Abstract
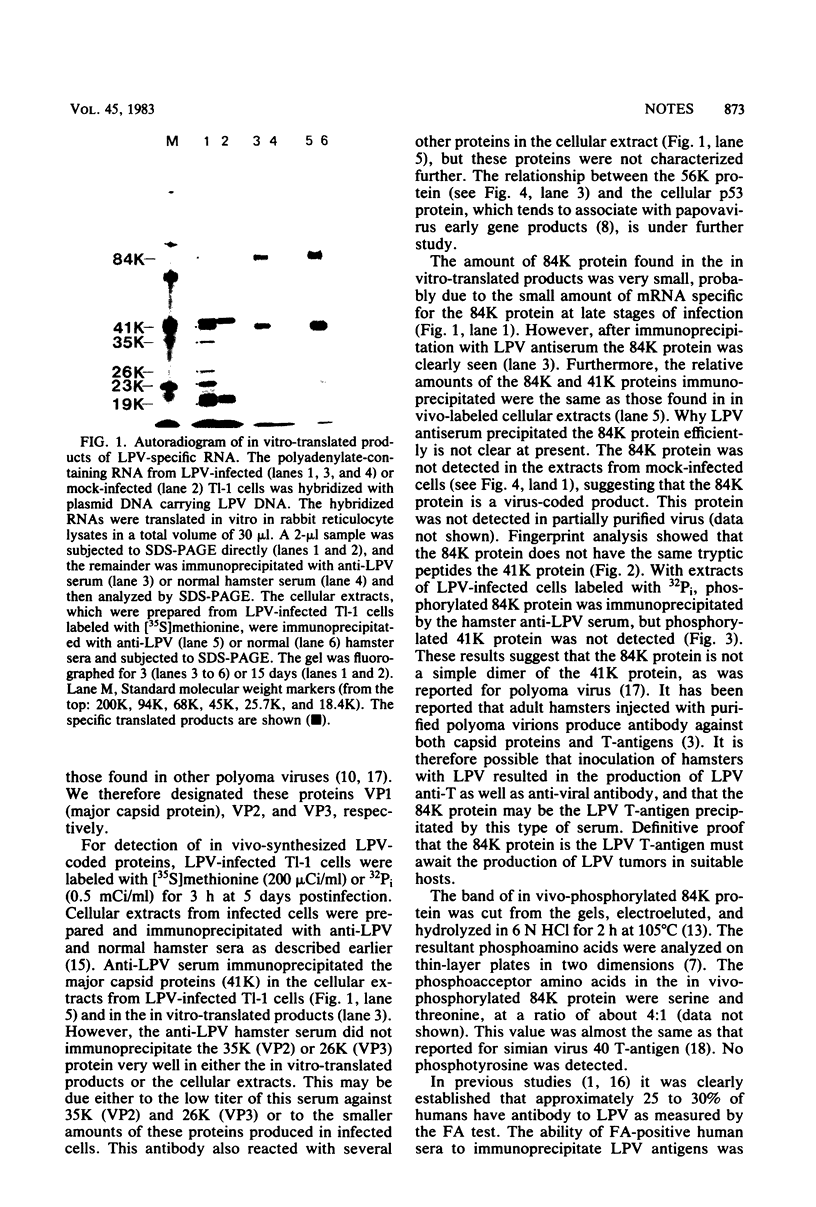
The lymphotropic papovavirus (LPV)-specific mRNAs were translated in vitro in rabbit reticulocyte lysates. The specific products were 84,000-dalton (84K), 41K, 35K, and 26K proteins. Immunoprecipitation with anti-LPV hamster sera and analysis of partially purified LPV virions showed that the last three proteins were the LPV capsid proteins, and we designated the 41K, 35K, and 26K proteins VP1 (major capsid protein), VP2, and VP3, respectively. Several characteristics, such as the small amount of mRNA for the 84K protein at late stages of infection, its absence from partially purified virus preparations, no common tryptic peptides between the 84K and 41K proteins, and the pattern of in vivo phosphorylation, suggest that the 84K protein is not a simple dimer of the 41K protein. Normal human sera and sera from certain leukemic patients positive for antibody to LPV viral antigens immunoprecipitated the 41K protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade L., Müller-Lantzsch N., zur Hausen H. B-lymphotropic papovavirus and possibility of infections in humans. J Med Virol. 1981;6(4):301–308. doi: 10.1002/jmv.1890060405. [DOI] [PubMed] [Google Scholar]
- Brade L., Vogl W., Gissman L., zur Hausen H. Propagation of B-lymphotropic papovavirus (LPV) in human B-lymphoma cells and characterization of its DNA. Virology. 1981 Oct 15;114(1):228–235. doi: 10.1016/0042-6822(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Coe J. E., Takemoto K. K. Immune response in the hamster. VI. Antibody response in polyoma oncogenesis. J Natl Cancer Inst. 1972 Jul;49(1):39–44. [PubMed] [Google Scholar]
- Girard M., Marty L., Suarez F. Capsid proteins of Simian virus 40. Biochem Biophys Res Commun. 1970 Jul 13;40(1):97–102. doi: 10.1016/0006-291x(70)91051-x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Matsumura Y., Nishihira T., Watanabe I., Ohi R., Kasai M., Kumagai K., Kamada N. Burkitt's lymphoma cell line bearing surface IgA and negative for nuclear antigen of Epstein-Barr virus (EBNA). Jpn J Exp Med. 1980 Dec;50(6):423–434. [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Mullarkey M. F., Hruska J. F., Takemoto K. K. Comparison of two human papovaviruses with simian virus 40 by structural protein and antigenic analysis. J Virol. 1974 May;13(5):1014–1019. doi: 10.1128/jvi.13.5.1014-1019.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M. Resolution of simian virus 40 proteins in whole cell extracts by two-dimensional electrophoresis: heterogeneity of the major capsid protein. Cell. 1976 Oct;9(2):289–298. doi: 10.1016/0092-8674(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Saito I., Shiroki K., Shimojo H. In vitro translation of adenovirus type 12-specific mRNA complementary to the transforming gene. Virology. 1980 Nov;107(1):61–70. doi: 10.1016/0042-6822(80)90272-x. [DOI] [PubMed] [Google Scholar]
- Segawa K., Yamaguchi N., Oda K. Simian virus 40 gene A regulates the association between a highly phosphorylated protein and chromatin and ribosomes in simian virus 40-transformed cells. J Virol. 1977 Jun;22(3):679–693. doi: 10.1128/jvi.22.3.679-693.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimizu K., Kubo Y., Segawa K., Natori S. Difference in phosphorylation of two factors stimulating RNA polymerase II of Ehrlich ascites tumor cells. Biochemistry. 1981 Apr 14;20(8):2286–2292. doi: 10.1021/bi00511a033. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Segawa K., Shimojo H. Two tumor antigens and their polypeptides in adenovirus type 12-infected and transformed cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2274–2278. doi: 10.1073/pnas.77.4.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Furuno A., Kato K., Yoshiike K. Biological and biochemical studies of African green monkey lymphotropic papovavirus. J Virol. 1982 May;42(2):502–509. doi: 10.1128/jvi.42.2.502-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Deppert W. Intermolecular disulfide bonds: an important structural feature of the polyoma virus capsid. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):255–257. doi: 10.1101/sqb.1974.039.01.033. [DOI] [PubMed] [Google Scholar]
- Walter G., Flory P. J., Jr Phosphorylation of SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):165–169. doi: 10.1101/sqb.1980.044.01.019. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Gissmann L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med Microbiol Immunol. 1979 Aug;167(3):137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]






