Abstract
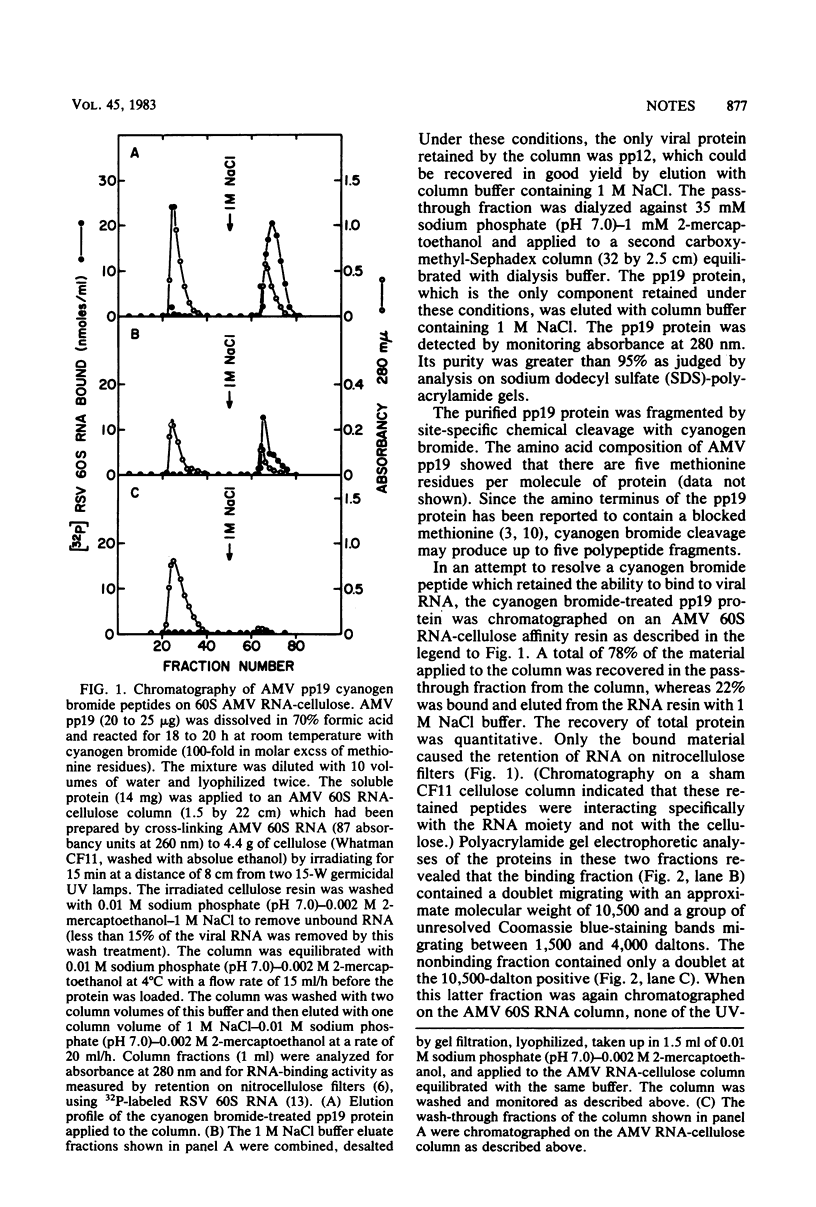
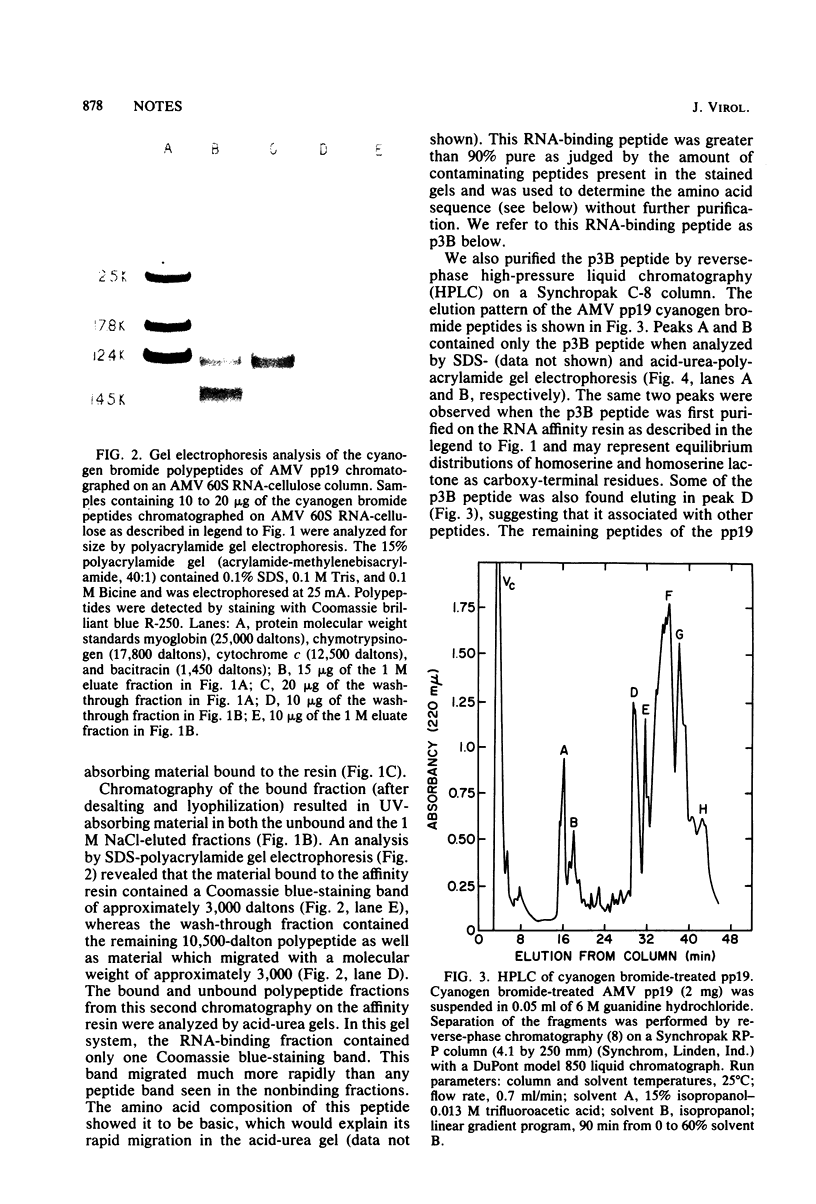
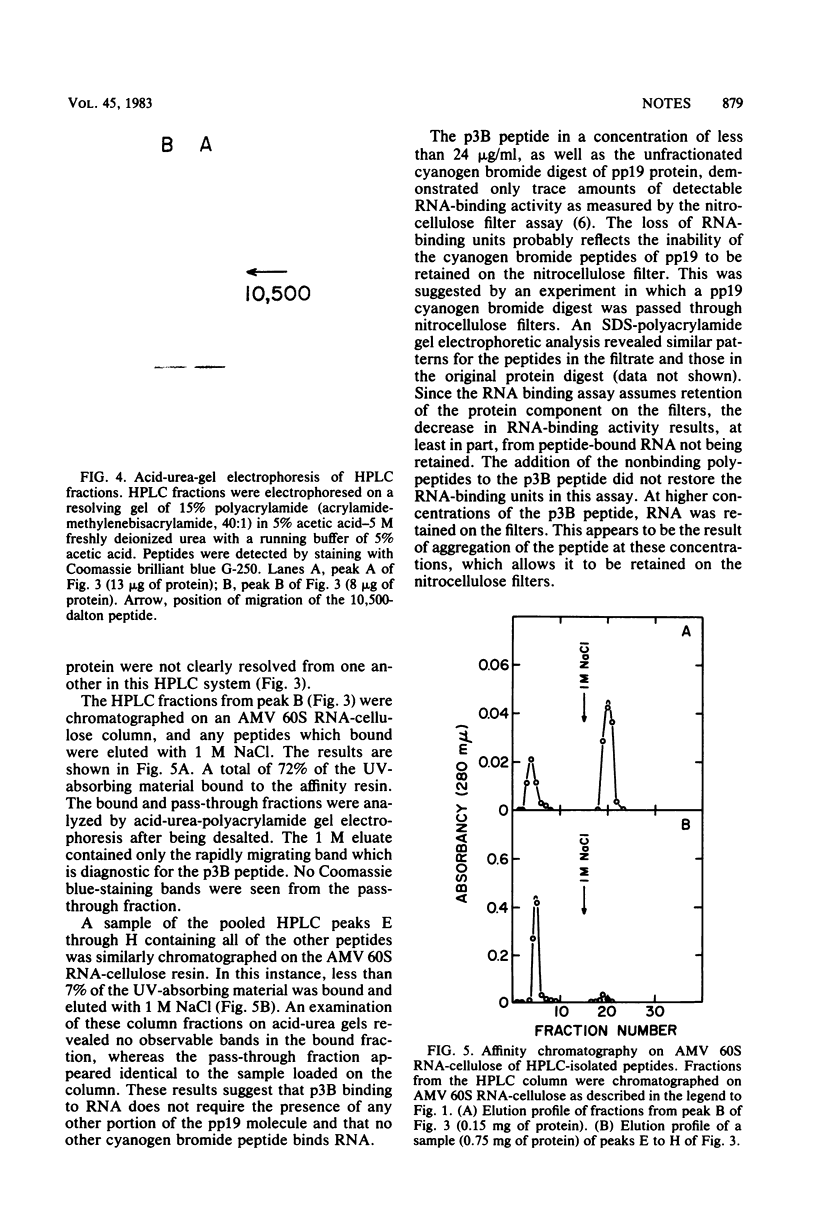
The avian myeloblastosis virus pp19 protein was separated from the other virus proteins by a rapid and simple purification procedure which yields milligram amounts of homogeneous protein. This protein was then fragmented by digestion with cyanogen bromide. When the mixture of the cyanogen bromide peptides was passed through a 60S avian myeloblastosis virus RNA-cellulose column, only one peptide bound with high affinity to the resin. The peptide migrated on a sodium dodecyl sulfate-polyacrylamide gel with an approximate molecular weight of 2,900 and will be referred to as the p3B peptide. This peptide was also isolated directly by chromatography of the cyanogen bromide-digested pp19 protein on a reverse-phase high-pressure liquid chromatography column. It was again the only cyanogen bromide peptide of the pp19 protein that bound to the RNA affinity resin. The p3B peptide is a basic peptide, as was seen by its rapid migration on acid-urea-polyacrylamide gels and its amino acid composition. A partial amino acid sequence analysis of the p3B peptide indicated that it was derived from the amino terminus of the intact protein. Although the p3B peptide bound to 60S RNA, it did not demonstrate the selective binding of native pp19 to regions of the RNA containing secondary structure.
Full text
PDF
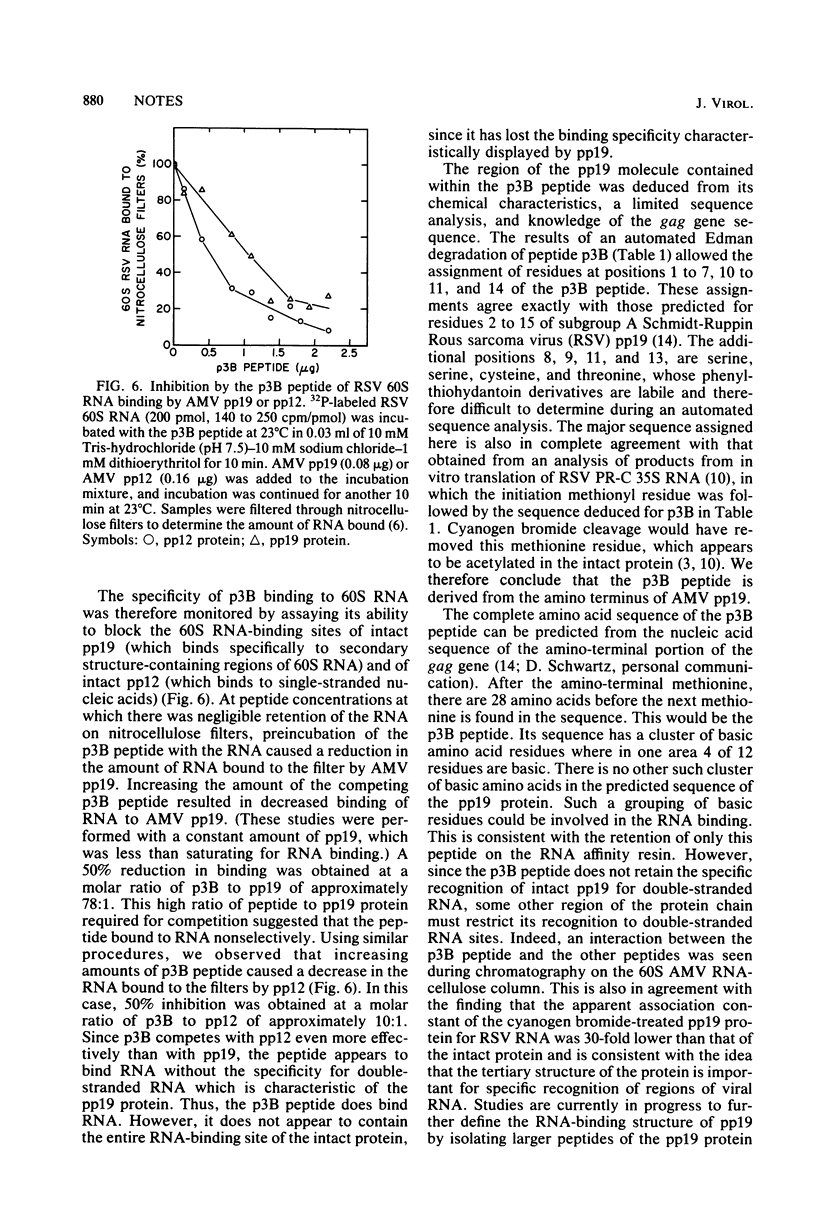
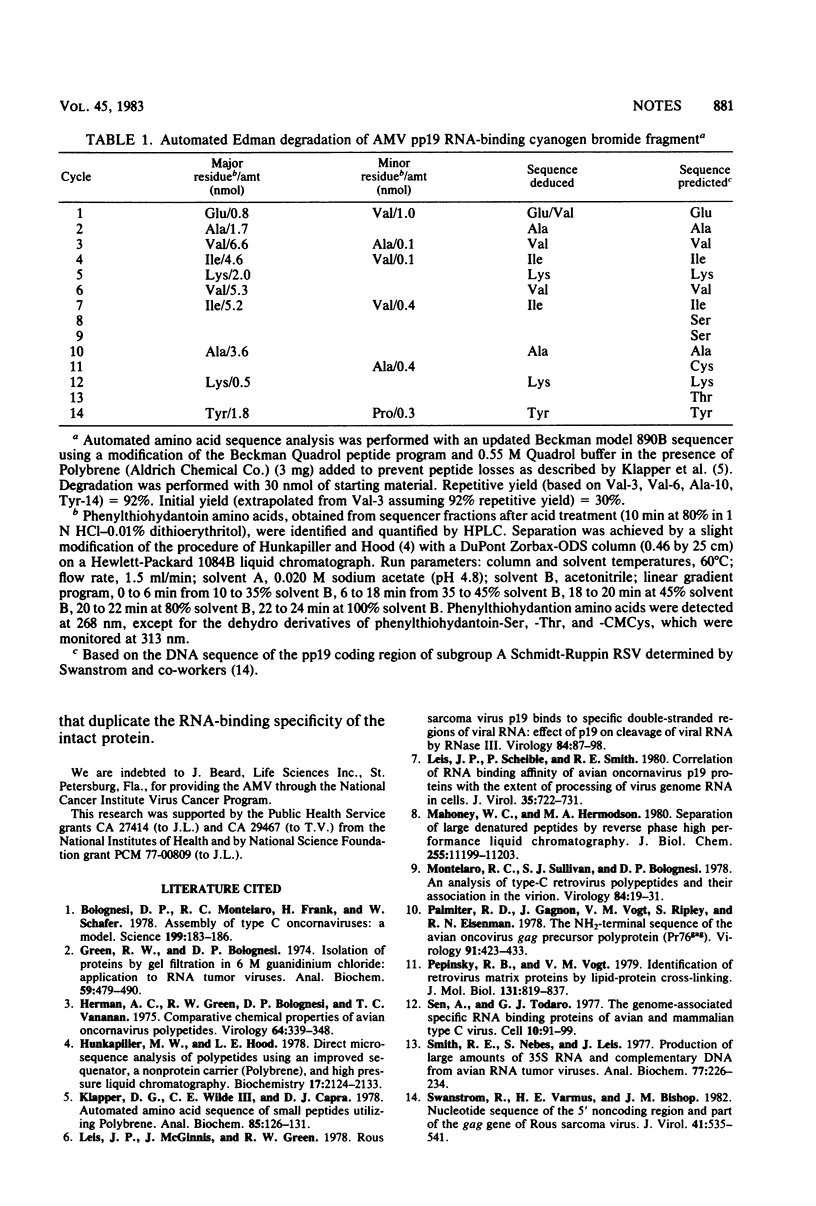
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Herman A. C., Green R. W., Bolognesi D. P., Vanaman T. C. Comparative chemical properties of avian oncornavirus polypeptides. Virology. 1975 Apr;64(2):339–348. doi: 10.1016/0042-6822(75)90110-5. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Leis J. P., McGinnis J., Green R. W. Rous sarcoma virus p19 binds to specific double-stranded regions of viral RNA: effect of p19 on cleavage of viral RNA by RNase III. Virology. 1978 Jan;84(1):87–98. doi: 10.1016/0042-6822(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Scheible P., Smith R. E. Correlation of RNA binding affinity of avian oncornavirus p19 proteins with the extent of processing of virus genome RNA in cells. J Virol. 1980 Sep;35(3):722–731. doi: 10.1128/jvi.35.3.722-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- Montelaro R. C., Sullivan S. J., Bolognesi D. P. An analysis of type-C retrovirus polypeptides and their associations in the virion. Virology. 1978 Jan;84(1):19–31. doi: 10.1016/0042-6822(78)90215-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Vogt V. M., Ripley S., Eisenman R. N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag). Virology. 1978 Dec;91(2):423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977 Jan;10(1):91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Nebes S., Leis J. Production of large amounts of 35S RNA and complementary DNA from avian RNA tumor viruses. Anal Biochem. 1977 Jan;77(1):226–234. doi: 10.1016/0003-2697(77)90308-6. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. Nucleotide sequence of the 5' noncoding region and part of the gag gene of Rous sarcoma virus. J Virol. 1982 Feb;41(2):535–541. doi: 10.1128/jvi.41.2.535-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]