Abstract
The generation of protective CD8 T-cell memory against tumor-expressed self-antigens is an important but elusive goal of cancer immunotherapy. The possibility that a progressive, poorly immunogenic tumor can induce T-cell memory against self-antigens has not previously been studied. Herein, we report that growth of the poorly immunogenic B16 melanoma in the absence of regulatory T cells (Treg) generates CD8 T-cell responses that develop into functional memory after the tumor has been surgically excised. Tumor-primed memory T cells recognized melanocyte differentiation antigens TRP-2/DCT and gp100 and persisted for as long as 5 months following surgical tumor excision. Phenotypic analysis showed that these cells develop into both central and effector memory T-cell subsets, which produce IFN-γ and interleukin-2 on reencounter with antigen. Most importantly, tumor-primed memory T cells mediated the rejection of intradermal and systemically disseminated challenge tumors given 30 to 60 days following surgery. Tumor-excised mice also developed autoimmune vitiligo, showing that Treg cells prevent tissue-specific autoimmunity in tumor-bearing hosts. This study establishes that Treg depletion in tumor-bearing hosts drives the natural development of protective T-cell memory. Generating such responses may aid in the clinical management of tumor recurrence and metastasis following surgery.
Introduction
Surgery is currently the leading cure for solid tumors, making the prevention of tumor recurrence and metastasis following surgery a vitally important goal. It was shown ~50 years ago that highly immunogenic tumors can spontaneously prime protective immunity, thus preventing the growth of the same tumor given after surgery, a phenomenon known as postsurgical (or postexcisional) tumor immunity (1). Studies in the 1980s further suggested that postsurgical immunity against highly immunogenic tumors is accompanied by protective T-cell memory (2). The generation of T-cell memory against tumor antigens may be a key to generating durable long-lived tumor protection (3). However, poorly immunogenic tumors do not spontaneously induce postsurgical tumor immunity (1) nor do they prime functional CD8 T-cell responses (4). Unfortunately, such tumors may more closely model cancers in humans, where antitumor T-cell responses are often detected but do not control tumor progression (5, 6).
The generation of functional T-cell responses in tumor-bearing hosts is tightly regulated by populations of naturally occurring and tumor-induced CD4+CD25+ regulatory T cells (Treg; ref. 7). We have previously shown that depletion of Treg cells during growth of the poorly immunogenic B16 melanoma enables the de novo priming of short-term tumor/self-antigen–specific CD8 T-cell responses (4). Importantly, these tumor-primed T cells mediated the rejection of the same tumor growing at a distal location in the same host, a phenomenon known as concomitant tumor immunity (4). However, whether these T-cell responses could be maintained after surgical excision of the primary tumor remained unknown.
One possibility was that the tumor-primed T-cell population would decline following surgery as a result of tumor antigen removal and/or due to the return of naturally occurring Treg cells. On the other hand, tumor-primed effectors could develop into functional memory and provide long-term postsurgical tumor protection. This latter hypothesis is supported by recent literature showing that Treg depletion enhances T-cell memory against foreign antigens (8, 9) as well as reports that vaccines can induce T-cell memory against tumor-expressed self-antigens (10–12). In contrast to vaccines, the growth of poorly immunogenic tumors has not been shown to drive the formation of functional T-cell memory against tumor antigens.
The present work addresses whether growth of the poorly immunogenic B16 melanoma is capable of inducing T-cell memory. Our results establish that tumor growth in the absence of Treg cells primes CD8 T cells, which are specific for tumor/self-antigens and develop into functional memory after surgical excision of the primary tumor. Findings described herein may have important implications for the design of immunotherapies that enhance the efficacy of surgical treatments for cancer.
Materials and Methods
Mice and tumors
Animal procedures were done in accordance with institutional animal care and use guidelines at Dartmouth College under an approved protocol. C57BL/6 mice (males, 6–10 weeks old) were obtained from The Jackson Laboratory. Homozygous pmel-1 Thy1.1+ mice, a gift from Nicholas Restifo and Doug Palmer (National Cancer Institute, Bethesda, MD), were provided through The Jackson Laboratory, bred at Dartmouth, and used at 6 to 10 weeks of age [pmel-1 mice express a transgenic T-cell receptor (TCR) that recognizes the mouse gp10025-33 peptide presented by H-2Db (13)].
B16 melanoma was selected for this study because it is an aggressive and poorly immunogenic tumor of spontaneous origin, which expresses very low levels of MHC molecules (14). The B16-F10 subline used in these experiments is nonmetastatic and grows as a well-encapsulated i.d. tumor. This cell line was originally obtained from Isaiah Fidler (M. D. Anderson Cancer Center, Houston, TX) and passaged i.d. in C57BL/6 mice five times to ensure reproducible tumor growth (hereafter referred to as B16). B16 cells were cultured in RPMI 1640 containing 7.5% fetal bovine serum, harvested after limited passage in vitro, and used only if viability exceeded 96%. All tumors were generated by inoculation of 1.0 × 105 to 1.2 × 105 live B16 cells into C57BL/6 mice. Primary tumors were inoculated i.d. on the right flank, and tumor diameters were measured with calipers thrice weekly. Challenge tumors were inoculated either i.d. on the left flank or i.v. through the tail vein (for lung metastases). Pigmented surface lung metastases were counted by eye using a dissection microscope.
Monoclonal antibody treatments
Hybridoma cell lines were obtained from the American Type Culture Collection (ATCC). All depleting antibodies were obtained from bioreactor supernatants, and treatments were given by i.p. injection. To deplete CD4 T cells, mice received 250 μg of clone GK1.5 monoclonal antibody (mAb; anti-CD4). To deplete CD8 T cells, mice received 250 μg of clone 2.43 mAb (anti-CD8). Flow cytometry was used to confirm depletion of >99% of target cells for at least 7 days following injection. To block CD25+ cell function, mice received 250 μg of clone PC61 mAb (anti-CD25).
Surgical tumor excision
I.d. B16 primary tumors were surgically excised on day 12 of growth at a size of approximately 5 to 10 mm diameter. Mice were anesthetized with isoflurane and tumors were removed with a 2-mm perimeter of healthy skin. Incisions were closed with steel wound clips, and mice were given 0.1 mg buprenorphine for pain. Less than 5% of tumors recurred following surgery, and mice with recurrent primary tumors were removed from the study.
Peptides and enzyme-linked immunospot assay
Peptides (>80% purity) were obtained from New England Peptide, Inc. Mouse tyrosinase-related protein 2/dopachrome tautomerase TRP-2/DCT180-188 peptide (SVYDFFVWL) is restricted by Kb (15). Mouse gp100/pmel 17 peptide gp10025-33 (EGSRNQDWL) is restricted by Db (16). The Kb-restricted epitope SIINFEKL from egg ovalbumin (ova257-264) was used as an irrelevant control.
IFN-γ enzyme-linked immunospot (ELISPOT; Mabtech) was done according to procedures described previously (4). Briefly, CD8 effector T cells were harvested from spleens or inguinal lymph nodes of mice with postsurgical immunity, purified using anti-CD8 MACS magnetic beads (Miltenyi Biotec), and plated at 2 × 105 per well. Naive splenocytes were used as a negative control. For antigen presentation, 2 × 104 irradiated B16 cells, or EL-4 leukemia cells (ATCC) that had been pulsed with 10 μg/mL peptide, were added to each well. Plates were incubated for 20 h at 37°C and then developed with aminoethylcarbazole chromogen. Spots were counted with an Automated ELISPOT Reader System with KS 4.3 software (Carl Zeiss).
Adoptive transfer and monitoring of gp100-specific pmel T cells
gp10025-33-specific CD8+ pmel cells were isolated from the combined lymph nodes and spleens of naive pmel-1 donor mice by positive selection using MACS CD8 magnetic beads. Greater than 95% of the CD8 T cells expressed the transgenic TCR Vβ13. T cells were immediately transferred i.v. (1 × 104 per mouse) into naive C57BL/6 recipients. Beginning 1 day after adoptive transfer, mice received tumors followed by anti-CD4 and surgery as described above.
Flow cytometry was used to detect Thy1.1+ pmel cells in tissues at various time points following surgery. Individual spleen and lymph node samples were harvested and mechanically dissociated. Lung samples were subjected to brief collagenase and liberase digestion followed by Percoll gradient centrifugation to isolate lymphocytes. Samples were stained with anti-CD8-PerCP (BD Biosciences), anti-Thy1.1-PE (eBioscience), anti-CD44-FITC (eBioscience), and anti-CD62L-PE-Cy7 (eBioscience). Flow cytometry was done on a BD FACSCanto, and data were analyzed using FlowJo software version 6.3.3.
Intracellular cytokine staining
Lymphocyte samples from spleens, lymph nodes, and lungs were aliquoted into 96-well plates, and mouse gp10025-33 or ova peptide was added to each well to a final concentration of 1 μg/mL. Interleukin-2 (IL-2; 10 units/mL) and brefeldin A (10 μg/mL) were added immediately, and cells were incubated for 5 h at 37°C. Cells were then washed and stained with anti-CD8-PerCP, anti-Thy1.1-APC, and anti-CD44-FITC. Finally, cells were fixed, permeabilized, and stained intracellularly with PE-conjugated antibodies to either IFN-γ or IL-2 (BD Biosciences).
Statistical calculations
To determine significance of differences in tumor-free survival between different groups of mice, log-rank analyses (comparisons pooled over strata) of Kaplan-Meier data were conducted using Statistical Package for the Social Sciences 12.0.1 software for Windows. Statistical differences between numbers of lung metastases, numbers of spots in the ELISPOT assay, or proportions of cells in flow cytometry were determined by two-tailed Student’s t test.
Results
Growth of a poorly immunogenic tumor in the absence of Treg cells induces systemic postsurgical tumor immunity
To determine if Treg depletion could induce postsurgical immunity, mice were inoculated with B16 primary tumors i.d. on the right flank, and then Treg cells were depleted by systemic administration of anti-CD4 beginning 4 days after tumor inoculation. We have previously shown that anti-CD4 treatment depletes CD4+CD25+ Treg cells but does not cause significant alteration in the growth of B16 primary tumors (4). To monitor postsurgical immunity, primary tumors were surgically excised on day 12, and mice were challenged with secondary tumors on the opposite flank 1 day later (Fig. 1A). This timing was chosen because we have previously found that CD4 T-cell depletion induces CD8 T-cell priming by day 12 of B16 tumor growth (4).
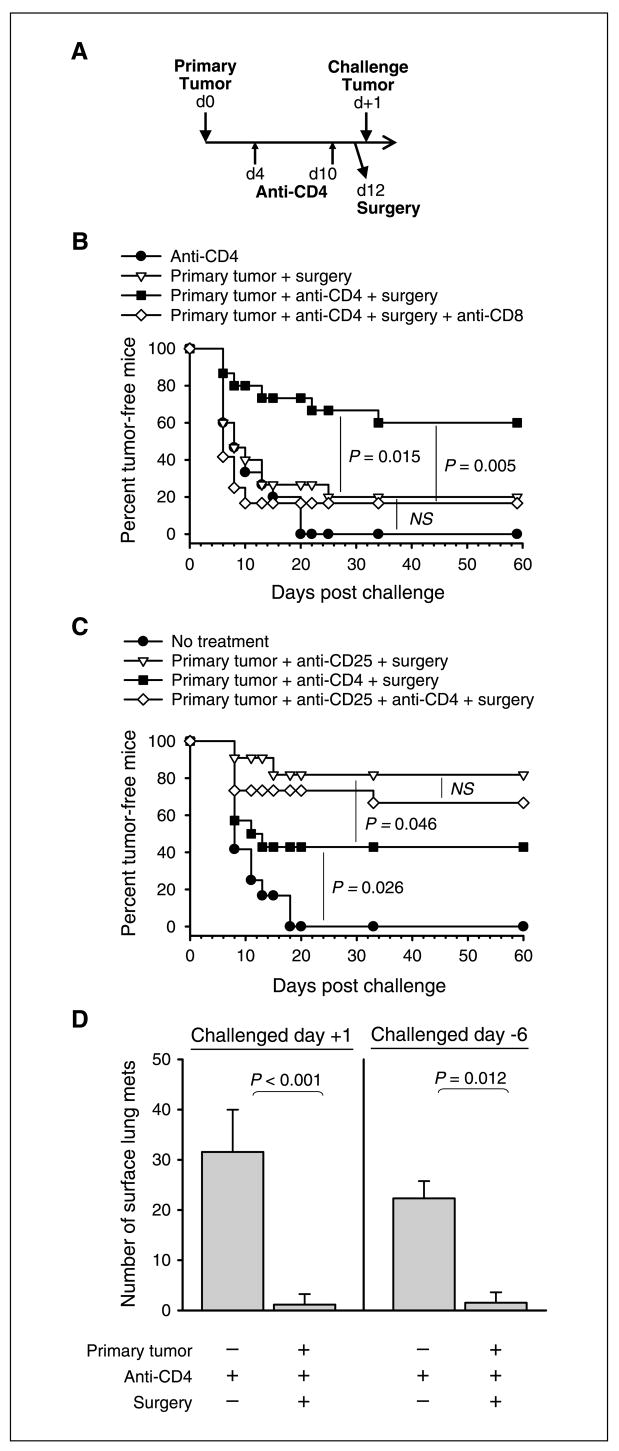
Figure 1.
Postsurgical immunity is prevented by CD4+CD25+ Treg cells and mediated by CD8 T cells. A, basic schematic outline of experiments: mice received primary B16 tumors in the right flank on day 0, anti-CD4 treatment on days 4 and 10, and surgery to remove primary tumors on day 12. Growth of challenge tumors. B, mice received treatments as specified in the figure and challenge tumors were given in the left flank 1 day after surgery. Anti-CD8 was also given in one group on days −2, +5, and +12 relative to the challenge tumor. C, mice received treatments as specified in the figure and challenge tumors were given in the left flank 1 d after surgery. Indicated groups also received anti-CD25 treatment on day −4 relative to the primary tumor. D, mice received treatments as specified below each bar, and challenge tumors were injected i.v. either 1 d following surgery (left) or 6 d before surgery (right). These mice were sacrificed ~22 d following challenge and pigmented surface lung metastases were counted. A to D, each experiment involved 10 to 16 mice per group and was conducted at least twice with similar results. Statistical significance was determined by log-rank analysis (B and C) or two-tailed Student’s t test (D). D, columns, average; bars, SD. NS, no significance (P > 0.05).
Indeed, 60% of the mice that had received primary tumors, anti-CD4, and surgery rejected challenge tumors given after surgery (Fig. 1B). The extent of protection varied, ranging from 40% to 80% tumor-free survival in five independent experiments (one representative experiment is shown). In contrast, no significant tumor protection was observed in mice that received only anti-CD4 (Fig. 1B) or anti-CD4 in conjunction with sham surgery.1 Similarly, no protection was observed in mice that received primary tumors and surgery alone, confirming that B16 tumors do not spontaneously prime postsurgical immunity (Fig. 1B). To determine if CD8 T cells were mediating postsurgical tumor immunity, tumor-primed mice were depleted of CD8 T cells beginning 2 days before tumor challenge. CD8 depletion significantly reduced protection in these mice (Fig. 1B). These data collectively show that postsurgical immunity against B16 melanoma is prevented by CD4 T cells and mediated by CD8 T cells.
To determine if CD4+CD25+ Treg cells were the suppressors of priming, mice were alternatively treated with anti-CD25 to specifically block Treg function (17). In contrast to anti-CD4, anti-CD25 was given prophylactically (4 days before primary tumor inoculation) to avoid the inhibition or depletion of activated CD25+ CD8 effectors. Prophylactic treatment with anti-CD25 induced postsurgical tumor protection in 80% of mice, which was greater than protection induced by therapeutic administration of anti-CD4 in the same experiment (Fig. 1C). However, mice cotreated with anti-CD25 and anti-CD4 showed similar protection compared with mice treated with anti-CD25 alone (Fig. 1C). These data suggest that temporary depletion of CD4 T cells (including helper T cells) does not impair the priming of postsurgical immunity. Importantly, the ability of either anti-CD4 or anti-CD25 to induce postsurgical immunity implicates CD4+CD25+ Treg cells as the suppressors of priming. Anti-CD4 was selected for subsequent experiments because of its effectiveness when given therapeutically.
For postsurgical immunity to be clinically relevant, hosts must be protected against systemically disseminated metastases that are already growing at the time of surgery. To model this, tumor-bearing mice were treated therapeutically with anti-CD4 and surgery (Fig. 1A), and then challenge tumors were given i.v. to generate lung metastases either 1 day after surgery or 6 days before surgery. These mice were protected regardless of whether lung tumors began growing after surgery (Fig. 1D, left) or were already established at the time of surgery (Fig. 1D, right). Therefore, it can be concluded that growth of an i.d. primary tumor, combined with therapeutic Treg depletion, can prevent the growth of systemically disseminated secondary tumors.
Mice with postsurgical immunity develop central and effector memory T-cell responses against tumor-expressed self-antigens
We have previously shown that Treg-depleted, B16 tumor-bearing mice naturally develop short-term CD8 T-cell responses against melanocyte differentiation antigens (4). However, the development of T-cell memory in tumor-bearing hosts has not been studied. To follow putative memory development following surgery, mice were again primed by tumor inoculation, anti-CD4 treatment, and surgery (Fig. 1A), and then CD8 T-cell responses were analyzed either 9 or 30 days following surgery using the IFN-γ ELISPOT assay. Nine days after surgery, significant responses to the mouse Kb-restricted TRP-2/DCT180-188 epitope, as well as whole B16 melanoma cells, were detected in tumor-draining lymph nodes, contralateral lymph nodes, and spleens (Fig. 2A). In lymph nodes, a small but significant proportion of CD8 T cells also responded to the mouse Db-restricted gp10025-33 epitope.
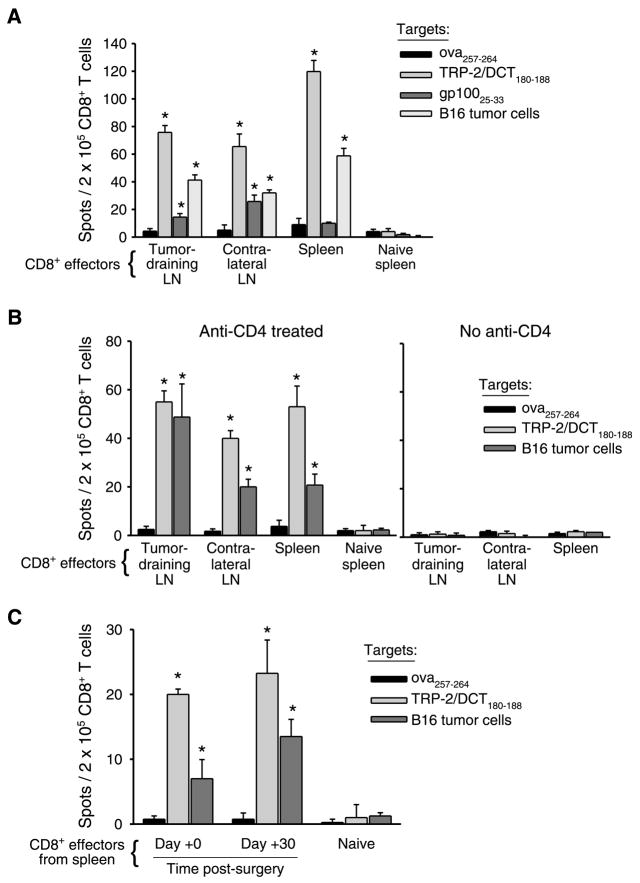
Figure 2.
Functional T-cell responses to melanocyte differentiation antigen TRP-2/DCT are maintained systemically 1 mo following surgery. Mice (eight per group, pooled) received primary tumors, anti-CD4 treatment, and surgery as described in Fig. 1A, but no challenge tumors were given. IFN-γ ELISPOT was done on purified CD8 T cells taken from lymph nodes (LN) and/or spleens either (A) 9 d following surgery, (B) 30 d following surgery (with or without anti-CD4 treatment), or (C) immediately before surgery (0 day) versus 30 d following surgery (spleens only). Naive splenocytes were used as a negative control in each ELISPOT. As specified in each panel, either EL-4 cells pulsed with the specified MHC I–restricted peptide epitopes or irradiated B16 cells were used as targets in the assays. Columns, average of four replicate wells per sample; bars, SD. *, P < 0.05, compared with the responses to irrelevant ova peptide. Each ELISPOT was conducted at least twice with similar results.
Surprisingly, 30 days following surgery, responses to TRP-2/DCT and B16 cells remained significant in both lymph nodes and spleens (Fig. 2B, left). Responses to gp100 were no longer detectable. No response was observed in mice that only received primary tumors and surgery, confirming that CD4 T-cell depletion was necessary for the induction of long-lived T-cell responses (Fig. 2B, right). We also directly compared responses in the spleen immediately before surgery (0 days) and 30 days following surgery. Notably, the number of responding CD8 T cells was similar at both time points (Fig. 2C). Thus, tumor-specific CD8 T cells developed into memory and did not contract within the month following surgery.
ELISPOT analyses indicated that the frequency of endogenous, epitope-specific memory T cells was very low [<0.06% of CD8 T cells (Fig. 2)]. Therefore, a more reliable phenotypic analysis of memory T cells was conducted using transgenic T cells from pmel-1 mice, which are specific for the mouse gp10025-33 epitope (13). Because T-cell responses against gp10025-33 develop naturally in mice with postsurgical immunity (Fig. 2A; ref. 4), the response of naive pmel cells is expected to model endogenous CD8 T-cell responses to tumor. To avoid artifacts due to high T-cell precursor frequencies (18), mice were adoptively transferred with only 10,000 naive pmel cells, which can be estimated to seed ~1,000 precursors in the periphery (19).
Following adoptive transfer, mice received primary tumors, anti-CD4, and surgery to prime postsurgical immunity, and then pmel responses were monitored at various time points after surgery using the congenic marker Thy1.1 (Fig. 3A). One day after surgery, pmel cells (CD8+Thy1.1+) were not detected in lymph nodes or spleens of mice that received primary tumors and surgery alone, but a small population expanded in tumor-draining lymph nodes (Fig. 3B). CD4 depletion in the absence of a primary tumor failed to induce any detectable expansion of pmel cells (Fig. 3B). In contrast, mice that received a combination of primary tumors, CD4 depletion, and surgery generated significant populations of CD44hi (antigen experienced) pmel cells in all tissues analyzed (Fig. 3B). These data confirm that tumor growth in the absence of CD4 T cells induces systemic priming of CD8 T cells specific for tumor/self-antigens.
Figure 3.
Memory T cells are generated and maintained in lymphoid and peripheral tissues of mice with postsurgical immunity. A, schematic diagram of experiments: mice received adoptive transfer of 104 CD8+Thy1.1+ pmel cells on day −1, primary B16 tumors on day 0, anti-CD4 treatment on days 4 and 10, and surgery on day 12. Flow cytometry was done at various times following surgery. B, mice that had received adoptive transfer were treated as described in the figure and tissues were analyzed 1 d following surgery. C, mice that had received adoptive transfer were treated as described in the figure and tissues were analyzed 30, 60, or 150 d following surgery. X axis, percentages of pmel cells (CD8+Thy1.1+) among total CD8 T cells in a live lymphocyte gate. Point, one mouse; horizontal bars, averages. *, P < 0.05, statistically significant differences. Each experiment was conducted twice with similar results.
Thirty days following surgery, significant proportions of pmel cells were still found in lymph nodes (0.8% of CD8 T cells) and spleens (0.2% of CD8 T cells) of mice that had received primary tumors in conjunction with CD4 depletion but not in mice that had only received primary tumors and surgery (Fig. 3C). Compared with day +1 (Fig. 3B), this population had contracted normally in the spleen (90% contraction) but to a lesser extent in the lymph nodes (50% contraction). Significant numbers of pmel cells were also found in the lungs (0.6% of CD8 T cells; Fig. 3C).
Remarkably, 1 month later (60 days following surgery), pmel populations remained significant and were only slightly reduced compared with proportions on day 30 (Fig. 3C). Even as long as 150 days (5 months) following surgery, pmel cells remained detectable in two of the three mice tested, although frequencies fell to ~10% of those observed on day 60 (Fig. 3C). Together, these data show that long-lived tumor antigen-specific memory T cells develop in mice with postsurgical immunity and diminish only very gradually with time.
We also analyzed whether memory T cells had differentiated into central (TCM) and/or effector (TEM) memory T-cell subsets because recent studies have suggested that TCM are more effective at eliminating established tumors (20). TEM and TCM cells both express CD44 but can be differentiated based on the expression of CD62L (21). On days 30 and 60 after surgery, phenotypically TEM cells (CD44hiCD62Llow) represented a majority of the pmel population in lymphoid tissues; however, phenotypically TCM cells (CD44hiCD62Lhigh) also represented a discrete population, comprising 15% to 20% of pmel cells (Fig. 4A). In accordance with the expectation that only TEM localize to peripheral tissues (22), we observed predominantly CD44hiCD62Llow pmel cells in the lungs. By day 150, TEM cells still dominated the pmel T-cell population in all tissues. However, a minor TCM population remained in lymph nodes and spleens (Fig. 4A). Thus, tumor-primed memory T cells developed predominantly into TEM cells but also contained a discrete and persistent subpopulation of TCM.
Figure 4.
Tumor-primed CD8 T cells develop into functional central and effector memory subsets. Mice received adoptive transfer, B16 tumors, anti-CD4, and surgery as shown in Fig. 3A, and analysis was done 30, 60, or 150 d following surgery. A, at each time point, dot plots (left) were gated on live CD8+ cells and corresponding contour plots (right) were gated on CD44hiThy1.1+ pmel cells (as shown in figure) with the percentage of CD62Lhi cells indicated in the upper-right quadrant. Values below the lines, average percentage of CD62Lhi pmel cells ~ SD of two to seven mice per group. One representative mouse is depicted at each time point. B, analysis of cytokine production: splenocytes of individual mice were isolated at various time points following surgery and restimulated for 5 h with either irrelevant peptide (ova257-264) or mouse gp10025-33 peptide as indicated. Five hours later, cells were stained intracellularly with antibodies to IFN-γ (top row) or IL-2 (bottom row), and flow cytometry was done to detect cytokine-producing T cells. All plots are gated on live CD8+Thy1.1+ pmel cells with the percentage of cytokine-secreting cells indicated in the upper-right quadrant. Values below the lines, average number of cytokine-secreting cells ~ SD of two to five mice per group, with asterisks (*) denoting statistically significant differences (P < 0.05) between ova-and gp100-stimulated wells. One representative mouse is depicted at each time point. These experiments were conducted twice with similar results.
Because it was possible that these memory cells became nonfunctional at longer time points due to constant antigen exposure (23), we also analyzed their ability to produce cytokines. At all time points following surgery, a majority of pmel cells (60–80%) produced IFN-γ (Fig. 4B, top row) in response to in vitro peptide restimulation. Notably, a smaller but significant proportion (4–13%) produced IL-2 (Fig. 4B, bottom row), which is indicative of a functional TCM subset (24). These results illustrate that tumor-primed central and effector memory CD8 T cells maintain antigen responsiveness for several months following Treg depletion and surgery.
Tumor-primed memory CD8 T cells provide long-term protection against local and disseminated tumors
The ability to provide protective immunity is a hallmark of functional CD8 memory T cells. Despite the persistence of tumor-specific memory T cells that were capable of producing cytokines, it remained unknown whether these T cells were protective. To assess this, mice were primed with i.d. tumors, anti-CD4, and surgery (Fig. 1A) but were then rested for 30 days before tumor challenge. Indeed, rejection of challenge tumors given 30 days after surgery was observed in 45% of these mice, and protection was completely abrogated on depletion of CD8 T cells (Fig. 5A). This result establishes that tumor-primed memory CD8 T cells are protective.
Figure 5.
Tumor-primed memory T cells provide long-lived systemic tumor protection following surgery. As shown, mice either received no treatment (Un-primed) or were primed by B16 i.d. primary tumors, anti-CD4 treatment, and surgery as shown in Fig. 1A (Primed). Growth of challenge tumors. A, challenge tumors were given i.d. 30 d following surgery. One group of mice also received anti-CD8 on days −2, +5, and +12 relative to the challenge tumor inoculation. B, challenge tumors were given i.v. 1 or 30 d following surgery. C, challenge tumors were given i.d. 1 or 60 d following surgery. All experiments involved 10 to 16 mice per group, and statistical significance was determined by log-rank analysis (A and C) or two-tailed Student’s t test (B). NS, no significance (P > 0.05).
To test if protective immune responses are also maintained systemically, mice were alternatively challenged 30 days after surgery with an i.v. inoculum of tumor cells. These mice showed significant rejection of lung metastases (Fig. 5B). Interestingly, mice challenged 30 days after surgery showed similar protection compared with mice challenged only 1 day after surgery in the same experiment (Fig. 5B). This undiminished level of tumor protection correlates with our observation that splenic T-cell responses to TRP-2 and B16 cells did not decline within the month following surgery (Fig. 2C).
Experimental monitoring of pmel T-cell responses had also shown that memory T cells declined gradually within the months following surgery (Fig. 3C). To determine if tumor protection also declines with time, mice were challenged i.d. 60 days following surgery. Impressively, 45% of these mice rejected challenge tumors (Fig. 5C). This protection, although significant, was lower than in mice challenged only 1 day after surgery in the same experiment (Fig. 5C). Therefore, it can be concluded that protective immunity is maintained for at least 2 months following surgery but seems to decline gradually with time.
Treg depletion in melanoma tumor-bearing hosts induces postsurgical vitiligo
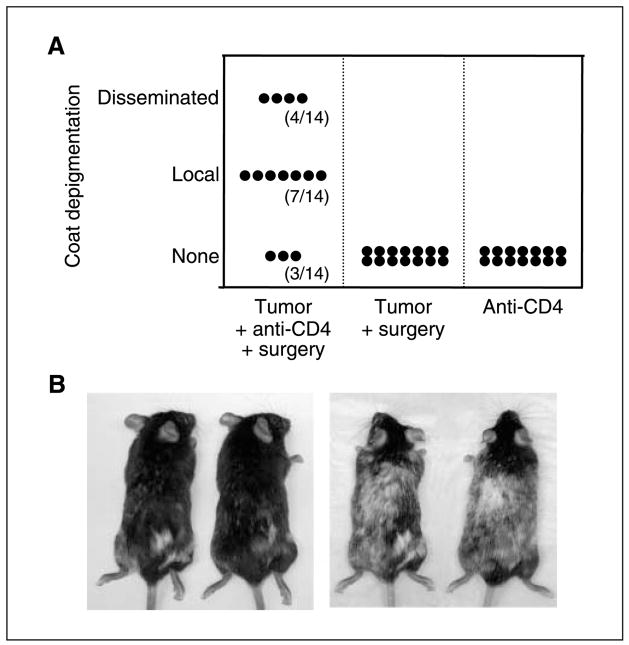
The appearance of autoimmune vitiligo has been shown to correlate with improved prognosis in animal models of melanoma (25) as well as in human patients with melanoma (26). Fifty days following surgery, we observed a surprising level of depigmentation in a majority of mice that had received primary tumors, anti-CD4, and surgery (Fig. 6A). In contrast, no depigmentation was observed in mice that had received anti-CD4 alone or primary tumors and surgery alone (Fig. 6A). Pigment loss began at the site of surgery but spread extensively to other locations in a proportion of mice (Fig. 6A and B). This result clearly shows that the growth of a poorly immunogenic tumor in the absence of Treg cells induces autoimmunity against the normal tissue counterpart of that tumor. Furthermore, the progressive and extensive destruction of host melanocytes supports our finding that mice with postsurgical immunity develop functional and systemic memory T-cell responses against antigens that are shared by melanoma cells and normal host melanocytes.
Figure 6.
Melanoma growth in the absence of Treg cells induces postsurgical autoimmunity against normal host melanocytes. A, mice (14 per group) received B16 primary tumors on the right flank (day 0) with or without anti-CD4 treatment (days 4 and 10) and surgery (day 12) as indicated on the X axis. No challenge tumor was given. Two days following surgery, mice were depilated on the left flank to induce hair regrowth at a site distal to the excised tumor. The extent of coat depigmentation was recorded 50 d following surgery (or 50 d following anti-CD4 treatment in mice that had not received surgery). Y axis, None, a completely black coat; Local, a patch of white fur on the right flank; Disseminated, a patch of white fur on the right flank in addition to white patches in other locations. B, photographs of two representative mice with local depigmentation were taken 50 d following surgery (left) and again 80 d after surgery, showing progressive dissemination of depigmentation (right). This experiment was conducted thrice with similar results.
Discussion
Generating protective T-cell memory against tumor-expressed self-antigens is a major goal in the development of cancer immunotherapies (3). Studies have shown that long-lived tumor protection can be induced through certain types of vaccination (10–12, 27) or by vaccination followed by the active rejection of a primary tumor (28, 29). However, it was previously thought that a progressively growing, poorly immunogenic tumor could not induce T-cell memory. The present study shows for the first time that unvaccinated, tumor-excised mice naturally generate memory CD8 T-cell responses if Treg cells are depleted during primary tumor growth. Treg depletion has previously been shown to enhance short-term inherent (4, 30) and vaccine-induced immune responses (29, 31, 32) against poorly immunogenic tumors. This work extends such observations to long-term immunity, illustrating that Treg depletion also drives the natural development of T-cell memory in tumor-bearing hosts.
Defining memory T-cell responses against self-antigens is not without challenges particularly because classic memory T cells have been defined based on their ability to persist in the absence of antigen (23, 33). Because self-antigens are inexorably persistent, we have used an operational definition of memory as antigen-experienced T cells that persist for at least 30 days following priming; that is, timing that exceeds primary effector T-cell responses (34). Based on this definition, we have shown the priming of memory T cells against at least two tumor-expressed self-antigens TRP-2/DCT and gp100. Interestingly, tumor-primed T-cell responses did not contract extensively following surgery. Accordingly, systemic tumor protection did not decrease significantly within the month following surgery. This may be due to the fact that T-cell responses are primed by the relatively noninflammatory process of tumor growth, which agrees with previously published studies showing that early inflammation during priming is required for T-cell contraction (35). Importantly, the lack of extensive T-cell contraction may account for the development of memory T-cell populations that persist for as long as 5 months following surgery.
Our results also show that tumor-primed T cells develop into both central and effector memory T-cell subsets. Recent studies have illustrated that adoptively transferred TCM cells are more effective at eliminating tumors than are TEM, lending particular importance to the generation of TCM in tumor-bearing hosts (20). However, unlike memory T cells induced by acute infections (33, 36), we show that tumor-primed memory T cells predominantly maintain a TEM phenotype and do not become dominated with TCM over time. Such a response is reminiscent of nonclassic antigen-dependent memory T cells induced by chronic viral infections, which have been characterized by various stages of functional impairment (24, 37, 38). Despite this, tumor-primed memory T cells are clearly not defective. They remain capable of cytokine production and, more importantly, provide long-term protection against both local and systemic tumor growth. This systemic postsurgical protection against secondary tumors, including tumors that are already established at the time of surgery, may prove to be an important component in the long-term prevention of metastatic disease.
Our study shows the importance of Treg depletion for the induction of functional T-cell memory. However, effective methods for selective Treg depletion in humans remain a subject of investigation. ONTAK (IL-2-diphtheria toxin conjugate) has been evaluated in cancer vaccine trials with promising results (39), although there is some controversy about its effectiveness (40), and it remains unclear if targeting the IL-2 receptor will impair memory T-cell responses. The CD4 depletion strategy used in the present study is advantageous because it does not harm CD8 effectors and is surprisingly inefficient at depleting activated CD4+ helper T cells in mice (41). Several notable studies have shown that CD4 T-cell help is crucial for the effective priming (42, 43) and/or maintenance (44) of CD8 T-cell memory. In our model, anti-CD4 treatment was given beginning 4 days after primary tumor inoculation to provide an early window for T-cell help. Depletion was then discontinued after day 10 to enable the return of CD4 T cells during the maintenance phase. Although this temporary CD4 depletion clearly gives rise to protective memory T-cell responses, future studies will be required to determine the importance of CD4 help during the priming and maintenance phases of postsurgical memory.
We found it interesting that tumor-primed memory T-cell responses were functional even on the repopulation of host Treg cells, which occurs gradually following CD4 depletion. This could be attributed to the fact that Treg cells return in a tumor-free environment. Studies published 20 years ago show that surgery before the growth of large highly immunogenic tumors prevents the generation of suppressor T cells (45), and more recent work has shown that surgery can reverse immune suppression in tumor-bearing hosts (46, 47). Indeed, B16 tumors have been shown to recruit Treg cells (48); however, further investigation will be required to determine if surgery prevents the induction of Treg cells that can suppress preprimed memory CD8 T-cell responses.
The fact that mice with postsurgical immunity also develop autoimmune hypopigmentation illustrates a previously unappreciated role for Treg cells in preventing tissue-specific autoimmunity in tumor-bearing hosts. Vitiligo has long been observed in a fraction of melanoma patients, and its appearance correlates with improved prognosis (49). The present study establishes that growth of a poorly immunogenic melanoma in the absence of Treg cells is sufficient for the induction of vitiligo. These findings could provide mechanistic support for clinical observations that anti-CTLA-4, which may alter Treg function, induces vitiligo in a fraction of melanoma patients (50).
A major obstacle of cancer immunotherapy has been the generation of functional and durable immunity against poorly immunogenic tumors. It has been known for many years that highly immunogenic tumors spontaneously induce postsurgical immunity (45). The present work establishes that natural suppression by host Treg cells prevents the development of postsurgical T-cell memory in hosts bearing poorly immunogenic cancers. Based on these findings, Treg depletion may have previously unrecognized but important implications for the long-term prevention of tumor recurrence and metastasis following surgery in patients with cancer.
Acknowledgments
We thank José Guevara, Alan Houghton, and Chrystal Paulos for helpful discussions; Jake Reder for editorial assistance; Nicholas Restifo and Doug Palmer for providing homozygous pmel-1 transgenic mice; Laurie Horne for assistance with breeding; and Gary Ward for assistance with flow cytometry.
Grant support: NIH grants R01 CA120777 and P20 RR 16437 (COBRE), Melanoma Research Foundation New Investigator Award (M.J. Turk), American Cancer Society Institutional grant award through the Norris Cotton Cancer Center, and NIH grant CA103642 (E.J. Usherwood).
Footnotes
Unpublished data.
References
- 1.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–78. [PubMed] [Google Scholar]
- 2.Bursuker I, North RJ. Immunological consequences of tumor excision: from active immunity to immunological memory. Int J Cancer. 1986;37:275–81. doi: 10.1002/ijc.2910370216. [DOI] [PubMed] [Google Scholar]
- 3.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–24. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–82. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero P, Rod Dunbar P, Valmori D, et al. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998;188:1641–50. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valmori D, Scheibenbogen C, Dutoit V, et al. Circulating tumor-reactive CD8+ T cells in melanoma patients contain a CD45RA+CCR7− effector subset exerting ex vivo tumor-specific cytolytic activity. Cancer Res. 2002;62:1743–50. [PubMed] [Google Scholar]
- 7.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 8.Kursar M, Bonhagen K, Fensterle J, et al. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J Exp Med. 2002;196:1585–92. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–34. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol. 2003;171:5940–7. doi: 10.4049/jimmunol.171.11.5940. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Zhou H, Mizutani M, et al. A DNA vaccine targeting Fos-related antigen 1 enhanced by IL-18 induces long-lived T-cell memory against tumor recurrence. Cancer Res. 2005;65:3419–27. doi: 10.1158/0008-5472.CAN-04-3120. [DOI] [PubMed] [Google Scholar]
- 13.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Res. 2001;61:1095–9. [PubMed] [Google Scholar]
- 15.Bloom MB, Perry-Lalley D, Robbins PF, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–9. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overwijk WW, Tsung A, Irvine KR, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self ”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohm AP, McMahon JS, Podojil JR, et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–5. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 18.Kemp RA, Powell TJ, Dwyer DW, Dutton RW. Cutting edge: regulation of CD8+ T cell effector population size. J Immunol. 2004;173:2923–7. doi: 10.4049/jimmunol.173.5.2923. [DOI] [PubMed] [Google Scholar]
- 19.Hataye J, Moon J, Khoruts A, Reilly C, Jenkins M. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–6. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 20.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8+ T cells. J Exp Med. 2001;194:953–66. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–9. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 25.Turk MJ, Wolchok JD, Guevara-Patino JA, Goldberg SM, Houghton AN. Multiple pathways to tumor immunity and concomitant autoimmunity. Immunol Rev. 2002;188:122–35. doi: 10.1034/j.1600-065x.2002.18811.x. [DOI] [PubMed] [Google Scholar]
- 26.Bystryn JC, Rigel D, Friedman RJ, Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123:1053–5. [PubMed] [Google Scholar]
- 27.He H, Wisner P, Yang G, et al. Combined IL-21 and low-dose IL-2 therapy induces anti-tumor immunity and long-term curative effects in a murine melanoma tumor model. J Transl Med. 2006;4:24. doi: 10.1186/1479-5876-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ercolini AM, Ladle BH, Manning EA, et al. Recruitment of latent pools of high-avidity CD8+ T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and auto-immunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 31.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–32. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–51. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 35.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–17. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 36.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–33. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obar JJ, Fuse S, Leung EK, Bellfy SC, Usherwood EJ. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J Virol. 2006;80:8303–15. doi: 10.1128/JVI.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tewari K, Sacha J, Gao X, Suresh M. Effect of chronic viral infection on epitope selection, cytokine production, and surface phenotype of CD8 T cells and the role of IFN-γ receptor in immune regulation. J Immunol. 2004;172:1491–500. doi: 10.4049/jimmunol.172.3.1491. [DOI] [PubMed] [Google Scholar]
- 39.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–92. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chace JH, Cowdery JS, Field EH. Effect of anti-CD4 on CD4 subsets. I Anti-CD4 preferentially deletes resting, naive CD4 cells and spares activated CD4 cells. J Immunol. 1994;152:405–12. [PubMed] [Google Scholar]
- 42.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 43.Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 44.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–33. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bursuker I, North R. Generation and decay of the immune response to a progressive fibrosarcoma. II Failure to demonstrate postexcision immunity after the onset of T cell-mediated suppression of immunity. J Exp Med. 1984;159:1312–21. doi: 10.1084/jem.159.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvadori S, Martinelli G, Zier K. Resection of solid tumors reverses T cell defects and restores protective immunity. J Immunol. 2000;164:2214–20. doi: 10.4049/jimmunol.164.4.2214. [DOI] [PubMed] [Google Scholar]
- 47.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–11. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 48.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordlund JJ, Kirkwood JM, Forget BM, Milton G, Albert DM, Lerner AB. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689–96. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- 50.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]