doi: 10.1111/j.1471-4159.2005.03612.x. [DOI] [PubMed] [Google Scholar]
- 11.Basselin M, Villacreses NE, Lee HJ, et al. Flurbiprofen, a cyclooxygenase inhibitor, reduces the brain arachidonic acid signal in response to the cholinergic muscarinic agonist, arecoline, in awake rats. Neurochem Res. 2007;32:1857–67. doi: 10.1007/s11064-007-9372-3. [DOI] [PubMed] [Google Scholar]
- 12.Bazinet RP, Weis MT, Rapoport SI, et al. Valproic acid selectively inhibits conversion of arachidonic acid to arachidonoyl-CoA by brain microsomal long-chain fatty acyl-CoA synthetases: relevance to bipolar disorder. Psychopharmacology (Berl) 2006;184:122–9. doi: 10.1007/s00213-005-0272-4. [DOI] [PubMed] [Google Scholar]
- 13.Bosetti F, Bell JM, Manickam P. Microarray analysis of rat brain gene expression after chronic administration of sodium valproate. Brain Res Bull. 2005;65:331–8. doi: 10.1016/j.brainresbull.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Bosetti F, Weerasinghe GR, Rosenberger TA, et al. Valproic acid down-regulates the conversion of arachidonic acid to eicosanoids via cyclooxygenase-1 and -2 in rat brain. J Neurochem. 2003;85:690–6. doi: 10.1046/j.1471-4159.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- 15.Bosisio E, Galli C, Galli G, et al. Correlation between release of free arachidonic acid and prostaglandin formation in brain cortex and cerebellum. Prostaglandins. 1976;11:773–81. doi: 10.1016/0090-6980(76)90186-6. [DOI] [PubMed] [Google Scholar]
- 16.Bowden CL. Valproate. Bipolar Disord. 2003;5:189–202. doi: 10.1034/j.1399-5618.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 17.Bowden CL, Karren NU. Anticonvulsants in bipolar disorder. Aust N Z J Psychiatry. 2006;40:386–93. doi: 10.1080/j.1440-1614.2006.01815.x. [DOI] [PubMed] [Google Scholar]
- 18.Bown CD, Wang JF, Young LT. Attenuation of N-methyl-D-aspartate-mediated cytoplasmic vacuolization in primary rat hippocampal neurons by mood stabilizers. Neuroscience. 2003;117:949–55. doi: 10.1016/s0306-4522(02)00743-1. [DOI] [PubMed] [Google Scholar]
- 19.Bymaster FP, Felder CC. Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Mol Psychiatry. 2002;7(suppl 1):S57–S63. doi: 10.1038/sj.mp.4001019. [DOI] [PubMed] [Google Scholar]
- 20.Caldeira MV, Melo CV, Pereira DB, et al. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–19. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Carli M, Afkhami-Dastjerdian S, Reader TA. Effects of a chronic lithium treatment on cortical serotonin uptake sites and 5-HT1A receptors. Neurochem Res. 1997;22:427–35. doi: 10.1023/a:1027355626355. [DOI] [PubMed] [Google Scholar]
- 22.Chang MC, Contreras MA, Rosenberger TA, et al. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. Journal of Neurochemistry. 2001;77:796–803. doi: 10.1046/j.1471-4159.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Zeng WZ, Yuan PX, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 24.Cipriani A, Rendell JM, Geddes JR. Haloperidol alone or in combination for acute mania. Cochrane Database Syst Rev. 2006;3:CD004362. doi: 10.1002/14651858.CD004362.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 8. Oxford University Press; Oxford: 2003. [Google Scholar]
- 26.DeGeorge JJ, Noronha JG, Bell JM, et al. Intravenous injection of [1-14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J Neurosci Res. 1989;24:413–423. doi: 10.1002/jnr.490240311. [DOI] [PubMed] [Google Scholar]
- 27.Delaney SM, Geiger JD. Enhancement of NMDA-induced increases in levels of endogenous adenosine by adenosine deaminase and adenosine transport inhibition in rat striatum. Brain Res. 1995;702:72–6. doi: 10.1016/0006-8993(95)01010-9. [DOI] [PubMed] [Google Scholar]
- 28.DeMar JC, Jr, Lee HJ, Ma K, et al. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochimica et Biophysica Acta. 2006;1761:1050–9. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Detich N, Bovenzi V, Szyf M. Valproate induces replication-independent active DNA demethylation. J Biol Chem. 2003;278:27586–92. doi: 10.1074/jbc.M303740200. [DOI] [PubMed] [Google Scholar]
- 30.Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem Res. 1997;22:759–65. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- 31.Dong E, Guidotti A, Grayson DR, et al. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci U S A. 2007;104:4676–81. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du J, Gray NA, Falke CA, et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–89. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumuis A, Sebben M, Haynes L, et al. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988;336:68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- 34.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 35.Fountoulakis KN, Vieta E, Siamouli M, et al. Treatment of bipolar disorder: a complex treatment for a multi-faceted disorder. Ann Gen Psychiatry. 2007;6:27. doi: 10.1186/1744-859X-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey BN, Andreazza AC, Cereser KM, et al. Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci. 2006;79:281–6. doi: 10.1016/j.lfs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Gean PW, Huang CC, Hung CR, et al. Valproic acid suppresses the synaptic response mediated by the NMDA receptors in rat amygdalar slices. Brain Res Bull. 1994;33:333–6. doi: 10.1016/0361-9230(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 38.Gobbi G, Janiri L. Sodium- and magnesium-valproate in vivo modulate glutamatergic and GABAergic synapses in the medial prefrontal cortex. Psychopharmacology (Berl) 2006;185:255–62. doi: 10.1007/s00213-006-0317-3. [DOI] [PubMed] [Google Scholar]
- 39.Goodnick PJ. Anticonvulsants in the treatment of bipolar mania. Expert Opin Pharmacother. 2006;7:401–10. doi: 10.1517/14656566.7.4.401. [DOI] [PubMed] [Google Scholar]
- 40.Groc L, Choquet D, Stephenson FA, et al. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–75. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Hassel B, Iversen EG, Gjerstad L, et al. Up-regulation of hippocampal glutamate transport during chronic treatment with sodium valproate. J Neurochem. 2001;77:1285–92. doi: 10.1046/j.1471-4159.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- 43.Johannessen CU. Mechanisms of action of valproate: a commentatory. Neurochem Int. 2000;37:103–10. doi: 10.1016/s0197-0186(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 44.Kábová R, Liptáková S, Slamberová R, et al. Age-specific N-methyl-D-aspartate-induced seizures: perspectives for the West syndrome model. Epilepsia. 1999;40:1357–69. doi: 10.1111/j.1528-1157.1999.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 45.Kanai H, Sawa A, Chen RW, et al. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J. 2004;4:336–44. doi: 10.1038/sj.tpj.6500269. [DOI] [PubMed] [Google Scholar]
- 46.Kim DK, Rordorf G, Nemenoff RA, et al. Glutamate stably enhances the activity of two cytosolic forms of phospholipase A2 in brain cortical cultures. Biochem J. 1995;310(Pt 1):83–90. doi: 10.1042/bj3100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko GY, Brown-Croyts LM, Teyler TJ. The effects of anticonvulsant drugs on NMDA-EPSP, AMPA-EPSP, and GABA-IPSP in the rat hippocampus. Brain Res Bull. 1997;42:297–302. doi: 10.1016/s0361-9230(96)00268-7. [DOI] [PubMed] [Google Scholar]
- 48.Kunig G, Niedermeyer B, Deckert J, et al. Inhibition of [3H]alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid [AMPA] binding by the anticonvulsant valproate in clinically relevant concentrations: an autoradiographic investigation in human hippocampus. Epilepsy Res. 1998;31:153–7. doi: 10.1016/s0920-1211(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 49.Lambert RC, Bessaih T, Leresche N. Modulation of neuronal T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:611–27. doi: 10.2174/187152706779025544. [DOI] [PubMed] [Google Scholar]
- 50.Lan MJ, McLoughlin GA, Griffin JL, et al. Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002130. [DOI] [PubMed] [Google Scholar]
- 51.Lazarewicz JW, Saliñska E, Stafiej A, et al. NMDA receptors and nitric oxide regulate prostaglandin D2 synthesis in the rabbit hippocampus in vivo. Acta Neurobiol Exp (Wars) 2000;60:427–35. doi: 10.55782/ane-2000-1362. [DOI] [PubMed] [Google Scholar]
- 52.Lazarewicz JW, Wroblewski JT, Palmer ME, et al. Activation of N-methyl-D-aspartate-sensitive glutamate receptors stimulates arachidonic acid release in primary cultures of cerebellar granule cells. Neuropharmacology. 1988;27:765–9. doi: 10.1016/0028-3908(88)90088-3. [DOI] [PubMed] [Google Scholar]
- 53.Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. J Biol Chem. 1997;272:12107–15. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- 54.Lerea LS, Carlson NG, Simonato M, et al. Prostaglandin F2a is required for NMDA receptor-mediated induction of c-fos mRNA in dentate gyrus neurons. J Neurosci. 1997;17:117–24. doi: 10.1523/JNEUROSCI.17-01-00117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li R, Wing LL, Wyatt RJ, et al. Effects of haloperidol, lithium, and valproate on phosphoinositide turnover in rat brain. Pharmacol Biochem Behav. 1993;46:323–329. doi: 10.1016/0091-3057(93)90360-6. [DOI] [PubMed] [Google Scholar]
- 56.Mahmood T, Silverstone T. Serotonin and bipolar disorder. J Affect Disord. 2001;66:1–11. doi: 10.1016/s0165-0327(00)00226-3. [DOI] [PubMed] [Google Scholar]
- 57.Martin ED, Pozo MA. Valproate reduced excitatory postsynaptic currents in hippocampal CA1 pyramidal neurons. Neuropharmacology. 2004;46:555–61. doi: 10.1016/j.neuropharm.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Mater MK, Thelen AP, Jump DB. Arachidonic acid and PGE2 regulation of hepatic lipogenic gene expression. J Lipid Res. 1999;40:1045–52. [PubMed] [Google Scholar]
- 59.McCullumsmith RE, Kristiansen LV, Beneyto M, et al. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. 2007;1127:108–18. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy DL, Brodie HK, Goodwin FK, et al. Regular induction of hypomania by L-dopa in “bipolar” manic-depressive patients. Nature. 1971;229:135–6. doi: 10.1038/229135a0. [DOI] [PubMed] [Google Scholar]
- 61.Muzina DJ, Elhaj O, Gajwani P, et al. Lamotrigine and antiepileptic drugs as mood stabilizers in bipolar disorder. Acta Psychiatr Neurol Scand. 2005;(Suppl):21–8. doi: 10.1111/j.1600-0447.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- 62.Nasrallah HA, Ketter TA, Kalali AH. Carbamazepine and valproate for the treatment of bipolar disorder: a review of the literature. J Affect Disord. 2006;95:69–78. doi: 10.1016/j.jad.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 63.O’Donnell T, Rotzinger S, Ulrich M, et al. Effects of chronic lithium and sodium valproate on concentrations of brain amino acids. Eur Neuropsychopharmacol. 2003;13:220–7. doi: 10.1016/s0924-977x(03)00070-1. [DOI] [PubMed] [Google Scholar]
- 64.Okada S, Murakami Y, Nishihara M, et al. Perfusion of the hypothalamic paraventricular nucleus with N-methyl-D-aspartate produces thromboxane A2 and centrally activates adrenomedullary outflow in rats. Neuroscience. 2000;96:585–90. doi: 10.1016/s0306-4522(99)00598-9. [DOI] [PubMed] [Google Scholar]
- 65.Ormandy GC, Song L, Jope RS. Analysis of the convulsant-potentiating effects of lithium in rats. Exp Neurol. 1991;111:356–361. doi: 10.1016/0014-4886(91)90103-j. [DOI] [PubMed] [Google Scholar]
- 66.Pal R, Eaton MJ, Islam S, et al. Immunocytochemical and in situ hybridization studies of the expression and distribution of three subunits of a complex with N-methyl-D-aspartate receptor-like properties. Neuroscience. 1999;94:1291–311. doi: 10.1016/s0306-4522(99)00386-3. [DOI] [PubMed] [Google Scholar]
- 67.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. Academic Press; New York: 1987. [DOI] [PubMed] [Google Scholar]
- 68.Peet M, Peters S. Drug-induced mania. Drug Saf. 1995;12:146–53. doi: 10.2165/00002018-199512020-00007. [DOI] [PubMed] [Google Scholar]
- 69.Pepicelli O, Fedele E, Bonanno G, et al. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E2 extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. J Neurochem. 2002;81:1028–34. doi: 10.1046/j.1471-4159.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- 70.Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 71.Post RM, Jimerson DC, Bunney WE, Jr, et al. Dopamine and mania: behavioral and biochemical effects of the dopamine receptor blocker pimozide. Psychopharmacology (Berl) 1980;67:297–305. doi: 10.1007/BF00431272. [DOI] [PubMed] [Google Scholar]
- 72.Qiang M, Ticku MK. Role of AP-1 in ethanol-induced N-methyl-D-aspartate receptor 2B subunit gene up-regulation in mouse cortical neurons. J Neurochem. 2005;95:1332–41. doi: 10.1111/j.1471-4159.2005.03464.x. [DOI] [PubMed] [Google Scholar]
- 73.Rao JS, Bazinet RP, Rapoport SI, et al. Chronic treatment of rats with sodium valproate downregulates frontal cortex NF-κ B DNA binding activity and COX-2 mRNA. Bipolar Disord. 2007;9:513–20. doi: 10.1111/j.1399-5618.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- 74.Rapoport SI. In vivo fatty acid incorporation into brain phospholipids in relation to plasma availability, signal transduction and membrane remodeling. J Mol Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. [DOI] [PubMed] [Google Scholar]
- 75.Rapoport SI, Chang MCJ, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- 76.Robinson PJ, Noronha J, DeGeorge JJ, et al. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: Review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- 77.Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- 78.Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry. 2005;58:879–84. doi: 10.1016/j.biopsych.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 79.Shen Y, Kishimoto K, Linden DJ, et al. Cytosolic phospholipase A2 alpha mediates electrophysiologic responses of hippocampal pyramidal neurons to neurotoxic NMDA treatment. Proc Natl Acad Sci U S A. 2007;104:6078–83. doi: 10.1073/pnas.0605427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sonnenberg JL, Mitchelmore C, Macgregor-Leon PF, et al. Glutamate receptor agonists increase the expression of Fos, Fra, and AP-1 DNA binding activity in the mammalian brain. J Neurosci Res. 1989;24:72–80. doi: 10.1002/jnr.490240111. [DOI] [PubMed] [Google Scholar]
- 81.Stefanovic B, Bosetti F, Silva AC. Modulatory role of cyclooxygenase-2 in cerebrovascular coupling. Neuroimage. 2006;32:23–32. doi: 10.1016/j.neuroimage.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 82.Steppuhn KG, Turski L. Modulation of the seizure threshold for excitatory amino acids in mice by antiepileptic drugs and chemoconvulsants. J Pharmacol Exp Ther. 1993;265:1063–70. [PubMed] [Google Scholar]
- 83.Stout SC, Owens MJ, Lindsey KP, et al. Effects of sodium valproate on corticotropin-releasing factor systems in rat brain. Neuropsychopharmacology. 2001;24:624–31. doi: 10.1016/S0893-133X(00)00243-8. [DOI] [PubMed] [Google Scholar]
- 84.Szupera Z, Mezei Z, Kis B, et al. The effects of valproate on the arachidonic acid metabolism of rat brain microvessels and of platelets. Eur J Pharmacol. 2000;387:205–10. doi: 10.1016/s0014-2999(99)00764-5. [DOI] [PubMed] [Google Scholar]
- 85.Tabachnick BG, Fidell LS. Computer-assisted research design and analysis. Boston: Allyn and Bacon; 2001. [Google Scholar]
- 86.Tanaka J, Miyakubo H, Kawakami A, et al. Involvement of NMDA receptor mechanisms in the modulation of serotonin release in the lateral parabrachial nucleus in the rat. Brain Res Bull. 2006;71:311–5. doi: 10.1016/j.brainresbull.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 87.Tapia-Arancibia L, Rage F, Recasens M, et al. NMDA receptor activation stimulates phospholipase A2 and somatostatin release from rat cortical neurons in primary cultures. Eur J Pharmacol. 1992;225:253–62. doi: 10.1016/0922-4106(92)90027-s. [DOI] [PubMed] [Google Scholar]
- 88.Teng CT, Demetrio FN. Memantine may acutely improve cognition and have a mood stabilizing effect in treatment-resistant bipolar disorder. Rev Bras Psiquiatr. 2006;28:252–4. doi: 10.1590/s1516-44462006000300020. [DOI] [PubMed] [Google Scholar]
- 89.Thurston JH, Hauhart RE. Valproate doubles the anoxic survival time of normal developing mice: possible relevance to valproate-induced decreases in cerebral levels of glutamate and aspartate, and increases in taurine. Life Sci. 1989;45:59–62. doi: 10.1016/0024-3205(89)90435-9. [DOI] [PubMed] [Google Scholar]
- 90.Toro C, Deakin JF. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–30. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 91.Turski L, Niemann W, Stephens DN. Differential effects of antiepileptic drugs and beta-carbolines on seizures induced by excitatory amino acids. Neuroscience. 1990;39:799–807. doi: 10.1016/0306-4522(90)90262-3. [DOI] [PubMed] [Google Scholar]
- 92.Ueda Y, Willmore LJ. Molecular regulation of glutamate and GABA transporter proteins by valproic acid in rat hippocampus during epileptogenesis. Exp Brain Res. 2000;133:334–9. doi: 10.1007/s002210000443. [DOI] [PubMed] [Google Scholar]
- 93.Weichel O, Hilgert M, Chatterjee SS, et al. Bilobalide, a constituent of Ginkgo biloba, inhibits NMDA-induced phospholipase A2 activation and phospholipid breakdown in rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:609–15. doi: 10.1007/s002109900131. [DOI] [PubMed] [Google Scholar]
- 94.Wu Y, Wang L. The effects of antiepileptic drugs on spatial learning and hippocampal protein kinase C in immature rats. Brain Dev. 2002;24:82–7. doi: 10.1016/s0387-7604(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 95.Young AM, Bradford HF. N-methyl-D-aspartate releases gamma-aminobutyric acid from rat striatum in vivo: a microdialysis study using a novel preloading method. J Neurochem. 1993;60:487–92. doi: 10.1111/j.1471-4159.1993.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 96.Zeise ML, Kasparow S, Zieglgansberger W. Valproate suppresses N-methyl-D-aspartate-evoked, transient depolarizations in the rat neocortex in vitro. Brain Res. 1991;544:345–8. doi: 10.1016/0006-8993(91)90078-a. [DOI] [PubMed] [Google Scholar]