Abstract
Amperometric immunosensor configurations featuring covalently bound anti-biotin antibodies (Ab) embedded into a polylysine (PLL)-single walled carbon nanotube (SWCNT) composite layer were evaluated. Assemblies were made by first oxidizing pyrolytic graphite (PG) electrodes to form surface carboxylic acid groups, to which PLL, SWCNTs and anti-biotin were covalently linked. Incorporating SWCNT into PLL-antibody assemblies improved the amperometric detection limit for biotin (Ag) labeled with horseradish peroxidase to 10 fmol mL−1. Anti-biotin embedded into the PLL matrix had improved thermal stability and retained its binding ability for biotin after exposure to temperatures of 42°C for up to 3 hours, while the noncrosslinked antibody was inactivated at this temperature in several minutes.
Keywords: Carbon nanotubes, Proteins, Immunosensor, Polylysine
1. Introduction
Sensitive, compact, stable immunosensor systems are valuable for detecting protein biomarkers, biological toxins and biowarfare agents in critical situations [1 −4]. Electrochemical immunosensors fit many of these criteria [5]. Device fabrication and operation is usually straightforward, requiring non-hazardous and relatively inexpensive materials and reagents. Immunosensors involve antibodies as recognition elements for antigen detection. Sensor shelf life, performance and number of attainable measurement cycles depend on the stability of these antibodies. Thus, antibody stability is critical for immunosensors if they are to be used reliably for point-of-care diagnostics or in field applications.
Antibody activity determines immunosensor performance and durability, and depends on reagents, reaction conditions and possible changes occurring in antibody conformation while the sensor is being used. Attempts have been made to stabilize antibodies for specific applications. For example, in immunosensors based on chemiluminescence detection, antibodies are susceptible to inactivation by the chemiluminescence reagent [6]. Activity of antibodies was retained when incubated with chemiluminescence substrate containing an antibody-specific hapten. Activity of three different monoclonal antibodies for triazines was totally preserved when it was incubated with heptanes – atrazine or semazine. The mechanism of this type of antibody stabilization may be due to the protection of the binding site by bound hapten or to antibody-hapten complex formation, which prevents the antibody from irreversible conformational changes [6, 7]. However, the same antibodies lost activity when incubated with the chemiluminescence reagents and heptanes in pH 2 buffer.
Smith-Gill et al. studied the effect of temperature change (6°C to 37°C) on the binding kinetics of two closely related antibodies, Fabs (H10 and H26), which recognize coincident epitopes on hen egg-white lysozyme (HEL) [8]. H10 and H26 binds HEL via a two step conformational change involving molecular encounter followed by docking. Temperature affected docking – in case of H10 due to significant increase in undocking rate constant where as in case of H26 it was due to decrease in forward docking rate constant and increase in the dissociation rate constant at higher temperatures.
Instability of biomolecules upon temperature changes or at higher temperatures is a common problem encountered during storage, transportation and use. Stabilization of enzymes in diluted form by their substrate is known, but is often not very effective [9, 10].
Herein we explore the possibility that antibodies can be stabilized in ways that have been successful for stabilizing enzymes. Improved thermal stability of enzymes has been achieved by chemical modification and directed evolution [11 − 14], and by immobilization on specialized solid supports [15 − 17]. We recently developed thin film approaches to enzyme stabilization towards extremes of pH, temperature, and oxidizing conditions [18 − 21]. The best stability for peroxidases and myoglobin was obtained by crosslinking within layers of poly-L-lysine (PLL) covalently bound to carboxylated surfaces. Circular dichroism and visible spectroscopy confirmed that crosslinked protein-PLL films retain near native conformations for 12 hours to days at acid and alkaline pH and at temperatures up to 90°C while retaining excellent catalytic activity. Crosslinking may fix the protein secondary structures in the PLL matrix, thereby inhibiting unfolding while retaining high activity.
We have developed vertically oriented single wall carbon nanotube (SWCNT) forests for sensitive amperometric immunosensors [22 − 24]. SWCNT electrodes provide high surface areas for antibody attachment, have excellent electrical conductivity, and when oriented correctly can promote vectorial electron exchange with enzymes [25 −27]. In the present paper, we evaluate the combination of SWCNTs crosslinked with antibodies into PLL matrices for stabilization of antibodies in amperometric immunosensors. As a model system, we attached anti-biotin antibodies onto PLL-coated SWCNTs on the sensor surfaces. Biotin labeled with horseradish peroxidase (HRP) was used as the model antigen. Analytical signals were developed after equilibration of the sensor with antigen and then by adding hydrogen peroxide, which activates HRP for electrochemical reduction. This approach involving PLL and SWCNTs provided significantly improved antibody stability at higher temperature and much better sensitivity and detection limit than an earlier approach [22] employing biotin antibodies absorbed onto SWCNT forest platforms.
2. Experimental
2.1. Chemicals and Materials
SWCNT (HiPCO) were from Tubes@rice (90% pure), and were oxidized by sonication in 3:1 HNO3−H2SO4 for 4 h at 70°C, then filtered, washed with water, and dried [23]. This treatment shortens the nanotubes and provided carboxylate functionality. AFM analysis showed that the dispersion contained nanotubes 30 – 200 nm long. Anti-biotin antibody (Ab) was a goat polyclonal (B-3640) and horseradish peroxidase labeled biotin (Ag) was biotinamidocaproyl-labeled peroxidase (P-9568) from Sigma. Poly (L-lysine) (MW 150000 – 300000), polyacrylic acid (PAA) (MW 2000), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (NHSS), bovine serum albumin (BSA) and all other reagents were from Sigma-Aldrich. Water was purified by a Hydro Nanopure system to specific resistance >15 MΩ cm−2.
2.2. Amperometry
Amperometry was done with CHI 420 electrochemistry module (CH Instruments, Texas US). A three-electrode cell was used with SCE as reference and a platinum wire as the counter electrode. Basal plane pyrolytic carbon (PG) disk electrodes (A = 0.16 cm2) were prepared as described previously [28].
Amperometric measurements were done after placing the sensors as prepared below in PBS, i.e., 0.1 M phosphate buffer pH 7.2 containing 0.137 M NaCl and 2.7 mM KCl. A nitrogen atmosphere was maintained during amperometry. Applied potential was −0.3 V vs. SCE with the sensor rotated at 2000 RPM. 100 μM H2Q in the PBS was used as mediator, and 4 μM hydrogen peroxide was injected to the stirring solution to develop the signal unless otherwise stated.
2.3. Sensor Preparation
2.3.1 (PLL-PAA)-Ab/Ag Assembly
PG electrodes were electrochemically oxidized as reported previously [19], then coated with 10 μL of 24 mM EDC and 10 μL of 4 mM PLL to form amide linkages between carboxylic groups on the electrode surface and amino groups of PLL. After 8 – 12 hours, electrodes were rinsed with water and a 10 μL drop of 27 mM polyacrylic acid and 10 μL of 24 mM EDC was placed on the surface. This step formed amide linkages between PLL amino groups and carboxylic groups of PAA. After, 8 – 12 hours, electrodes were rinsed with DI water. A 10 μL drop of 400 mM EDC and 100 mM NHSS was placed on top of the PAA layer for 10 min, followed by rinsing with water. To this surface was added a 20 μL drop of 0.5 mg mL−1 anti-biotin in PBS. After 3 h, the surface was rinsed 2X with 0.05 (v/v %) Tween 20 in PBS followed by 2X PBS to remove unbound antibody. To further adsorbed antibody, 20 μL of 2% BSA in PBS was added as a blocking solution for an hour and then rinsed off. This immunosensor was then incubated with 20 μL of biotin-HRP (2 μg mL−1, i.e., 50 pmol mL−1) for 1 h, then rinsed 2X with Tween 20 in PBS followed by 2X PBS.
2.3.2 (PLL-SWCNT)-Ab/Ag Assembly
Here, a composite layer of PLL coated SWCNTs was used to immobilize antibody. PG electrodes were oxidized and coated with PLL as above. Then, i) 5 μL of carboxylated SWCNTs dispersed in DMF (0.1 mg mL−1) and 15 μL EDC was deposited on the PLL-coated surface to covalently link PG-PLL with carboxylate groups on the SWCNTs to form PLL-SWCNT composite layer. This assembly was called PLL-SWCNT1. The amount of SWCNT was also varied by using ii) 10 μL functionalized SWCNT dispersed in DMF (0.1 mg mL−1) and 10 μL EDC, called PLL-SWCNT2, and iii) 15 μL of functionalized SWCNT dispersed in DMF (0.1 mg mL−1) and 5 μL EDC, called PLL-SWCNT3.
Unreacted carboxylic acid groups on SWCNTs on these surfaces were activated by 10 μL of 400 mM EDC and 100 mM NHSS for 10 min. The electrode was rinsed with water, and then 20 μL of anti-biotin was deposited on the electrode surface for 3 h. Then electrode was washed and then 20 μL of blocking agent was deposited to prevent nonspecific binding as above. The electrode was washed in PBS again, and incubated with 20 μL of biotin-HRP for an hour followed by washing and then tested by amperometry.
2.3.3 (PLL-SWCNT2)-(PLL-Ab)/Ag Assembly
For (PLL-SWCNT2) electrodes, carboxylic groups were activated as above, then PLL and anti-biotin was deposited on the surface in varying amounts: i) 3 μL PLL and 20 μL Ab; ii) 5 μL PLL and 20 μL Ab; iii) 10 μL PLL and 20 μL Ab; iv) 15 μL PLL and 20 μL Ab; v) 20 μL PLL and 20 μL Ab; vi) 25 μL PLL and 20 μL Ab; in order to optimize the assembly for stability and activity.
2.4. Thermal Stability Studies
Changes in binding ability of the antibody on exposure to elevated temperatures were investigated by first placing the sensor assembly in PBS or in an air incubator at 32°C or 42°C for maximum of 3 h for temperature equilibration and then measuring the amperometric response. These four experimental configurations were used i) fully assembled PLL-SWCNT2-Ab/Ag was equilibrated at 32°C and 42°C in buffer and periodically tested; ii) (PLL-SWCNT2)-Ab assembly was equilibrated with buffer at 32°C followed by BSA blocking step, Ag incubation and then tested; iii) (PLL-SWCNT2)-(PLL-Ab) assembly was equilibrated at 32°C and 42°C in phosphate buffer and in air followed by BSA blocking step, Ag incubation and tested; iv) (PLL-SWCNT2)-(PLL-Ab) assembly was equilibrated at 42°C in an air incubator for maximum of 3 hours followed by BSA blocking step, Ag incubation and tested.
3. Results and Discussion
3.1. Assembly Characterization
The PLL-SWCNT2 assembly was used for antibody immobilization and for subsequent detection and stability studies. Tapping mode AFM revealed a relative flat PLL layer, but a PLL-SWCNT2 composite layer featuring domains ca. 23 nm in height and ca. 380 nm wide. The fully assembled PLL-SWCNT2-Ab/Ag assembly showed chemical reversible cyclic voltammetry for the H2Q mediator with midpoint potential of 0.015 V, and peak separation of 0.05 V to 0.19 V when scan rate was varied from 0.01 – 0.3 V s−1 (Fig. 1).
Fig. 1.
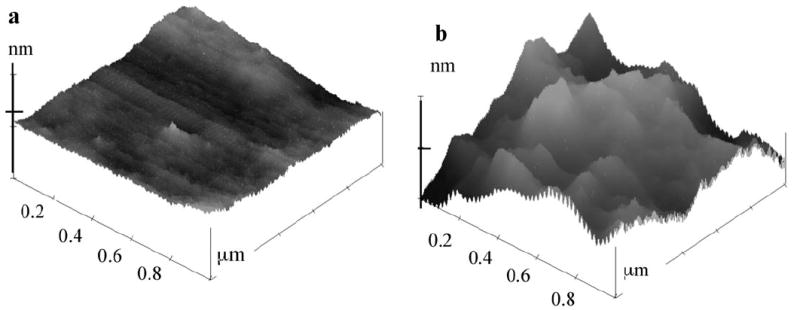
Tapping mode AFM image of a) PLL film and b) PLL-SWCNT2 film on mica. PLL-SWCNT2 assembly showed average protrusion height of 23 ± 5 nm and average domain width of 382 ± 68 nm. X-axis = 0.2 μm/division; Z-axis = 35 nm/division.
3.2. Optimization of Immunosensor Assembly and Variables
We first constructed a control immunosensor with covalently bound layers of polyions and (PLL-PAA)-Ab which had no SWCNTs. The detection limit and sensitivity obtained are compared below to our previous configuration of anti-biotin adsorbed onto a SWCNT forest platform [22], which involved no crosslinking with PLL. Observation of steady state currents for a sensor equilibrated with biotin-HRP antigen upon addition of hydrogen peroxide was used as a convenient method to monitor relative sensitivity of the various sensor configurations.
Our previous SWNT forest immunosensor for biotin-HRP gave a steady state amperometric current increase of 0.08 μA/μM H2O2 for H2O2 concentrations between 0.2 mM and 1 mM in presence of 100 μM hydroquinone (H2Q) as mediator [22]. The current response was independent of H2Q in the concentration range 100 μM to 1 mM. Under the same conditions for 50 pmol mL−1 biotin-HRP, the (PLL-PAA)-Ab sensor gave a steady state current about twice that of the zero antigen control (Fig. 2a). In addition, a sensitivity of 0.002 μA/μM H2O2 was found for the H2O2 concentration range 4 – 400 μM at 0.2 mM H2Q, 40 times less than for the previous SWCNT forest immunosensors [22]. This poor response for the (PLL-PAA)-Ab/Ag assembly may be due to a limited amount of antibody on the surface, and/or to electron transfer limitations.
Fig. 2.
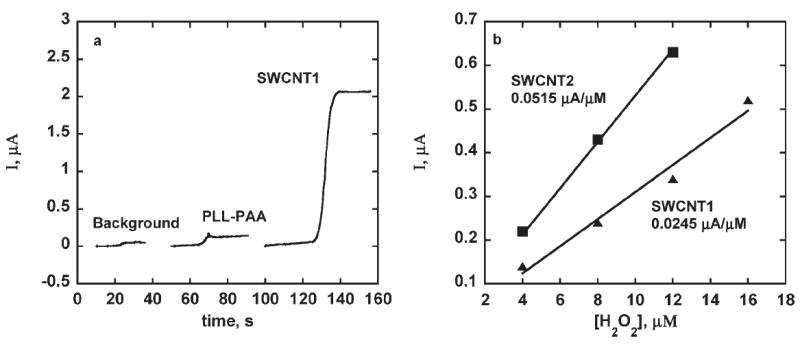
a) Amperograms at −0.3 V vs. SCE from injection of 40 μM H2O2 for sensors in 0.1 mM H2Q in PBS rotated at 2000 rpm for. 50 pmol mL−1 biotin-HRP (Ag): A) background PLL-PAA-Ab, no Ag, B) PLL-PAA-Ab-Ag, C) SWCNT1 sensor assembly (see Sec. 2). b) Amperograms showing effect of hydrogen peroxide concentrations on steady state currents obtained with sensor assemblies made with 0.5 mg mL−1 Ab. Conditions: 2000 rpm, 0.1 mM H2Q, 4 μM H2O2, 50 pmol mL−1 biotin-HPR.
The SWCNT1 assembly (see Sec. 2 and Table 1), featuring a composite layer of SWCNT-PLL with Ab attached, greatly improved sensitivity as shown in Figure 2a. SWCNT1 has larger surface area and more carboxylic groups on the SWCNTs most likely resulting in more antibodies attached, and possibly more efficient electron transport through the SWCNTs compared to the polyion layer in (PLL-PAA)-Ab.
Table 1.
Assembly types, acronyms and details.
| Ab crosslinked to the PLL-PAA composite layer | |
| PLL-PAA-Ab/Ag | |
| Ab crosslinked to the PLL-SWCNT composite layer | Amount of SWCNT used to form PLL-SWCNT composite layer |
| PLL-SWCNT1-Ab/Ag | 5 μL SWCNT |
| PLL-SWCNT2-Ab/Ag | 10 μL SWCNT |
| PLL-SWCNT3-Ab/Ag | 15 μL SWCNT |
| PLL-Ab composite layer crosslinked to the PLL-SWCNT2 layer | |
| (PLL-SWCNT2)-(PLL-Ab)/Ag | |
| Proportion of PLL and Ab mixed to form PLL-Ab | gm% of PLL in PLL & Ab mix |
| i) 3 μL PLL and 20 μL Ab | 90 |
| ii) 5 μL PLL and 20 μL Ab | 93.7 |
| iii) 10 μL PLL and 20 μL Ab | 96.7 |
| iv) 15 μL PLL and 20 μL Ab | 97.8 |
| v) 20 μL PLL and 20 μL Ab | 98.3 |
| vi) 25 μL PLL and 20 μL Ab | 98.6 |
The SWCNT1 sensor gave a sensitivity of 0.025 μA/μM H2O2 at 100 μM H2Q. Sensitivity was further improved in PLL-SWCNT2, with an increased amount of SWNCTs, which gave sensitivity of 0.052 μA/μM H2O2 (Fig. 2b). However, the sensitivity to peroxide dropped for the PLL-SWCNT3 assembly, even though more nanotubes were used in this construction. Thus PLL-SWCNT2 was used and optimized in subsequent studies.
3.3. Stability at Elevated Temperatures
Stability at elevated temperature was tested in two ways. First, fully assembled PLL-SWCNT2-Ab/Ag sensors were equilibrated in buffer at 32°C and 42°C, and tested by amperometry (Fig. 3). Amperometry of the exposed assembly showed a ca. 10% decrease in the response at 30 minutes but for equilibration at longer times, the amperometric response decreased by ca. 70%. Re-incubation with Ag didn’t improve the response. Assembly exposed to 42°C for 15 minutes showed ca. 80% loss in amperometric response.
Fig. 3.
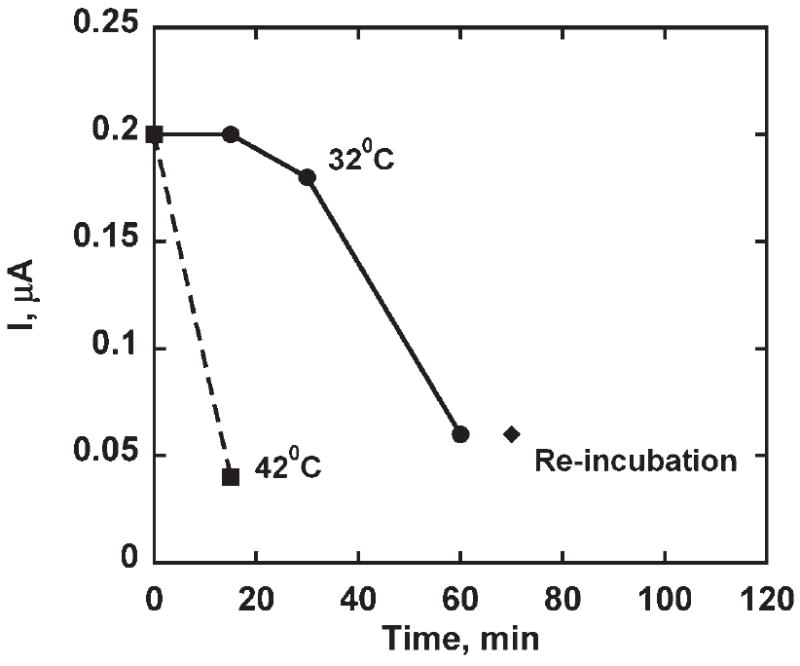
Steady state amperometric response of PLL-SWCNT2-Ab/Ag assembly upon addition of 4 μM hydrogen peroxide. Assembly was equilibrated in phosphate buffer at 32°C and 42°C for different time intervals (−0.3 V vs. SCE at 2000 RPM, 0.1 mM H2Q, 50 pmol mL−1 Ag, 4 μM H2O2 injection).
Second, to evaluate the effect of temperature on binding ability of Ab, PLL-SWCNT2-Ab was equilibrated in buffer at 32°C for different time intervals. Electrodes were then subjected to a bovine serum albumin (BSA) blocking protocol and incubated with biotin-HRP as previously. Amperometric response showed approximately 90% loss of binding ability of Ab for 15 minutes of exposure in phosphate buffer at 32°C.
Electrodes were assembled as (PLL-SWCNT2)-(PLL-Ab), i.e., a composite layer of PLL and Ab was build on top of the PLL-SWCNT2 assembly. The amount of PLL was varied to give 90% and 93.7% PLL content in PLL-Ab composite layer (procedure (i) and (ii) in Table 1). (PLL-SWCNT2)-(PLL-Ab) assemblies were equilibrated in buffer and in air at 32°C for desired time interval. Then, sensors were subjected to BSA blocking, incubated with biotin-HRP and tested amperometrically (Fig. 4). (PLL-SWCNT2)-(PLL-Ab) assembly with 93.7% PLL content showed good binding ability for Ag when exposed to 32°C in phosphate buffer for ≥40 min. However, (PLL-SWCNT2)-(PLL-Ab) with 90% PLL content showed loss in binding ability for Ag after 20 minutes of exposure at 32°C in phosphate buffer. Both assemblies showed loss of binding ability for Ag when exposed to the air at 32°C for 20 minutes or more in an air incubator.
Fig. 4.
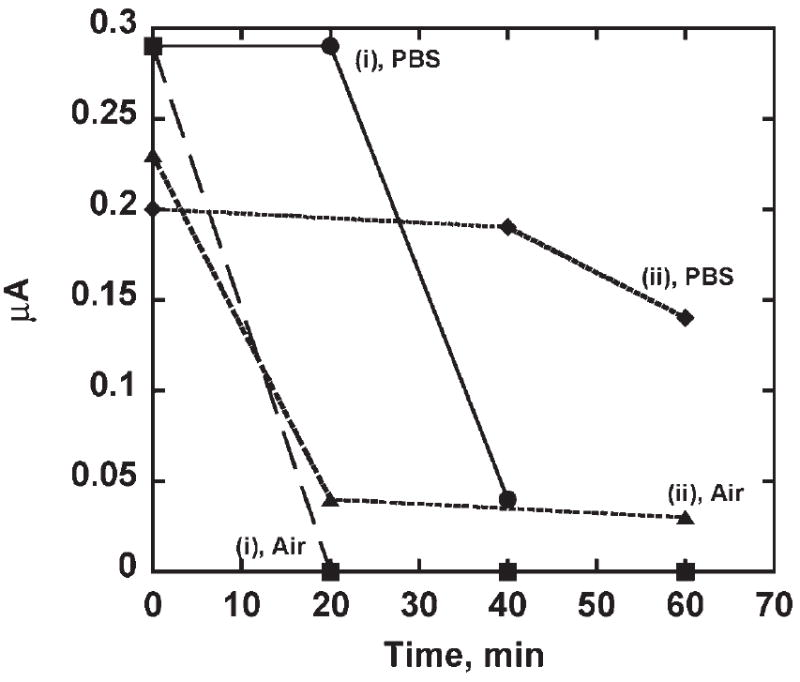
(PLL-SWCNT2)-(PLL-Ab) assemblies equilibrated in buffer and in air incubator at 32°C for different time intervals for sensors with i) 90% PLL and ii) 93.7% PLL. After equilibration, sensor was subjected to BSA blocking step and then incubated with 50 pmol mL−1 Ag and tested amperometrically. Steady state amperometric response of PLL-SWCNT2-Ab/Ag was developed by addition of hydrogen peroxide (−0.3 V vs. SCE at 2000 RPM, 0.1 mM H2Q, 4 μM H2O2 injection). Response at zero time serves as control and is from fully assembled PLL-SWCNT2-(PLL-Ab)/Ag assembly with i) 90% PLL and ii) 93.7% PLL that was not exposed to buffer or air 32°C.
The amount of PLL in the PLL-Ab composite layer influenced thermal stability. The change in the response of electrodes exposed in air at 42°C was compared with electrodes not exposed to 42°C in an air incubator. PLL helped in stabilizing Ab against high temperature denaturation as shown in Figure 5.
Fig. 5.
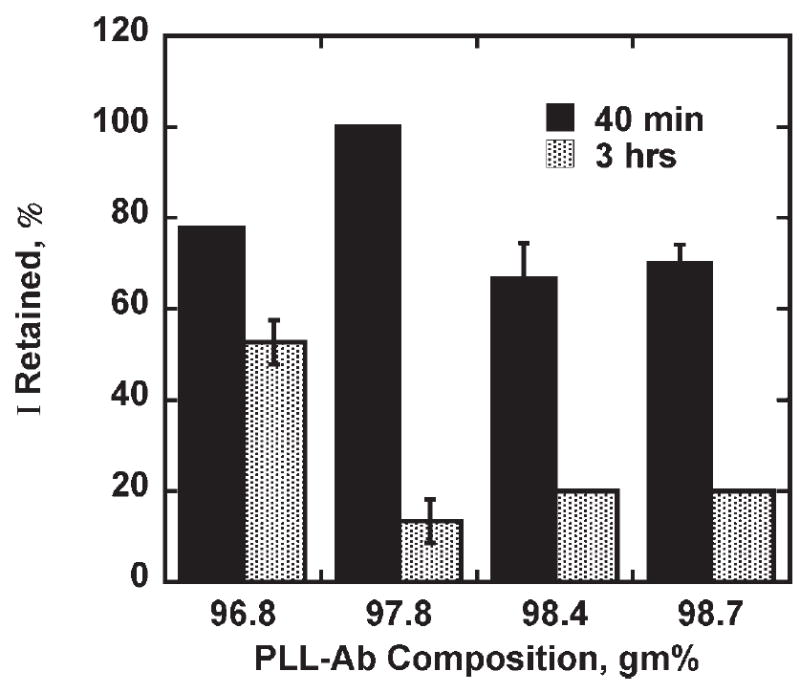
Retention of binding ability of (PLL-SWCNT2)-(PLL-Ab) assembly on equilibrating in air at 42°C. Thermally equilibrated assembly was subjected to BSA blocking, then incubated with Ag as described earlier and steady state amperometric response on addition of hydrogen peroxide was measured (2000 rpm, 0.1 mM H2Q, 4 μM H2O2 additions, 50 pmol mL−1 Ag, and 0.5 mg mL−1 Ab).
Increasing the amount of PLL in the PLL-Ab composite layer had an effect on the amperometric response of the assemblies not exposed to temperature equilibration. (PLL-SWCNT2)-(PLL-Ab)/Ag with 90 – 96.7% PLL (cf. Table 1) produced a current increase ca. 0.2 μA and (PLL-SWCNT2)-(PLL-Ab)/Ag with 97.8% and higher PLL amount in the PLL-Ab composite layer produced a response of ca. 0.1 μA.
(PLL-SWCNT2)-(PLL-Ab) made with procedure 96.7% PLL in the PLL-Ab composite layer offered better thermal stability to the Ab at 42°C and was used for detection limit and sensitivity studies. A linear increase in steady state current with increasing Ag concentration was observed with sensitivity of 3.10±0.16 μA pmol−1 mL−1 of biotin-HRP and detection limit of 10 fmol mL−1 of Ag (Figs. 6a, b).
Fig. 6.
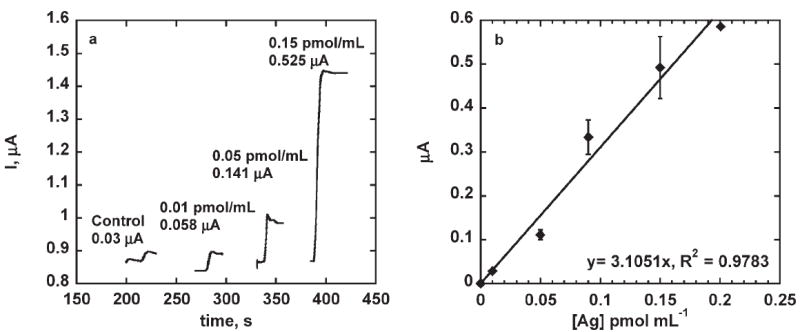
a) Steady state amperometric response of (PLL-SWCNT2)-(PLL-Ab)/Ag assembly on addition of hydrogen peroxide (2000 rpm, 0.1 mM H2Q, 40 μM H2O2 additions and 0.5 mg mL−1Ab (except for control) and 50 pmol mL−1 Ag). b) Steady state amperometric response of (PLL-SWCNT2)-(PLL-Ab)/Ag sensor with varying biotin-HRP concentration on addition of hydrogen peroxide (2000 rpm, 0.1 mM H2Q, 40 μM H2O2, and 0.5 mg mL−1 Ab). Response corrected for control.
Results above show that of three compositions of PLL/ SWCNT immunosensor tested, SWCNT2 showed sensitivity comparable to the previously reported SWCNT forest assembly, but a much better detection limit of 10 fmol mL−1 of Ag compared to the previous value of 2.5 pmol mL−1 of Ag [22]. As with our previous amperometric biosensors for biotin, the larger response may be due to high Ab loading because of roughness introduced by SWCNTs and also their higher conductivity [29]. Adding SWCNTs improved sensitivity by 10 times (for SWCNT1) and 25 times (for SWCNT2) over PLL-PAA assembly (Fig. 2b). Further increases in SWCNT content decreased the sensitivity (SWCNT3<SWCNT2).
PLL-PAA-Ab assembly showed linear increase in current with increasing peroxide concentration. However, the sensitivity was lower than previously reported SWCNT forest assembly under similar amperometric conditions (Fig. 2a) [22]. While physical stability of the Ab assembly was achieved via crosslinking to the polyion underlayers, this approach did not attain high sensitivity.
While reasons for lower sensitivity offered by SWCNT3 despite having higher SWCNT content are unclear, a similar phenomenon was observed with SWCNT forest assembly when higher concentrations of Ag were used [22]. A linear increase in current was observed with increasing Ag concentration up to 75 pmol mL−1 but with further increase the sensitivity dropped. This behavior may involve steric hindrance resulting from densely packed biomolecules on the electrode surface, thereby impeding the mediator diffusion or binding of antigen.
Activity of PLL-SWCNT2-Ab/Ag was affected when exposed to buffer at 32°C for longer times as shown in Figure 3. Reincubation with Ag didn’t improve the response, and that suggested either there was a loss in binding ability of the Ab or HRP tags on Ag bounded to Ab are not active. Thus PLL-SWCNT2-Ab equilibrated with phosphate buffer at 32°C for 30 minutes was then incubated with Ag and then tested amperometrically. The current response was negligible suggesting loss in binding ability of Ab compared to the response of fully assembled PLL-SWCNT2-Ab/Ag equilibrated in buffer at 32°C for same time duration. Antibodies or Immunoglobulins have a common Y shaped core structure that consists of two identical light chains of amino acids (ca. 25 kDa) and two identical heavy chains of amino acids (ca. 50– 75 kDa). The V region of heavy chain and light chain features complementary determining regions (CDRs) which contain most antigen binding amino acid residues. Thermally induced unfolding of amino acid chains in CDRs affects the binding capacity if refolding is incorrect [30]. Winkklmair et al. [6] have suggested that Ab-Ag complex formation restrict unfolding of polypeptide chains in CDRs. It could be the reason that PLL-SWCNT2-Ab/Ag sustained activity for longer time on equilibration in phosphate buffer at 32°C.
We extended this idea by incorporating PLL in the Ab binding stage so as to form a matrix of PLL around immobilized antibodies in order to restrict unfolding and to maintain the hydrophilic environment around antibody. Initial experiments showed some improvement in the retention of Ab activity. PLL-SWCNT2-(PLL-Ab) with 93.7% PLL in the PLL-Ab composite layer when equilibrated in phosphate buffer at 32°C for 40 minutes followed by Ag incubation produced the amperometric response comparable to the similar assembly that was not equilibrated in phosphate buffer at 32°C. In general, (PLL-SWCNT2)-(PLL-Ab) showed better retention of Ab binding ability on equilibration at 32°C in buffer environment than in air. Dumoulin et al. have studied unfolding of six single domain antigen binders derived from camelid antibodies with specificities for lysozyme, beta-lactamases and dye (RR6). Thermally induced unfolding of conventional antibody fragments resulted in exposure of hydrophobic interfaces of heavy and light chains causing aggregation and precipitation resulting in nonfunctional molecules. This indicates importance of native conformation which features a hydrophilic interface suitable for antigen binding. Probably, the aqueous phase environment in PBS at 42°C, compared to air at the same temperature, appears to better maintain the hydrophilic environment retaining the native structure and the binding ability of Ab due to exposed polar residues.
As seen in Figure 5, on exposure to air at 42°C for 3 hrs, PLL-Ab composite layer with 96.7% PLL content showed 50% loss in binding ability where as higher PLL content showed 80% drop in the binding ability. A relatively small change in the PLL content has larger effect on Ab stabilization. It is more evident from the amperometric response of the (PLL-SWCNT2)-(PLL-Ab)/Ag assembly when PLL content in the PLL-Ab composite layer was varied from the 90% to 98.6%. Amperometric response of the (PLL-SWCNT2)-(PLL-Ab)/Ag assembly unexposed to higher temperature was ca. 0.2 μA for 0% to 96.7% PLL content of the PLL-Ab composite layer where as at higher PLL content, the response dropped to ca. 0.1 μA even though assemblies were made with same Ab amounts and were exposed to the same Ag concentration. When a PLL and Ab were mixed and coated on the activated PLL-SWCNT2 layer to form a PLL-Ab composite layer, amino groups on Ab as well as PLL compete to form an amide bond with the activated carboxylic groups on the SWCNT. It suggests that either i) lower amounts of Ab was co-immobilized with PLL since their linking to activated carboxylic groups on SWCNT was competitive or ii) there was less active Ab at high PLL content in the composite layer or iii) denser PLL matrix lowered H2Q regeneration. It can be seen from the results that the composite layer with optimum PLL content of 96.7% does not affect amperometric response, suggesting maximum activity or accessibility or amount of the antibody in the composite layer. However, the 80% drop in the binding ability of Ag for PLL content higher than 96.7% suggest that probably Ab were not well embedded into the matrix due to competitive immobilization of PLL and Ab. Understanding of lower thermal stability of the Ab at higher PLL content in the composite layer needs further investigation. The amperometric responses of (PLL-SWCNT2)-Ab/Ag assembly and of (PLL-SWCNT2)-(PLL-Ab)/Ag assembly with 96.7% PLL content in PLL-Ab composite layer were the same. It appears to be the optimum PLL content in the composite layer which doesn’t affect Ab binding to the PLL-SWCNT2 layer and probably Ab molecules were surrounded by PLL thereby holding their native structure.
4. Conclusion
Herein we have presented an approach to build electrochemical immunosensor assemblies with capture antibodies stabilized to some extent against temperature variations by crosslinking into a PLL-SWCNT composite matrix. The test PLL-SWCNT composite layer system examined showed high sensitivity and improved detection limit for biotin-HRP compared to an alternative configuration in which capture antibodies were adsorbed to SWCNT electrodes. We expect that this methodology is general, and are currently testing it for detection of proteins.
Scheme 1.
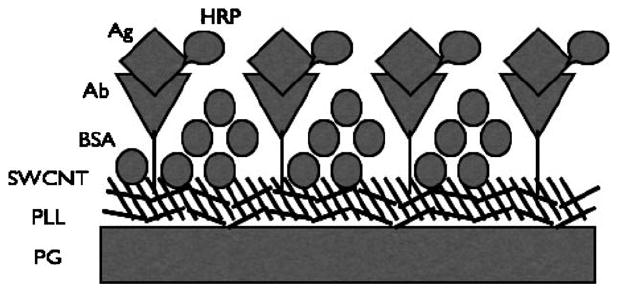
Simplified illustration of PLL-SWCNT-Ab/Ag assembly.
Acknowledgments
This work was supported jointly by PHS grant ES013557 from NIEHS/NIH and by NSF Grant No. CTS 0335345. V. C. acknowledges the NSF REU Program for a summer fellowship.
References
- 1.Weston AD, Hood L. J Proteome Res. 2004;3:179. doi: 10.1021/pr0499693. [DOI] [PubMed] [Google Scholar]
- 2.Walt DR, Franz DR. Anal Chem. 2000;72:739A. doi: 10.1021/ac003002a. [DOI] [PubMed] [Google Scholar]
- 3.Hood E. Environ Health Perspectives. 2003;111:A817. doi: 10.1289/ehp.111-a692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitch JP, Raber E, Imbro DR. Science. 2003;302:1350. doi: 10.1126/science.1085922. [DOI] [PubMed] [Google Scholar]
- 5.Warsinke A, Benkert A, Scheller FW. Fresenius J Anal Chem. 2000;366:622. doi: 10.1007/s002160051557. [DOI] [PubMed] [Google Scholar]
- 6.Winklmair M, Schuetz AJ, Weller MG, Niessner R. Fresenius J Anal Chem. 1999;363:619. [Google Scholar]
- 7.Davis DR, Padlan EA, Sheriff S. Ann Rev Biochem. 1990;59:439. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- 8.Lipscultz CA, Yee A, Mohan S, Li Y, Smith-Gill SJ. J Mol Recognition. 2002;15:44. doi: 10.1002/jmr.559. [DOI] [PubMed] [Google Scholar]
- 9.Maes RF. 5183751. US Pat. 1993
- 10.Wehner R, Mattersberger J, Klenner D, Deutsch-Weyer G. 4764468. US Pat. 1988
- 11.Bokhari SA, Afzal AJ, Rashid HH, Rojaka MI, Siddiqui KS. Biotechnol Prog. 2002;18:276. doi: 10.1021/bp010146h. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Blasco G, Sanz-Aparicio J, Gonzalez B, Hermoso JA, Polaina J. J Biol Chem. 2000;275:13708. doi: 10.1074/jbc.275.18.13708. [DOI] [PubMed] [Google Scholar]
- 13.Reading NS, Aust SD. Biotechnol Prog. 2000;20:326–333. doi: 10.1021/bp0000151. [DOI] [PubMed] [Google Scholar]
- 14.Joe K, Borgford TJ, Bennet AJ. Biochemistry. 2004;43:7672. doi: 10.1021/bi0496337. [DOI] [PubMed] [Google Scholar]
- 15.Kumar CV, Chaudhari A. Chem Commun. 2002:2382. doi: 10.1039/b206988a. [DOI] [PubMed] [Google Scholar]
- 16.Pessela CC, Mateo C, Fuentes M, Vain A, Garcia JL, Carrascosa AV, Guisan JM, Fernandez-Lafluente R. Biotechnol Prog. 2004;20:388. doi: 10.1021/bp034183f. [DOI] [PubMed] [Google Scholar]
- 17.Jagannadham V, Bhambhani A, Kumar CV. Micropor Mesopor Mater. 2006;88:275. [Google Scholar]
- 18.Panchagnula V, Kumar CV, Rusling JF. J Am Chem Soc. 2002;124:12515. doi: 10.1021/ja020683d. [DOI] [PubMed] [Google Scholar]
- 19.Vaze AS, Parizo M, Rusling JF. Langmuir. 2004;20:10943. doi: 10.1021/la048712g. [DOI] [PubMed] [Google Scholar]
- 20.Guto PM, Rusling JF. J Phys Chem B. 2005;109:24457. doi: 10.1021/jp054621w. [DOI] [PubMed] [Google Scholar]
- 21.Guto PM, Kumar CV, Rusling JF. J Phys Chem B. 2007 doi: 10.1021/jp071525h. ASAP Article. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor M, Kim SN, Killard AJ, Foster RJ, Smyth MR, Papadimitrakopoulos F, Rusling JF. Analyst. 2004;129:1176. doi: 10.1039/b412805b. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, Kim S-N, Papadimitrakopoulos F, Rusling JF. Molec Biosys. 2005;1:70. doi: 10.1039/b502124c. [DOI] [PubMed] [Google Scholar]
- 24.Yu X, Munge B, Patel V, Jensen G, Bhirde A, Gong JD, Kim S-N, Gillespie J, Gutkind JS, Papadimitrakopoulos F, Rusling JF. J Am Chem Soc. 2006;128:11199. doi: 10.1021/ja062117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz E, Willner I. ChemPhysChem. 2004;5:1084. doi: 10.1002/cphc.200400193. [DOI] [PubMed] [Google Scholar]
- 26.Wang J. Analyst. 2005;130:421. doi: 10.1039/b414248a. [DOI] [PubMed] [Google Scholar]
- 27.Gooding JJ. Electrochim Acta. 2005;50:3049. [Google Scholar]
- 28.Njue CK, Rusling JF. J Am Chem Soc. 2000;122:6459. [Google Scholar]
- 29.Davis JJ, Coleman KS, Azamian BR, Bagshaw CB, Green MLH. Chem Eur J. 2003;9:3732. doi: 10.1002/chem.200304872. [DOI] [PubMed] [Google Scholar]
- 30.Dumoulin M, Conrath K, Meirhaeghe AV, Meersman F, Heremans K, Frenken LGJ, Muyldermans S, Wyns L, Matagne A. Protein Sci. 2002;11:500. doi: 10.1110/ps.34602. [DOI] [PMC free article] [PubMed] [Google Scholar]


