Summary
Brown fat cells are specialized to dissipate energy and can counteract obesity; however, the transcriptional basis of their determination is largely unknown. We show here that the zinc-finger protein PRDM16 is highly enriched in brown fat cells compared to white fat cells. When expressed in white fat cell progenitors, PRDM16 activates a robust brown fat phenotype including induction of PGC-1α, UCP1 and type 2 deiodinase expression, and a remarkable increase in uncoupled respiration. Transgenic expression of PRDM16, at physiological levels, in white fat depots stimulates the formation of brown fat cells. Depletion of PRDM16 through shRNA expression in brown fat cells causes a near-total loss of the brown characteristics. PRDM16 activates brown fat cell identity, at least in part, by simultaneously activating PGC-1α and PGC-1β through direct protein binding. These data indicate that PRDM16 can control the determination of brown fat fate.
Keywords: PRDM16, brown fat, UCP1, PGC-1α, PGC-1β, PPARγ, obesity, thermogenesis, mitochondrial biogenesis
Introduction
Adipose cells and tissue are of great interest in view of the worldwide epidemic of obesity and its associated metabolic disorders, such as insulin resistance, diabetes, dyslipidemia and hypertension. Fat cells occur in two distinct subtypes: white fat cells, that function primarily to store energy in the form of triglycerides and brown fat cells that oxidize fuels and dissipate energy in the form of heat. Brown adipose tissue (BAT) is important for small mammals such as mice to defend against the cold. In humans, brown fat is abundant at birth, but is rapidly replaced by white adipose tissue (WAT) and is relatively scarce in the adult as an identifiable tissue (Lean and James, 1986). On the other hand, several reports indicate that brown fat cells are interspersed within WAT of rodents and humans (Cousin et al., 1992; Garruti and Ricquier, 1992; Guerra et al., 1998; Lean et al., 1986; Oberkofler et al., 1997; Xue et al., 2007). Identifying the mechanisms that stimulate the development or function of human BAT could, therefore, provide new therapeutic avenues to reduce obesity and its associated diseases.
Brown fat cells are characterized by densely packed mitochondria that contain uncoupling protein-1 (UCP1) in their inner mitochondrial membrane. UCP1, almost exclusive to brown fat cells, is a proton transporter that allows protons to leak across the mitochondrial inner membrane, thereby dissipating the electrochemical gradient normally used for ATP synthesis (reviewed by Klingenberg, 1999). The thermogenic capacity of brown fat cells, in which over 50% of cellular respiration is uncoupled from ATP synthesis, is unique among mammalian cell types (Nedergaard et al., 1977; Prusiner et al., 1968; Reed and Fain, 1968).
Brown fat cells also depend upon an extensive vascular bed and a high degree of sympathetic innervation to produce and distribute heat. Cold, sensed in the CNS, causes sympathetic nerves to release catecholamines which, through the β-adrenergic receptors, increases cyclic-AMP levels in brown fat cells (reviewed by Cannon and Nedergaard, 2004). Cyclic-AMP is a critical second messenger in the function of brown fat, stimulating the levels of key thermogenic factors such as PGC-1α, type 2 deiodinase and UCP1. Chronic adrenergic stimulation can also cause a proliferation of brown adipose preadipocytes and the emergence of pockets of brown fat cells in white fat depots (Bronnikov et al., 1992; Cannon and Nedergaard, 2004; Ghorbani and Himms-Hagen, 1997; Himms-Hagen et al., 2000; Nedergaard et al., 1994).
As noted above, a small number of brown adipocytes and detectable levels of UCP1 mRNA are found in white fat of adult humans (Garruti and Ricquier, 1992; Oberkofler et al., 1997). Indeed, BAT and oxidative activity can be increased by chronic cold exposure in humans (Huttunen et al., 1981). A re-emergence of BAT is also observed in adult humans with pheochromocytoma, where neuroendocrine tumors cause excessive catecholamine secretion (Ricquier et al., 1982). Experiments in rodents have also demonstrated that chronic cold exposure or β3-adrenergic stimulation induces the expression of UCP1 in classic white fat depots (Himms-Hagen et al., 2000). Interestingly, particular mouse strains have more brown adipose cells in their white fat depots, which correlates with a lower susceptibility to obesity and diabetes (Guerra et al., 1998). Moreover, ectopic deposits of BAT in mouse skeletal muscle protect against diet-induced obesity (Almind et al., 2007).
The extent to which BAT functions in the regulation of energy balance has been well studied in rodents. Overfeeding activates BAT thermogenesis through the stimulation of sympathetic nerves (Cannon and Nedergaard, 2004; Lupien et al., 1985; Rothwell and Stock, 1979). Increased UCP1 expression in mouse fat promotes energy expenditure, reduces adiposity and protects animals from diet-induced and genetic forms of obesity (Cederberg et al., 2001; Ghorbani et al., 1997; Ghorbani and Himms-Hagen, 1997; Guerra et al., 1998; Kopecky et al., 1995; Kopecky et al., 1996; Tsukiyama-Kohara et al., 2001). Furthermore, specific ablation of brown fat, through expression of a toxigene, leads to reduced whole body energy expenditure and increased obesity (Hamann et al., 1996; Lowell et al., 1993).
The developmental origin of fat, either brown or white, has not been fully elucidated; however, much is known about the process of adipogenic differentiation from preadipocytes. Extensive studies in cultured cell and mouse models have revealed the central role of PPARγ in the differentiation of both brown and white adipose cells (Barak et al., 1999; Kubota et al., 1999; Nedergaard et al., 2005; Rosen et al., 2002; Rosen et al., 1999; Tontonoz et al., 1994). Thus, PPARγ is apparently not the primary determinant of whether a fat cell assumes a brown versus white phenotype. In fact, brown and white preadipocytes appear to be fully “committed” at that stage since fibroblastic cells cultured from brown depots differentiate as brown fat cells whereas those from white fat tissue differentiate as white adipocytes (Klaus, 1997; Klaus et al., 1995; Kozak and Kozak, 1994). The cold-induced emergence of brown adipocytes in WAT, and replacement of BAT with WAT in newborn humans and obese rodents does, however, suggest some degree of plasticity, at least at the tissue level. However, whether mature adipocytes can interconvert, or whether separate pools of white and brown precursors mediate “transdifferentiation” of these tissues has not been resolved.
The influence of brown fat on energy balance has thus motivated strong interest in finding a dominant regulator of brown fat cell determination. To date, a “master” regulator of brown fat has not been found, in terms of a single, tissue-selective factor that can positively regulate the entire program of brown fat in a cell-autonomous manner and which is required for the brown fat cell phenotype. Several transcription-related factors have, however, been shown to influence the brown fat cellular phenotype. Notably, the transcriptional coactivator PGC-1α is highly expressed in brown fat compared to white fat, and can activate the adaptive thermogenic gene program when expressed in white fat cells (Puigserver et al., 1998; Tiraby et al., 2003). However, genetic experiments in cultured brown fat cells and mice have clearly indicated that PGC-1α, while critical for cAMP-dependent thermogenic aspects of the brown fat program, does not give cells their essential brown fat identity (Lin et al., 2004; Uldry et al., 2006).
In addition to PGC-1α, other factors have been shown to selectively influence the function of brown versus white fat. FOXC2, when expressed by transgenesis in WAT, induces many features of BAT tissue (Cederberg et al., 2001). However, FOXC2 is not expressed preferentially in BAT versus WAT, and its role in this interconversion between tissues is probably linked to its stimulation of adrenergic signaling when FOXC2 is overexpressed in vivo (Dahle et al., 2002; Gronning et al., 2006). A cell-autonomous role for FOXC2 in white to brown fat cell conversion has not been shown. Other factors, such as Rb (retinoblastoma), p107 and RIP140 have been shown to suppress the brown fat phenotype in white fat cells or tissue (Christian et al., 2005; Hansen et al., 2004; Leonardsson et al., 2004; Powelka et al., 2006; Scime et al., 2005). Quantitative increases in the levels of Rb, p107 or RIP140 have not, however, been demonstrated to preferentially favor the differentiation of white fat adipocytes. These data suggest that while each of these factors may participate, to various degrees, in the formation or function of brown fat cells, they are unlikely to be the central factor that determines an adipocyte to specifically commit to a brown adipose fate.
Using global expression analysis of murine transcriptional components, we found a very small number of factors that are selectively expressed in brown versus white fat cells. One of these was PRDM16, a 140 kD PR- (PRD1-BF-1-RIZ1 homologous) domain containing protein, that was first identified at a chromosomal breakpoint in myeloid leukemia (Nishikata et al., 2003). We show here that PRDM16 activates a broad program of brown fat differentiation when expressed in cultured white fat preadipocytes or in white fat depots in vivo. Uncoupled respiration accounts for the majority of the very high oxygen consumption of PRDM16-expressing cells, a classic hallmark of brown fat. Furthermore, shRNA-mediated knock-down of PRDM16 in bona fide brown fat preadipocytes allows normal differentiation to fat cells but results in a total loss of the brown fat character. Our experiments thus reveal a dominant regulatory function for PRDM16 in the brown fat lineage.
Results
PRDM16 is expressed selectively in brown adipocytes
With the goal of identifying transcriptional components that regulate the development and function of brown adipocytes, we performed a global expression screen of all genes known to be involved in transcription and those having a molecular signature suggestive of transcriptional function. White and brown fat tissue RNA samples from C57Bl6 mice (n=5) at 10–12 weeks of age were examined by RT-PCR using primer-sets corresponding to all transcription-related mouse genes (Gray et al., 2004). Genes differentially regulated in these tissues were then assayed for their expression in cultured white and brown fat cells from established cell lines. Three genes, Lhx8, Zic1 and PRDM16 ultimately met the criteria of being preferentially expressed in brown versus white fat and having elevated (>5 fold) mRNA expression levels in immortalized brown fat cells (Uldry et al., 2006) compared to any of three immortalized white fat cell lines (3T3-L1, 3T3-F442A, C3H10T1/2) (data not shown). As described in detail below, ectopic expression demonstrated a function for PRDM16 in stimulating the brown fat phenotype.
Quantitative analyses of PRDM16 expression at the mRNA level showed a 15-fold enrichment in brown (BAT) relative to white fat tissue (WAT) (n=6) (Figure 1A). Fractionation of BAT showed that PRDM16 was mainly expressed in mature brown adipocytes as compared to stromal-vascular fraction that contains preadipocytes and other cell types (Figure 1A). Importantly, PRDM16 was selectively expressed in adipocytes from two immortalized brown fat cell lines (Uldry et al., 2006) as compared to its expression in white fat cells from 3T3-F442A, 3T3-L1 and C3H-10T1/2 fibroblasts (Figure 1B). Moreover, PRDM16 mRNA expression increased 20-fold during the differentiation of brown fat cells in culture (Figure 1C). Northern blot analysis indicated that PRDM16 was expressed at its highest levels in BAT and was virtually undetectable in WAT (Figure 1D). PRDM16 transcripts were also detected in heart, lung, kidney and brain. Acute cold-exposure of mice (4°C for 4 hours) activated the thermogenic program of BAT without any significant regulation of PRDM16 mRNA expression (p=0.13) (Figure 1E). Similarly, PRDM16 expression was not affected by cAMP treatment of cultured cells (data not shown). These results suggest that PRDM16 expression in brown fat is linked to determination or differentiation but not to adaptive thermogenesis. Collectively, these data identify PRDM16 as a gene that is very selectively expressed in brown versus white adipocytes, but is not entirely specific to brown fat tissue.
Figure 1. PRDM16 is expressed selectively in brown fat cells.
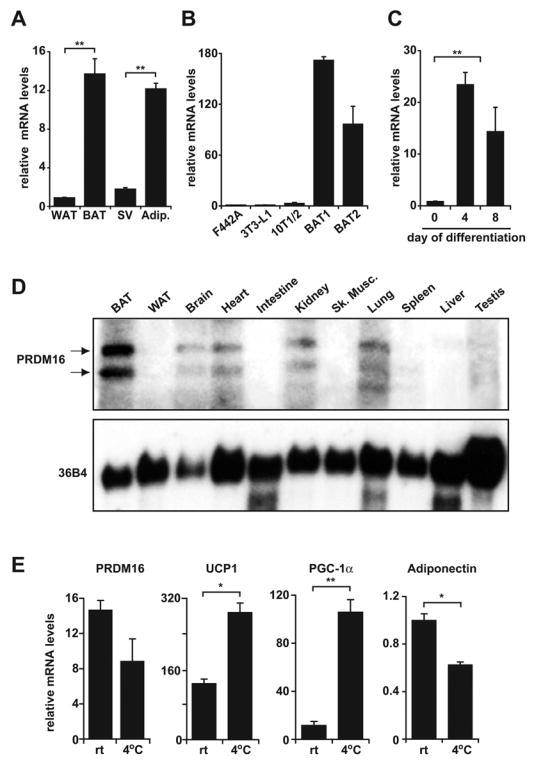
(A) Real-time PCR analysis of PRDM16 mRNA expression in BAT relative to WAT; and its expression in the adipocyte fraction (Adip.) compared to the stromal vascular fraction (SV) of BAT (n= 6, mean ± SD). (B) PRDM16 mRNA expression in adipocytes from immortalized BAT cell lines (BAT1 and BAT2) and three WAT cell lines (3T3-F442A, 3T3-L1, C3H-10T1/2) (n=3–5 samples per cell line, mean ± SD). (C) PRDM16 mRNA levels during the differentiation of immortalized BAT cell lines (n=3 for each of 2 separate cell lines, mean ± SD). (D) Northern blot analysis of PRDM16 mRNA and control, 36B4 mRNA, in adult mouse tissues. (E) Expression of PRDM16, UCP1, PGC-1α and adiponectin in BAT after a 4°C cold exposure of mice for 4 hours (n=5 mice per group, mean ± SD) (rt: room temperature). * p < 0.05; ** p < 0.01.
PRDM16 drives the molecular phenotype of brown fat cells
To study its role in brown fat cells, we first expressed PRDM16 in cells with no preadipose (white or brown) character whatsoever, using fibroblasts genetically deficient in PPARγ (Rosen et al., 2002). A retroviral vector expressing PRDM16 or control vector was introduced together with a PPARγ2-expressing retroviral vector into subconfluent fibroblasts and selected for stable viral integration. Cells transduced with retroviral-PRDM16 expressed mRNA levels which were 4 to 6-fold higher than its endogenous expression in cultured brown fat cells. These cell cultures were grown to confluence and stimulated to undergo adipogenic differentiation driven by the ectopic PPARγ2. At day 6 of differentiation, cultures expressing PPARγ2 were composed mainly of adipocytes whether or not they also expressed PRDM16; ectopic PRDM16 did not appear to promote nor inhibit cellular differentiation per se (Figure 2A). At the molecular level, expression of genes common to both white and brown fat lineages were either not affected (PPARγ and adiponectin) or moderately elevated (aP2) by PRDM16 expression (Figure 2A).
Figure 2. PRDM16 expression induces the gene program of brown fat cells.
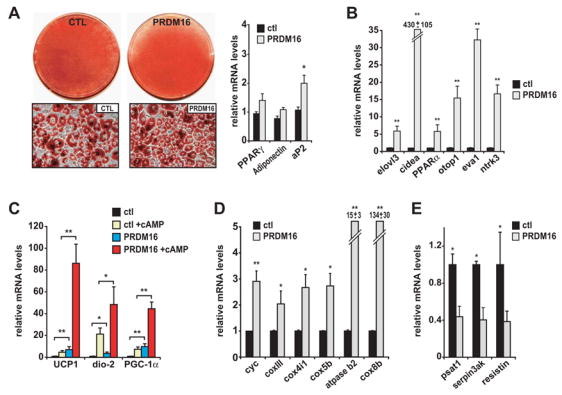
(A) Oil-red-O staining of mature adipocytes (day 6) from PPARγ-deficient cells expressing retroviral PPARγ2 and either retroviral- PRDM16 or vector control (ctl). These adipocyte cultures were analyzed by real-time PCR for their expression of: differentiation markers common to WAT and BAT (A); brown fat cell-selective genes (as indicated) (B); brown fat- thermogenic genes (UCP1, deiodinase-d2 (dio-2), PGC-1α) with and without cAMP treatment (C); mitochondrial components (as indicated) (D); and white fat cell-selective markers (psat1, serpin3ak, resistin) (E). (n=3, mean ± SD). * p < 0.05; ** p < 0.01
To analyze the molecular phenotype of mature fat cells from PRDM16-expressing cultures in detail, we first performed global, unbiased expression analyses to develop a more complete set of brown versus white fat-selective genes. To this end, brown and white fat tissues from three adult C57Bl6 mice were expression profiled using Affymetrix microarrays. The mRNA levels for the 40-most highly enriched genes in both brown and white fat tissue were analyzed by Q-PCR in samples from three independent mice to validate the microarray data. These genes were then assayed for their expression in mature fat cells from three white (3T3-L1, 3T3-F442A, C3H10T1/2) and two brown fat cell lines. Altogether, we identified 19 BAT-selective and 9 WAT-selective genes whose expression correlated completely with the BAT or WAT- adipocyte phenotype in tissue and in cultured cells (by all pair wise comparisons) (Table S1).
Strikingly, most brown fat selective mRNAs were specifically induced in adipocytes from PRDM16-expressing cells. For example, elovl3 (previously called cig30), a very long chain fatty acid elongase that is expressed in brown but not white fat and is implicated in brown fat hyperplasia (Tvrdik et al., 1997; Westerberg et al., 2006), was induced 6-fold by PRDM16 expression (Figure 2B). Cidea, a gene predominantly expressed in brown fat where it is thought to regulate UCP1 activity (Zhou et al., 2003), was induced 430-fold by PRDM16 (Figure 2B). PRDM16 also elevated PPARα expression, a brown fat selective gene and an important regulator of fatty acid oxidation (Barbera et al., 2001; Braissant et al., 1996), by 5-fold (Figure 2B). In addition, several genes, not previously known to be expressed selectively in brown versus white adipocytes, such as otopetrin1 (otop1), epithelial like antigen-1 (eva1) and neurotrophic tyrosine kinase receptor type 3 (ntrk3) were significantly increased by PRDM16-expression (Figure 2B). Moreover, PRDM16-transduced cells expressed several of the important thermogenic genes in a cAMP-dependent manner, similar to what bona fide brown fat cells do in culture and in vivo. The mRNA levels of UCP1, deiodinase-d2 and PGC-1α were increased by PRDM16 in unstimulated fat cells and induced to very high levels by PRDM16 in response to cAMP (Figure 2C). UCP1, for instance, was elevated 20-fold by cAMP in PRDM16-expressing cells, compared to only a 3-fold increase in control cells. PRDM16 expression also elevated the mRNA levels for many genes of mitochondrial oxidative phosphorylation, that are known to be enriched in brown fat cells and tissue, such as cytochrome c (cyc), cox4i1, and cox5b (Figure 2D). In particular, cox8b, a highly brown fat-selective mitochondrial gene (see Table S1) was expressed at 134-fold higher levels in PRDM16- compared to control- adipocyte cultures.
Interestingly, the mRNA levels of several genes that are preferentially expressed in white relative to brown fat cells were significantly repressed by PRDM16 expression, including psat1, serpin3ak and resistin (Figure 2E). In particular, resistin, a WAT-secreted protein in mice that promotes insulin resistance (Steppan et al., 2001), was reduced by 60% at the mRNA level as a result of PRDM16 expression. Altogether, 20/28 or 71% of the brown/white fat cell selective genes that we identified in an unbiased global expression analysis were regulated in a brown fat-specific manner by PRDM16 (Table S1). A robust regulation of brown fat cell genes by PRDM16 was observed in other cellular models including 3T3-F442A white preadipocytes and immortalized brown preadipocytes (Figure S1-A,B). In particular, PRDM16 increased the mRNA levels of UCP1 and cidea by almost 200-fold and repressed resistin expression by 70% in 3T3-F442A adipocytes (Figure S1-A). These data, therefore, demonstrate that the action of PRDM16 in activating brown fat gene expression was not unique to PPARγ−/− fibroblasts and also not dependent on exogenous PPARγ expression. Moreover, PRDM16 dramatically induced the expression of key brown fat genes UCP1 and PGC-1α, but not cidea or mitochondrial components in the genetic absence of PPARγ Figure S1-C) These results show that certain genes characteristic of the brown fat gene program do not absolutely require the fat cell environment and/or PPARγ function. Altogether, these data thus strongly suggest a role for PRDM16 as a positive regulator of the brown fat cell gene program.
An important question is whether PRDM16 can stimulate the transdifferentiation of mature white fat cells into brown fat. This question was addressed by analyzing the effect of PRDM16 expression in stromal-vascular cells and mature adipocytes from the same white fat tissue. When PRDM16 was expressed in stromal-vascular cells prior to differentiation, this factor efficiently activated the brown fat gene program in differentiated cultures, including an induction of UCP1 mRNA by more than 200-fold (Figure S2-A). However, expression of PRDM16 using adenovirus in mature white fat cells failed to elicit a significant induction of brown fat marker genes, despite robust expression of PRDM16 (Figure S2-B). Importantly, PRDM16 protein expressed by adenovirus was able to induce BAT-related genes when introduced into cells before their differentiation (data not shown). Altogether, the strong induction of brown fat genes in adipocytes from PRDM16-expressing primary stromal vascular cells, and lack of any effect in mature cells from the same tissue strongly suggests that PRDM16 function is required before and/or during adipogenic differentiation to promote brown fat cell character.
PRDM16 stimulates mitochondrial biogenesis and uncoupled cellular respiration
A defining feature of brown fat cells is their very abundant mitochondria and associated high rates of cellular respiration, particularly uncoupled respiration. To assay the functional consequences of PRDM16 action in fat cells, PPARγ−/− cells transduced with PRDM16 or vector control, together with PPARγ2, were induced to differentiate into adipocytes. Representative electron micrographs of control and PRDM16 expressing cells before and after adipogenic differentiation showed a very significant elevation of mitochondrial density by PRDM16 only in differentiated fat cells (Figure 3A). Quantitative analysis of this data revealed a 2-fold increased mitochondrial volume in PRDM16-expressing adipocytes compared to control cultures (p<0.001) (Figure 3B). Oxygen consumption was measured in adipocytes using an oxygen sensitive electrode to calculate relative rates of respiration. These experiments revealed a remarkable effect of PRDM16 on the levels of both total and uncoupled cellular respiration. Specifically, adipocytes from PRDM16-expressing cells displayed a 40% increase in total respiration and 2-fold increase in uncoupled respiration relative to control cultures (n=4) (Figure 3C). After 12 hour treatment with cAMP, PRDM16-expressing adipocytes displayed a 70% increase in total respiration relative to control cells and >60% of their respiration was uncoupled from ATP production (Figure 3D). PRDM16 expression, therefore, induced mitochondrial biogenesis and uncoupled respiration to levels that are characteristic of brown fat cells (Uldry et al., 2006) but not other mammalian cell types. These results demonstrate that PRDM16 expression enables fat cells to develop the respiratory activity characteristic of brown adipocytes.
Figure 3. PRDM16 stimulates mitochondrial biogenesis and uncoupled respiration.
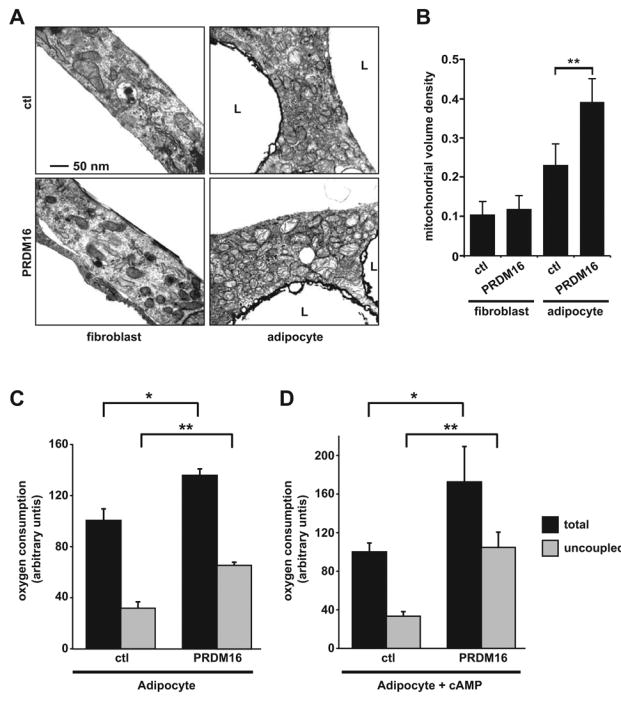
(A) Representative transmission electron micrographs of fibroblasts and mature adipocytes (day 6) from PPARγ-deficient cells expressing retroviral PPARγ2 and either retroviral- PRDM16 or vector control (ctl). (B) Comparison of mitochondrial volume densities from cells depicted in (A) (n= >20 micrographs per group, mean ± SD). (C, D) Total mitochondrial oxygen consumption and uncoupled respiration in mature adipocytes expressing either PRDM16 or control vector under basal conditions (n=4, mean ± SD) (C), or after stimulation with a cAMP analog for 12 hours (n=4, mean ± SD) (D). L: lipid droplet. * p < 0.05; ** p < 0.01
In vivo differentiation of PRDM16-expressing fibroblasts into “brown” fat
Since the environmental cues that stimulate adipocyte differentiation in cell culture and in vivo are presumably different, we sought a more physiological setting to study PRDM16 action. To this end, we utilized transplantation studies in mice. Specifically, 107 PPARγ−/− fibroblasts, expressing PPARγ2 and PRDM16 or PPARγ2 alone, were implanted subcutaneously into nude mice. As shown originally by Howard Green and colleagues (Green and Kehinde, 1979), implanted fibroblasts were able to differentiate into fat tissue. PRDM16 and vector-expressing cells formed equivalent sized ectopic fat pads after 6–8 weeks (data not shown). Histological analysis showed no obvious differences in the morphology and appearance of the tissues derived from control or PRDM16 expressing cells with both fat pads containing largely unilocular adipocytes (Figure 4A). Importantly, fat pads from PRDM16-expressing cells displayed an average 16-fold increase in PRDM16 mRNA compared to control fat pads (Figure 4B), well matched to the ~14 fold enrichment in brown relative to white fat tissue (Figure 1A). Immunohistochemical analysis showed that only fat tissue from PRDM16-expressing cells and endogenous BAT contained easily detectable levels of the brown fat-specific cidea protein (Figure 4A). PRDM16 did not influence the tissue expression of general adipogenic markers PPARγ, or adiponectin (Figure 4C). However, the brown-selective genes UCP1, cidea, PGC-1α, elovl3 and PPARα were all induced by at least 5-fold in fat pads from PRDM16 transduced cells relative to control fat pads (Figure 4D). Interestingly, PRDM16 also induced the expression of the endogenous PRDM16 gene (PRDM16-3′UTR) suggesting that PRDM16 regulates itself in a positive feed-back loop (Figure 4D). Of note, the mRNA levels of resistin, a white fat selective gene, were reduced by 90% in PRDM16 relative to control- expressing tissue (Figure 4D). These data demonstrate that a physiological level of PRDM16 expression induces the gene expression program but not the multilocular appearance of BAT. This is as expected, since the morphology of BAT is not cell autonomous and depends on a high degree of sympathetic innervation (Minokoshi et al., 1986; Rothwell and Stock, 1984), which presumably could not take place during the time frame of these experiments.
Figure 4. Differentiation of PRDM16-expressing cells into brown fat in vivo.
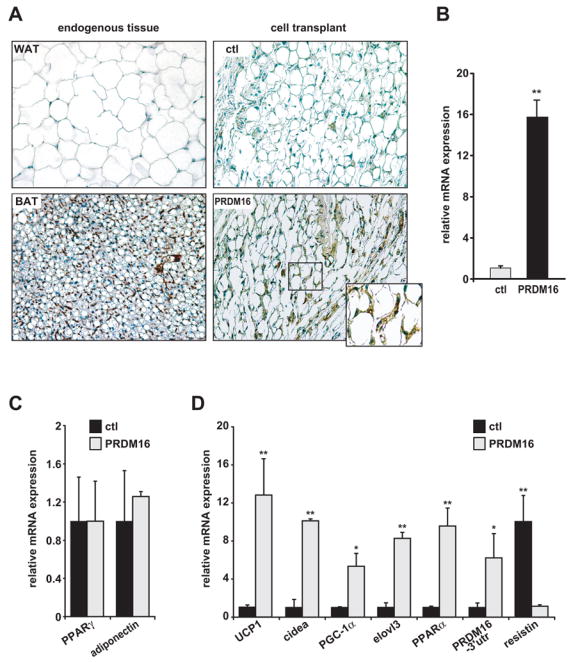
(A) Immunohistochemistry for cidea protein expression in endogenous WAT and BAT, and in ectopic subcutaneous fat pads formed from fibroblasts expressing PPARγ2 and either vector control (ctl) or PRDM16. (B–D) 8-weeks after transplantation, ectopic fat pads were analyzed by real-time PCR for expression of: PRDM16 (B); differentiation markers common to white and brown fat (PPARγ and adiponectin) (C); brown fat-selective genes (UCP1, cidea, PGC-1α, elovl3, PPAR-α, endogenous PRDM16 (-3′UTR) and the white fat selective marker, resistin (D). (n=10 mice per cell line, mean ± SE). * p < 0.05; ** p < 0.01
PRDM16 functions as a coregulator to activate PGC-1α and PGC-1β
Since PGC-1α has a prominent role as a regulator of brown fat thermogenic function and its mRNA is induced by the action of PRDM16, we studied PRDM16 function on this gene promoter. The expression of PRDM16 enhanced the activity of the -2kb PGC-1α promoter, but only in the presence of differentiation- inducing cocktail (Figure 5A). Additional experiments showed that activation of the PGC-1α promoter by PRDM16 required a cAMP stimulus, either by adding IBMX or forskolin (data not shown). PRDM16 similarly activated the -4kb upstream region of the UCP1 gene in forskolin-treated cells (not shown). Since PGC-1α is known to potently regulate its own expression in a feed-back loop (Handschin et al., 2003), we asked if PGC-1α is required for the action of PRDM16 on the PGC-1α promoter. In PGC-1α-deficient brown preadipocytes, the PGC-1α reporter gene was only activated by PRDM16 after re-expression of PGC-1α (Figure 5B). These data thus reveal a requirement for PGC-1α protein in the activation of the -2kb PGC-1α promoter by PRDM16.
Figure 5. PRDM16 activates PGC-1α and PGC-1β via direct binding.
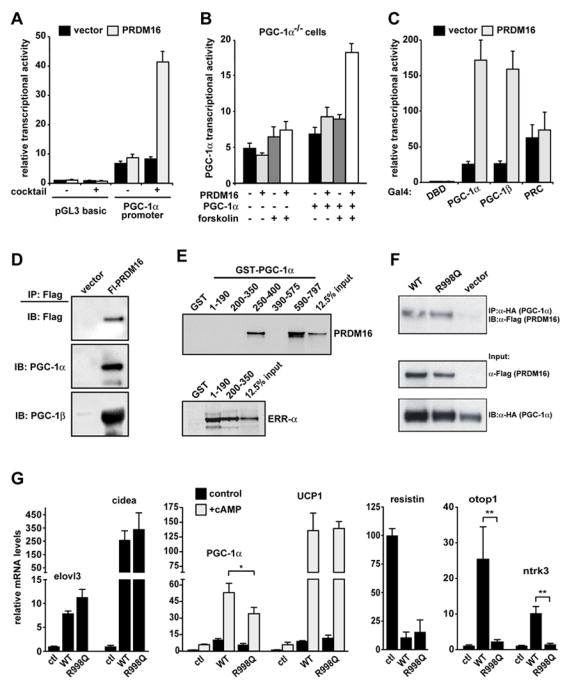
(A) The transcriptional activity of the -2 kb region of PGC-1α in response to PRDM16 or vector control expression in brown fat preadipocytes (n=3, mean ± SD). (B) PGC-1α promoter activity in response to PRDM16 or vector expression in PGC-1α-deficient cells (n=3, mean ± SD). (C) The transcriptional activity of Gal4-DNA binding domain (DBD) fusion proteins containing PGC-1α, PGC-1β, or PRC in response to PRDM16 or vector expression (n=3, mean ± SD). (D) Flag-PRDM16 and its associated proteins were immunoprecipitated from brown fat preadipocytes and analyzed by Western blot to detect PGC-1α and PGC-1β (E) GST fusion proteins containing different regions of PGC-1α were incubated with 35S- labeled PRDM16 protein. ERR-α was used to demonstrate binding to the 1–190 and 200–350 regions of PGC-1α. (F) PGC-1α and PRDM16 were co-precipitated from cos7 cells that had been transfected with HA-PGC-1α and either wildtype (WT) or R998Q mutant PRDM16. The input was 2% of the cell lysate used for immunoprecipitation. (G) WT or R998Q mutant PRDM16 were expressed with PPARγ2 in PPARγ−/− fibroblasts. After differentiation into adipocytes (day 6), real-time PCR was used to measure the mRNA expression of: brown fat-selective genes (as indicated); and resistin, a white fat selective gene (n=3, mean ± SD). * p < 0.05; ** p < 0.01
Previous studies showed that cells lacking PGC-1α and PGC-1β lose brown fat character (Uldry et al., 2006), so we investigated whether PRDM16 could enhance the transcriptional activity of PGC-1α and PGC-1β. To do this, PGC-1α, PGC-1β, PRC, SRC1 and VP16 were expressed as fusion proteins covalently bound to the Gal4-DNA binding domain (DBD), together with either PRDM16 or control vector. The transcriptional activity of Gal4- fusions was assayed using a reporter gene driven by the Gal1-UAS sequence. In these assays, PRDM16 robustly activated both PGC-1α and PGC-1β but did not activate the related coactivator protein PRC (Figure 5C), or the unrelated coactivators SRC1 and VP16 (not shown).
We then assessed whether the enhancement of PGC-1α and PGC-1β activity by PRDM16 was linked to a physical interaction between these proteins. Using co-immunoprecipitation assays in brown adipocytes, endogenous PGC-1α and PGC-1β proteins were detected in a complex with PRDM16 protein (Figure 5D). The PRDM16-PGC-1α interaction appeared to be direct since purified GST-PGC-1α fusion protein but not GST alone binds to in vitro translated PRDM16 (Figure 5E). Domain mapping experiments identified two regions in PGC-1α (250–400 and 590–797) that interact with PRDM16. Correct folding of PGC-1α fragments 1–190 and 200–350 was verified by their binding to another PGC-1α interacting protein, ERR-α. Taken together, these data strongly suggest that PRDM16 directs at least some aspects of brown fat determination and function through a direct interaction with the PGC-1α and PGC-1β transcriptional coactivator proteins.
PRDM16 contains two distinct DNA binding domains that consist of 7 C2H2 repeats at the N-terminus and 3 repeats at C-terminus. Since previous work has shown that PRDM16 binds directly to DNA (Nishikata et al., 2003), we asked whether PRDM16 requires direct sequence-specific DNA-binding to induce the brown fat gene program. Based on the structural characteristics of zinc finger motifs (Pavletich and Pabo, 1991), we created a series of mutants with point mutations in the conserved amino acids typically required for DNA binding. Among them, we found that an R998Q mutant allele completely loses DNA binding to the known consensus sequences of PRDM16 (Figure S3, A). Importantly, the R998Q mutant allele retains the ability to interact with PGC-1α (Figure 5F). We next examined whether the R998Q mutant protein was able to activate the brown fat gene program when expressed in PPARγ−/− cells together with PPARγ2. Importantly, adipocytes from these cultures expressed equivalent levels of PRDM16 mRNA (Figure S3, B). Strikingly, a large number of brown fat-selective genes including elovl3, cidea, PGC-1α and UCP1 were induced to similar extents by R998Q mutant and wildtype PRDM16 (Figure 5G). Moreover, resistin, a WAT-selective gene was markedly repressed by both wildtype PRDM16 and the R998Q allele (Figure 5G). By contrast, wildtype PRDM16 but not the R998Q allele induced the expression of otop1 and ntrk3 (Figure 5G). Taken together, these results indicate that PRDM16 does not require sequence-specific DNA binding to activate many, but not all, brown fat genes, and suggest that it functions in BAT determination, at least in part, through direct interaction with the PGC-1s and other proteins.
Knockdown of PRDM16 ablates the genetic program of brown fat
The requirement for PRDM16 in determining the brown fat phenotype was investigated using siRNA technology to specifically knockdown PRDM16 in the brown fat cell lineage. To this end, a siRNA sequence was identified that efficiently depleted PRDM16 mRNA and protein levels in murine cells (data not shown). Expression of the corresponding shRNA (short-hairpin) by retrovirus efficiently reduced PRDM16 expression in brown fat preadipocytes and completely blocked the differentiation-linked induction of PRDM16 mRNA in these cells (Figure 6A). Importantly, depletion of PRDM16 in immortalized brown fat cells did not suppress morphological differentiation, nor did it impact the expression of adipocyte markers that are not specific for brown fat, such as aP2, adiponectin or PPARγ (Figure 6B). Strikingly, however, PRDM16-depletion resulted in a broad loss of brown fat gene expression including severely reduced levels of UCP1, cidea, PGC-1α, elovl3 and PPARα relative to cells infected with a sh-scramble (SCR) control (Figure 6C). Notably, UCP1 and cidea, whose expression defines the identity of “brown fat”, were both decreased by 95% in PRDM16-depleted cells (Figure 6C). Furthermore, some white fat selective mRNAs, such as resistin were increased in PRDM16-depleted cells (Figure 6B).
Figure 6. Knockdown of PRDM16 in brown fat cells ablates their brown fat characteristics.
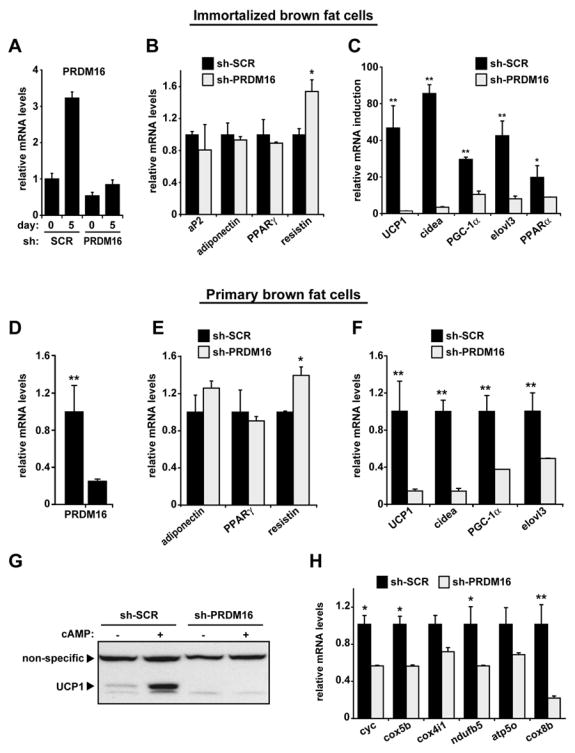
(A) PRDM16 mRNA levels in immortalized brown fat cells expressing shRNA targeted to PRDM16 or a scrambled (SCR) control- shRNA before (day 0) and after their differentiation (day 5) into adipocytes. (B) Gene expression in brown fat cells (day 5) expressing sh-PRDM16 or sh-SCR including: markers common to white and brown fat cells (aP2, PPARγ, adiponectin) and resistin, a white fat cell selective gene. (C) The differentiation-linked mRNA induction (day 0 to day 5) of brown fat- selective genes (as indicated) in sh-PRDM16 and sh-SCR expressing cells. (D–F) Gene expression in adipocytes (day 6) from sh-PRDM16 and sh-SCR expressing primary brown preadipocytes including mRNA levels of: PRDM16 (D); adiponectin, PPARγ and resistin (E); brown fat- selective genes (as indicated) (F). (G) Western blot analysis of UCP1 protein levels in primary brown fat cells expressing sh-PRDM16 or sh-SCR control with and without cAMP treatment. (H) mRNA levels of various mitochondrial components in adipocytes from sh-PRDM16 and sh-SCR expressing primary brown preadipocytes. (n= 3–5, mean ± SD). * p < 0.05; ** p < 0.01
Loss of function experiments were also performed in primary brown preadipocytes immediately after their isolation. Transduction of primary brown preadipocytes with sh-PRDM16 caused a ~70% reduction of PRDM16 mRNA at day 6 of differentiation (Figure 7D). Once again, this caused no visible decrease in morphological differentiation (not shown) and no difference in the expression of mRNAs common to white and brown fat cells, such as adiponectin and PPARγ (Figure 6E). As in the immortalized cells, the expression of most, if not all, brown fat selective genes, including UCP1, cidea, elovl3 and PGC-1α, were significantly decreased in PRDM16-depleted primary brown fat cells (Figure 6F). Specifically, loss of PRDM16 caused an 85% reduction in UCP1 and cidea mRNAs and a 60% decrease in the expression of PGC-1α. Furthermore, PRDM16-depleted brown fat cells exhibited close to a complete loss of UCP1 protein expression that was readily apparent in control cultures after cAMP stimulation (Figure 6G). Importantly, expression of a broad set of mitochondrial genes, that are vital to brown fat function, was significantly reduced by knockdown of PRDM16 in immortalized and primary brown fat cells (Figure 6H). This included a 70% reduction in the mRNA levels of cox8b, a highly brown fat selective mitochondrial gene (Figure 6H). The effect of PRDM16-depletion on the expression of the brown-white fat selective gene set in both cellular models is summarized in Table S1. These data establish a near absolute requirement for PRDM16 in determining the identity of brown fat cells. PRDM16, however, is not required for aspects of adipogenesis that are common to brown and white fat cells.
Figure 7. Transgenic expression of PRDM16 in WAT depots induces the formation of BAT cells.
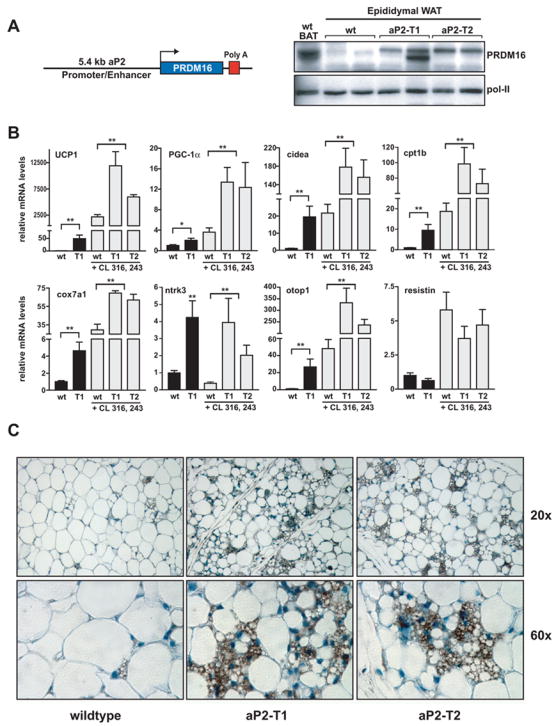
(A) The fat-specific aP2 promoter/enhancer was used to express PRDM16 in WAT depots. Western blot analysis for PRDM16 protein expression in: non-transgenic, wildtype (wt) BAT; wt WAT; and WAT from two strains of aP2-PRDM16 transgenic mice (aP2-T1 and aP2-T2). POL-II protein expression was used to control for loading. (B) Expression of BAT-selective genes (as indicated) and resistin in WAT from wildtype (wt) and aP2-T1 transgenic mice. This gene set was also measured in WAT from wt, aP2-T1 and aP2-T2 mice that had been treated with CL 316, 243 (n= 7–10 mice per group, mean ± SE). (C) Immunohistochemistry for UCP1 protein (brown stain) in sections of WAT from wt and transgenic mice (T1 and T2) after treatment with CL 316, 243. * p < 0.05; ** p < 0.01
Transgenic PRDM16 expression in WAT stimulates the formation of brown fat cells
PRDM16 can drive the transcriptional program of BAT when expressed in white fat progenitors before differentiation; however its ability to stimulate BAT cell determination in a purely in vivo context remained unclear. To address this question, we sought to express PRDM16 in WAT by transgenesis. Since there are no known fat-selective promoters that express well in preadipose cells, we utilized the aP2 promoter/enhancer, which is active during fat cell differentiation (Graves et al., 1992; Ross et al., 1990). We developed several transgenic mouse strains and the two highest expressing strains (aP2-T1 and aP2-T2) had levels of PRDM16 protein in epididymal WAT that were equivalent to or somewhat less than its expression in wildtype interscapular BAT (Figure 7A). PRDM16 is expressed in wildtype BAT and in one transgenic WAT sample as a closely spaced doublet of 150–170 kD; the molecular basis for this doublet is not known. Notably, transgenic PRDM16 expression induced a broad set of BAT-selective genes in epididymal white fat depots from 3–4 month old aP2-PRDM16 (T1) (n=7), as compared to non-transgenic littermates (n=10) (Figure 7B). For example, the mRNA levels of UCP1, cidea, cpt1b, and otop1 were increased by 50-, 20-, 10-, and 20-fold respectively in WAT from aP2-PRDM16 relative to non-transgenic mice. PGC-1α, cox7a1 and ntrk3 were also significantly elevated by transgenic expression of PRDM16. Furthermore, expression of resistin, a white fat cell selective gene, was slightly decreased (though not statistically significantly) in aP2-PRDM16 transgenic fat. These data demonstrate a robust induction of the genetic program of brown fat by PRDM16 in a classic white fat depot.
WAT is poorly innervated, at least compared to BAT, and the action of PRDM16 as a determinant of BAT in cellular models was enhanced by cAMP treatment, which mimics sympathetic (adrenergic) input. We therefore treated wildtype and aP2-PRDM16 mice (aP2-T1 and aP2-T2) with CL 316, 243, a selective β3-adrenergic agonist, for 6 days. In wildtype WAT, this treatment increased PRDM16 mRNA expression by 4-fold (not shown) coincident with the emergence of small clusters of brown fat cells (see later) and increased expression of many brown fat-selective genes (Figure 7B). Notably, the induction of these BAT-related genes in WAT was greatly enhanced in both strains of aP2-PRDM16 mice (Figure 7B). In particular, UCP1, PGC-1α and cidea were expressed to 5-fold, 4-fold and 9-fold higher levels respectively in the WAT of CL316, 243 treated aP2-PRDM16 relative to non-transgenic mice (n=8 (T1), n=6 (T2)) (Figure 7B). Importantly, BAT-selective genes were expressed in WAT of aP2-PRDM16 animals at a significant fraction of their levels in similarly treated bona fide interscapular BAT. For example, PGC-1α, UCP1 and cidea were expressed in transgenic WAT at about 80%, 15% and 30% of wildtype BAT levels (not shown).
The transgenic mice were also investigated for the morphological emergence of brown fat cells. As shown in Figure 7C and as demonstrated before (Ghorbani and Himms-Hagen, 1997; Himms-Hagen et al., 1994), WAT from CL 316, 243 treated control animals showed few cells with a multilocular appearance that also stained positively for UCP1. In striking contrast, WAT depots from aP2-PRDM16 mice had a genuine chimeric appearance with very abundant clusters of multilocular BAT-type cells that stained intensely for UCP1 protein (Figure 7C). Importantly, all the multilocular cells in the WAT of aP2-PRDM16 transgenic mice expressed UCP1 protein and all UCP1-expressing cells had a multilocular appearance, suggesting that these cells are bona fide brown fat cells. Approximately 10–20% of all cells in epididymal WAT depots of aP2-PRDM16 transgenic mice were BAT-type fat cells, while equivalent depots of the control animals had less than 1% of BAT-type cells. These data show that PRDM16, when expressed at or below authentic BAT levels, can control the determination of brown adipose cells in a classic white fat depot.
Discussion
A detailed understanding of the brown fat cell and its unique ability to dissipate chemical energy may offer new treatment avenues for obesity and associated diseases. In small mammals that retain distinct brown fat pads as adults, the heat produced by brown fat is an important contributor to overall energy balance (Cannon and Nedergaard, 2004; Cederberg et al., 2001; Ghorbani et al., 1997; Ghorbani and Himms-Hagen, 1997; Guerra et al., 1998; Kopecky et al., 1995; Kopecky et al., 1996; Lowell et al., 1993; Rothwell and Stock, 1979; Tsukiyama-Kohara et al., 2001). Although adult humans do not have distinct brown fat depots, they do appear to have small numbers of brown adipocytes in their white fat depots, and these brown cells proliferate under certain circumstances, such as chronic cold exposure (Garruti and Ricquier, 1992; Huttunen et al., 1981; Lean et al., 1986; Oberkofler et al., 1997). Thus, the molecular mechanisms that regulate brown fat cell determination are of significant and escalating biomedical interest.
A great deal is known about the metabolism and function of mature white and brown adipocytes; however, the developmental origins of these cell lineages have remained elusive. The majority of brown fat develops prenatally and is mature and fully functional at birth, when thermogenic requirements are particularly high (Nedergaard et al., 1986). Most WAT, on the other hand, develops postnatally in response to relative nutritional excess. It has been presumed that white and brown fat cells are closely related to each other developmentally because they express many common enzymes, and both require PPARγ for their differentiation. Interestingly, recent fate-mapping experiments in mice show that interscapular brown fat but not white fat arises from a population of Engrailed-1 expressing cells in the dermomyotome, a structure that also gives rise to muscle and skin (Atit et al., 2006). These data suggests that the two types of fat cells may have quite different origins.
PRDM16 is expressed very selectively in brown fat cells versus white fat cells and stimulates nearly all the key characteristics of authentic brown fat cells when expressed at or near physiological levels. This includes enhanced mitochondrial gene expression and mitochondrial density, increased expression of PGC-α, UCP1 and a very large increase in the uncoupled fraction of respiration. Importantly, the expression of UCP1 and PGC-1α induced by PRDM16 is further enhanced by cAMP, as it is in authentic brown fat cells. At a global scale, a large majority of genes that are selectively expressed in brown adipocytes are positively regulated by PRDM16 (Table S1). Conversely, PRDM16 expression suppressed the mRNA levels of several genes that are selectively enriched in white fat such as resistin and serpin3ak. Notably, PRDM16 expression does not influence the expression of those genes that are common to both brown and white fat cells. Interestingly, several BAT genes including UCP1 and PGC-1α were induced by PRDM16 in the genetic absence of PPARγ (and therefore fat cell differentiation). Importantly, PRDM16 is shown here to activate a brown fat gene program in many different kinds of adipocytes as long as it is introduced before cell differentiation. These results suggest a model in which PRDM16 functions to establish “brown” identity including UCP1 expression and increased mitochondria that is, at least partly, separable from the adipogenic differentiation pathway common to white and brown fat cells (Figure S4). Why PRDM16 is not effective when expressed after adipogenic differentiation in these experiments is not known at present.
A very effective shRNA directed against PRDM16 allowed us to ask about the requirement for this factor in the expression of brown fat-selective genes in established brown fat cell lines, and in primary brown fat cells. The reduction of PRDM16 levels has no effect on morphological differentiation of these cells, but causes an almost complete suppression of brown fat-selective genes, including UCP1 mRNA and protein, while leaving intact the expression of genes common to both white and brown fat cells such as PPARγ and aP2. Clearly, PRDM16 is required for the expression of the brown fat phenotype in isolated cells. Examination of this feature will be important in mice ablated for PRDM16 in vivo.
A key question is how does PRDM16 stimulate the development of a brown fat gene program? This protein is annotated in databases as a potential transcription factor because it possesses two zinc-fingers. However, while zinc finger proteins are often DNA binding factors, it is also clear that zinc finger domains can mediate protein-protein interactions (Leon and Roth, 2000). We have confirmed that PRDM16 does indeed bind to DNA in a sequence-specific manner, but this is not required for its regulation of many BAT-selective genes. On the other hand, it is clear that PRDM16 activates the expression as well as the transcriptional function of both PGC-1α and PGC-1β, apparently through direct physical binding. While functions of PRDM16 in other aspects of brown fat regulation are by no means ruled out, the PRDM16-stimulated activity of PGC-1α and PGC-1β may explain many actions of PRDM16 in brown fat determination (Figure S4).
As shown previously, PGC-1α can activate many of the genes that comprise the thermogenic program of brown fat, such as UCP1 and Deiodinase d2 (Puigserver et al., 1998). On the other hand, PRDM16 is shown to have certain actions here that are not consistent with a function solely through modulation of PGC-1α expression and/or function. In fact, genetic studies have shown conclusively that mice lacking PGC-1α retain identifiable, though abnormal, brown fat tissue (Lin et al., 2004). Similarly, isolated brown fat cells lacking PGC-1α still express several genes characteristic of brown fat (Uldry et al., 2006). However, it is notable that shRNA-mediated suppression of PGC-1β in the cells lacking PGC-1α caused a further loss of the brown fat phenotype (Uldry et al., 2006). Thus, it is possible that PRDM16 functions as a brown fat determination factor, at least in part, by robustly stimulating PGC-1α and PGC-1β simultaneously. PRDM16 may also increase PGC-1 coactivator function in other tissues where it is expressed, such as heart, brain and kidney, with important physiological consequences.
How PRDM16 achieves this activation of the PGC-1s remains to be determined. The PRDI-BF1 and RIZ homology (PR) domain present in a subclass of zinc finger proteins, including PRDM16, is highly homologous to the SET domain that is noted for its histone lysine methyltransferase activity and diverse functions in regulating chromatin structure (Huang et al., 1998; Rea et al., 2000). Other PR-domain containing factors such as RIZ1 (Retinoblastoma interacting zinc finger-1) and Meisitz have intrinsic histone methyltransferase activity, while PRISM (PR-domain in smooth muscle) binds and recruits a histone methyltransferase (Davis et al., 2006; Hayashi et al., 2005; Kim et al., 2003). Interestingly, another methyltransferase protein, PRMT1, has been shown to bind to PGC-1α and activate it via arginine methylation (Teyssier et al., 2005). Whether the PR-domain of PRDM16 has enzymatic function and whether this activity is required for stimulating brown fat gene expression remains to be established.
Components that can influence the brown fat phenotype, in addition to the PGC-1s, have been identified, such as FOXC2, Rb, p107 and RIP140 (Cederberg et al., 2001; Christian et al., 2005; Hansen et al., 2004; Leonardsson et al., 2004; Powelka et al., 2006; Scime et al., 2005). It will be important to investigate their genetic interactions with PRDM16. The absolute requirement for PRDM16 in the formation of brown adipocytes suggests that the mechanism of action of these other factors may involve PRDM16. Whether they act upstream or downstream of PRDM16 in the differentiation program of brown fat remains to be elucidated.
The replacement of BAT with WAT in humans, and in obese mice and rats, has shown that these tissues can interconvert to some extent in vivo. Similarly, prolonged exposure to cold or β–adrenergic agonists induces the appearance of many brown fat cells within classic white fat depots (Himms-Hagen et al., 2000). This so-called “transdifferentiation” of fat by cold-exposure or β–adrenergic agonists could be due to the acquisition of brown fat cell features in preformed white fat cells and/or by the differentiation of resident committed brown preadipocytes into mature brown fat cells. While this important issue is not entirely closed, the latter scenario is supported by the observation that preadipocytes contained in fat tissues are committed to the brown or white fate (Klaus, 1997; Klaus et al., 1994; Klaus et al., 1995; Kozak and Kozak, 1994). In light of these data, it is very intriguing that PRDM16 expression did not convert white mature adipocytes into brown type cells. However, the white fat cell precursors present in whole fat tissues could be converted to a brown fat-like phenotype very efficiently when PRDM16 was introduced before differentiation. Furthermore, the emergence of UCP1-positive brown fat cells in the white fat depots of transgenic mice suggests strongly that PRDM16 directs the differentiation of resident white fat progenitors into the BAT fate. The small clusters of BAT cells in wildtype WAT most likely arise from BAT progenitors that are present in small numbers within white depots. The stimulation of PRDM16 action by β-adrenergic signaling is not surprising, given the known importance of this cascade in BAT development (Cannon and Nedergaard, 2004). The mechanism by which cAMP signaling modulates the activity of PRDM16 is an important outstanding question from this study.
Taken together, inducing PRDM16 expression in preadipocytes could constitute a strategy to raise whole body energy expenditure and prevent excess fat accumulation. This can be done, in theory, by using drugs that raise PRDM16 levels in fat cell precursors, or by engineering preadipocytes ex vivo, and then reinjecting them, analogous to the transplantation experiments done here. More experimentation will be necessary to determine how this brown fat determination factor might be used to fight obesity in the context of a whole animal.
Experimental Procedures
Plasmids and viral vectors
Full-length coding sequence of mouse PRDM16 (GeneID: 70673) was amplified from brown fat RNA by PCR, and cloned into the Xho1/EcoR1 sites of pMSCV-puro retroviral vector (Stratagene) in frame with an N-terminal FLAG tag. PRDM16 cDNA was also cloned into the Xho1/EcoR1 sites of pcDNA3.1 (Invitrogen) for expression in mammalian cells. DNA-binding mutants of PRDM16, including the R998Q allele, were created by site-directed mutagenesis (Quickchange, Stratagene). Adenoviral vectors for PRDM16 expression were made in pAdTrack-CMV using the AdEasy system as described elsewhere (He et al., 1998). Adenoviral vectors for PGC-1α and GFP (Yoon et al., 2001); Gal4 fusion plasmids containing PGC-1β (Lin et al., 2002), PGC-1α and SRC-1 (Puigserver et al., 1999) have been described previously. The siRNA sequence identified for use in PRDM16-depletion experiments was: 5′-GAAGAGCGUGAGUACAAAU-3′ (Dharmacon). The corresponding double-stranded DNA sequence was ligated into pSUPER-Retro (GFP-Neo) (Oligoengine) for retroviral shRNA expression. This siRNA sequence was able to specifically knockdown PRDM16 protein and mRNA expression by over 75%.
Cell culture
3T3-L1, C3H-10T1/2 and cos7 cells were obtained from ATCC. Immortalized brown fat preadipocytes and PPARγ−/− fibroblasts have been described by others (Rosen et al., 2002; Uldry et al., 2006). 3T3-F442A cells were from H. Green (Green and Kehinde, 1979). Primary brown fat preadipocytes were obtained from mice by collagenase digestion as described elsewhere (Tseng et al., 2004). Primary white fat stromal vascular and mature fat cells were fractionated according to published methods (Rodbell, 1964; Soukas et al., 2001). For retrovirus production, φnx packaging cells (Kinsella and Nolan, 1996) were transfected at 70% confluence by Calcium Phosphate co-precipitation with 10 μg retroviral vectors; viral supernatant was harvested 48 hours later. For retroviral transduction, cells were incubated overnight with viral supernatant supplemented with 8 μg/mL polybrene. For adenoviral infection of mature adipocytes, the fat cell fraction (see above) from epididymal WAT of 10–12 week old C57Bl6 mice (N=4) was incubated with PRDM16 (GFP) or GFP-expressing adenovirus (M.O.I=100) in 0.5 mL eppendorf tubes for 2 hours in 10% FBS/DMEM. The medium was then replaced, and cells were maintained at 5% C02, 37°C for an additional 48 hours prior to RNA extraction and expression analysis. GFP expressed from the adenoviral vectors was used to monitor infection efficiency, which was typically over 50%.
Adipocyte differentiation was induced by treating confluent cells for 48 hours in medium containing 10% FBS, 0.5 mM isobutylmethylxanthine, 125 nM indomethacin, 1 μM dexamethosone, 850 nM insulin, 1 nM T3 and 1 μM rosiglitazone (Cayman Chemical). Two days after induction, cells were switched to maintenance medium containing 10% FBS, 850 nM insulin, 1 nM T3 and 1 μM rosiglitazone. To stimulate thermogenesis, cells were incubated with 0.5 mM dibutyrl cyclic-AMP for 4 hours. All chemicals for cell culture were obtained from Sigma, unless otherwise indicated.
Global expression screen of transcription-related factors
RNA was isolated from brown adipose tissue (BAT) and epididymal white fat tissue (WAT) of 5 adult male C57Bl6 mice at 10–12 weeks of age. First strand cDNA was prepared from RNA samples using oligo-d(T) and Superscript III (Invitrogen). cDNAs were normalized for equal loading by real-time PCR using TBP and HPRT mRNA expression levels. 30 cycles of PCR (94°C-30 sec, 62°C-45 sec, 68°C-45 sec) with primer-sets described in (Gray et al., 2004) were performed in 50 μl reactions using Platinum Taq (Invitrogen). PCR products (average of 500 bp) were separated on 96 well-2% agarose E-gels (Invitrogen) containing ethidium bromide, imaged, and analyzed using E-Editor software (Invitrogen).
Reporter Gene Assays
Reporter gene assays were performed in cos7 cell or brown fat preadipocytes. Briefly, the -2kb-PGC-1α reporter gene (Handschin et al., 2003) or the UCP1 -4kb reporter (a gift from L. Kozak) were transiently transfected with PRDM16 and/or PGC-1α expression plasmids in 12 well plates using Fugene6 (Roche). Forty-eight hours after transfection, cells were harvested and reporter gene activity was measured using the Dual-luciferase Reporter Assay System (Promega). Forskolin (100 μM) or differentiation cocktail was added for 4 or 24 hours prior to harvesting cells, respectively. Gal4 based reporter gene assays were performed in cos7 cells by co-transfecting Gal4-DBD or Gal4-DBD fusion constructs with PRDM16 or control expression constructs. Firefly luciferase reporter gene measurements were normalized using Renilla luciferase activity.
Binding studies and electrophoretic mobility shift assays (EMSA)
To study protein–protein interactions, brown fat preadipocytes were transduced with retroviral-PRDM16 or control vector, and induced to differentiate into adipocytes. At day 6 of differentiation, whole cell extracts were incubated with flag-M2 agarose (Sigma) overnight at 4°C. Immunoprecipitates were washed three times with washing buffer (20 mM Tris HCl, 150 mM NaCl, 10% glycerol, 2 mM EDTA, 0.1% NP40, 0.1 mM PMSF), resolved by SDS–PAGE, transferred to PVDF membrane (Millipore) and blotted with anti-PGC-1α (Puigserver et al., 1999) and PGC-1β (Lin et al., 2005) antibodies. For GST-immunoaffinity assays, GST-PGC-1α fusion proteins immobilized to glutathione sepharose beads were purified as previously described (Wallberg et al., 2003). 35S-labeled PRDM16 or ERRα proteins were in vitro translated using TNT coupled transcription-translation system (Promega). To examine the binding of PGC-1α with the R998Q mutant allele of PRDM16, cos7 cells were co-transfected with HA-PGC-1α and Flag-PRDM16 (wt and R998Q). Whole cell extracts were immunoprecipitated with HA antibody (Roche), resolved by SDS-PAGE and blotted with anti-Flag antibody to detect PRDM16. For EMSA, an oligonucleotide probe containing the PRDM16 binding site (5′-GATCCGACAAGATAAGATAAGGATCTATAAGAAGATGAGGTATG-3′) (Nishikata et al., 2003) was end-labeled with γ32P-ATP and incubated (5 fmol) with in vitro translated wildtype and R998Q mutant PRDM16 in binding buffer (10 mM Tris-HCl (pH 7.5), 50mM NaCl, 1mM MgCl2, 0.5mM EDTA, 4% glycerol, 0.5mM DTT, and 0.5 μg poly(dI-dC)-poly(dI-dC)). 50 or 500 fmol of unlabeled probe was added to the binding reaction for competition assays. DNA-protein complexes were separated by electrophoresis on a 4% PAGE gel then dried and exposed to X-ray film.
Oxygen Consumption Assays
PPARγ−/− fibroblasts transduced with retroviral-PPARγ2 and either PRDM16 or vector control were grown to confluence and induced to differentiate into adipocytes. At day 6 of differentiation, oxygen consumption was measured in fat cells as described previously (St-Pierre et al., 2003). For cyclic-AMP-induced respiration assays, fully differentiated fat cells were incubated with 0.5 mM dibutyrl cyclic-AMP for 12 hours before measuring oxygen consumption.
Electron Microscopy
Electron microscopy was performed as described previously (St-Pierre et al., 2003) on PPARγ−/− cells expressing PPARγ2 and PRDM16 or vector control before and after their differentiation into adipocytes (day 6). To calculate the mitochondrial volume density, a grid was laid on randomly selected micrographs (n>20), and the number of points falling onto mitochondria was expressed as a fraction of those landing on cell area.
Real-time PCR analysis and Western blotting
Total RNA from cultured cells was isolated using Qiagen Rneasy mini columns according to manufacturer’s instruction. Tissue RNA samples were prepared by the Trizol method (Invitrogen). Northern blot analysis was performed as described before (Maniatis et al., 1982). For real-time PCR analysis, RNA was reverse transcribed using the IScript cDNA synthesis kit (BioRad), and used in quantitative PCR reactions containing SYBR-green fluorescent dye (ABI). Relative expression of mRNAs was determined after normalization with TBP levels using the ΔΔ-Ct method. Q-PCR was performed using the ABI-9300 PCR machine. As a point of reference, the Ct values for both PRDM16 and TBP mRNA expression in BAT were typically 24–26. Student’s t-test was used for comparisons and to obtain statistics. Primers used for real-time PCR are shown in Table S2. For western blot analysis, cells or tissues were lysed in RIPA buffer (0.5% NP-40, 0.1% sodium deoxycholate, 150 mM NaCl, 50 mM Tris-Cl, pH 7.5). Lysates were resolved by SDS-PAGE, transferred to PVDF membrane (Millipore) and probed with anti-UCP1 (Chemicon), anti-Flag M2 (Sigma), anti-PRDM16 (rabbit polyclonal), and anti-pol-II (Santa-Cruz biotechnology).
Animals
All animal experiments were performed according to procedures approved by the Dana-Farber Cancer Institute’s Institutional Animal Care and Use Committee. Mice were maintained on a standard rodent chow diet with 12 hr light and dark cycles. For acute cold-exposure studies, BAT was obtained from five 3–4 week old male C57Black6 mice that were housed at 4°C for 4 hours. For transgenic mice, the complete PRDM16 cDNA was cloned 3′ to the 5.4 kb aP2 promoter/enhancer and the human growth hormone polyadenylation site was inserted 3′ to the cDNA. FVB Mouse oocytes were injected with this construct by the Dana-Farber Cancer Institute Core Facility. CL316, 243 (Sigma) at 0.5 mg/kg was injected intraperitoneally into mice daily for 6 days. Mice were euthanized for analysis of tissues 4 hours following the final injection on day 6. Transgenic and non-transgenic control littermates used for all experiments were 4–6 month old males. For cell transplantion, 107 fibroblasts, transduced with retroviral-PPARγ2 and either PRDM16 or vector control, were suspended in 200 μl of 10% FBS/DMEM and implanted subcutaneously just above the sternum of nude (Nu/Nu) mice (Taconic) (N=10 mice/stable cell line) using an insulin syringe attached to a 28 gauge needle. This injection site was chosen due to the almost complete absence of endogenous subcutaneous fat. For Immonohistochemistry, paraffin-embedded sections were incubated with anti-cidea (Chemicon) or anti-UCP1 (Abcam) antibodies for 30 min at room temperature, followed by detection using the ABC Vectastain-Elite kit (Vector Labs) according to manufacturer’s instructions.
Transcriptional profiling
Total RNA was isolated from the epididymal WAT and interscapular BAT of 3 male C57Bl6 mice at 10–12 weeks of age. Array hybridization and scanning were performed by the Core Facility at Dana-Farber Cancer Institute using Affymetrix mouse 430 high-density oligonucleotide arrays according to established methods (Lockhart et al., 1996). The array data were normalized using the DNA-Chip Analyzer (dChip) software (Li and Wong, 2001). The statistical significance of differences in gene expression was assessed by unpaired t test (p < 0.05). The microarray data set has been deposited in the Gene Expression Omnibus repository (http://www.ncbi.nlm.gov/geo/), accession no. GSE8044.
Supplementary Material
Acknowledgments
We thank Pere Puigserver for helpful discussions and critical reading of the manuscript; and Carine Valle (Inserm U858) for expert technical assistance. PS is supported by a fellowship from the American Heart Association and previously by a Canadian Institutes of Health Research Fellowship. S.K. is supported by a fellowship from the Japan Society for the promotion of Science. This work is supported by NIH grants DK31405-24 to BMS; Inserm, the Agence Nationale de la Recherche ANR-05-PCOD-012-04 to DL; and the European Commission FP6 project HEPADIP (LSHM-CT-2005-018734 to DL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–1493. doi: 10.1074/jbc.M006246200. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Bronnikov G, Houstek J, Nedergaard J. Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. J Biol Chem. 1992;267:2006–2013. [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG. RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol. 2005;25:9383–9391. doi: 10.1128/MCB.25.21.9383-9391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- Dahle MK, Gronning LM, Cederberg A, Blomhoff HK, Miura N, Enerback S, Tasken KA, Tasken K. Mechanisms of FOXC2- and FOXD1-mediated regulation of the RI alpha subunit of cAMP-dependent protein kinase include release of transcriptional repression and activation by protein kinase B alpha and cAMP. J Biol Chem. 2002;277:22902–22908. doi: 10.1074/jbc.M200131200. [DOI] [PubMed] [Google Scholar]
- Davis CA, Haberland M, Arnold MA, Sutherland LB, McDonald OG, Richardson JA, Childs G, Harris S, Owens GK, Olson EN. PRISM/PRDM6, a transcriptional repressor that promotes the proliferative gene program in smooth muscle cells. Mol Cell Biol. 2006;26:2626–2636. doi: 10.1128/MCB.26.7.2626-2636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garruti G, Ricquier D. Analysis of uncoupling protein and its mRNA in adipose tissue deposits of adult humans. Int J Obes Relat Metab Disord. 1992;16:383–390. [PubMed] [Google Scholar]
- Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- Graves RA, Tontonoz P, Platt KA, Ross SR, Spiegelman BM. Identification of a fat cell enhancer: analysis of requirements for adipose tissue-specific gene expression. J Cell Biochem. 1992;49:219–224. doi: 10.1002/jcb.240490303. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979;101:169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- Gronning LM, Baillie GS, Cederberg A, Lynch MJ, Houslay MD, Enerback S, Tasken K. Reduced PDE4 expression and activity contributes to enhanced catecholamine-induced cAMP accumulation in adipocytes from FOXC2 transgenic mice. FEBS Lett. 2006;580:4126–4130. doi: 10.1016/j.febslet.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci U S A. 2003;100:7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JB, Jorgensen C, Petersen RK, Hallenborg P, De Matteis R, Boye HA, Petrovic N, Enerback S, Nedergaard J, Cinti S, et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci U S A. 2004;101:4112–4117. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yoshida K, Matsui Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005;438:374–378. doi: 10.1038/nature04112. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Huang S, Shao G, Liu L. The PR domain of the Rb-binding zinc finger protein RIZ1 is a protein binding interface and is related to the SET domain functioning in chromatin-mediated gene expression. J Biol Chem. 1998;273:15933–15939. doi: 10.1074/jbc.273.26.15933. [DOI] [PubMed] [Google Scholar]
- Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46:339–345. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- Kim KC, Geng L, Huang S. Inactivation of a histone methyltransferase by mutations in human cancers. Cancer Res. 2003;63:7619–7623. [PubMed] [Google Scholar]
- Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Klaus S. Functional differentiation of white and brown adipocytes. Bioessays. 1997;19:215–223. doi: 10.1002/bies.950190307. [DOI] [PubMed] [Google Scholar]
- Klaus S, Choy L, Champigny O, Cassard-Doulcier AM, Ross S, Spiegelman B, Ricquier D. Characterization of the novel brown adipocyte cell line HIB 1B. Adrenergic pathways involved in regulation of uncoupling protein gene expression. J Cell Sci. 1994;107(Pt 1):313–319. doi: 10.1242/jcs.107.1.313. [DOI] [PubMed] [Google Scholar]
- Klaus S, Ely M, Encke D, Heldmaier G. Functional assessment of white and brown adipocyte development and energy metabolism in cell culture. Dissociation of terminal differentiation and thermogenesis in brown adipocytes. J Cell Sci. 1995;108(Pt 10):3171–3180. doi: 10.1242/jcs.108.10.3171. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Uncoupling protein--a useful energy dissipator. J Bioenerg Biomembr. 1999;31:419–430. doi: 10.1023/a:1005440221914. [DOI] [PubMed] [Google Scholar]
- Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky J, Rossmeisl M, Hodny Z, Syrovy I, Horakova M, Kolarova P. Reduction of dietary obesity in aP2-Ucp transgenic mice: mechanism and adipose tissue morphology. Am J Physiol. 1996;270:E776–786. doi: 10.1152/ajpendo.1996.270.5.E776. [DOI] [PubMed] [Google Scholar]
- Kozak UC, Kozak LP. Norepinephrine-dependent selection of brown adipocyte cell lines. Endocrinology. 1994;134:906–913. doi: 10.1210/endo.134.2.7905411. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Lean ME, James WP. Brown adipose tissue in man. In: Trayhurn DGNP, editor. Brown Adipose Tissue. London, Baltimore, MD: E. Arnold; 1986. pp. 339–365. [Google Scholar]
- Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue in patients with phaeochromocytoma. Int J Obes. 1986;10:219–227. [PubMed] [Google Scholar]
- Leon O, Roth M. Zinc fingers: DNA binding and protein-protein interactions. Biol Res. 2000;33:21–30. doi: 10.4067/s0716-97602000000100009. [DOI] [PubMed] [Google Scholar]
- Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R, Parker MG. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci U S A. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wong L. Emerging patterns and gene expression data. Genome Inform. 2001;12:3–13. [PubMed] [Google Scholar]
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta ), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem. 2002;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Lockhart DJ, Dong H, Byrne MC, Follettie MT, Gallo MV, Chee MS, Mittmann M, Wang C, Kobayashi M, Horton H, Brown EL. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- Lowell BB, V SS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Lupien JR, Glick Z, Saito M, Bray GA. Guanosine diphosphate binding to brown adipose tissue mitochondria is increased after single meal. Am J Physiol. 1985;249:R694–698. doi: 10.1152/ajpregu.1985.249.6.R694. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Sambrook J, Fritsch EF. Molecular cloning : a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- Minokoshi Y, Saito M, Shimazu T. Metabolic and morphological alterations of brown adipose tissue after sympathetic denervation in rats. J Auton Nerv Syst. 1986;15:197–204. doi: 10.1016/0165-1838(86)90063-9. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bronnikov G, Houstek J, Golozoubova V, Tvrdik P. Norepinephrine-induced proliferation of brown fat cell precursors. Exp Nephrol. 1994;2:135. [PubMed] [Google Scholar]
- Nedergaard J, Cannon B, Lindberg O. Microcalorimetry of isolated mammalian cells. Nature. 1977;267:518–520. doi: 10.1038/267518a0. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Connally E, Cannon B. Brown adipose tissue in the mammalian neonate. In: Trayhurn P, Nicholls DG, editors. Brown Adipose Tissue. Baltimore: Edward Arnold; 1986. pp. 1–30. [Google Scholar]
- Nedergaard J, Petrovic N, Lindgren EM, Jacobsson A, Cannon B. PPARgamma in the control of brown adipocyte differentiation. Biochim Biophys Acta. 2005;1740:293–304. doi: 10.1016/j.bbadis.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Nishikata I, Sasaki H, Iga M, Tateno Y, Imayoshi S, Asou N, Nakamura T, Morishita K. A novel EVI1 gene family, MEL1, lacking a PR domain (MEL1S) is expressed mainly in t(1;3)(p36;q21)-positive AML and blocks G-CSF-induced myeloid differentiation. Blood. 2003;102:3323–3332. doi: 10.1182/blood-2002-12-3944. [DOI] [PubMed] [Google Scholar]
- Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res. 1997;38:2125–2133. [PubMed] [Google Scholar]
- Pavletich NP, Pabo CO. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991;252:809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, Czech MP. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB, Cannon B, Ching TM, Lindberg O. Oxidative metabolism in cells isolated from brown adipose tissue. 2. Catecholamine regulated respiratory control. Eur J Biochem. 1968;7:51–57. doi: 10.1111/j.1432-1033.1968.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Reed N, Fain JN. Stimulation of respiration in brown fat cells by epinephrine, dibutyryl-3′,5′-adenosine monophosphate, and m-chloro(carbonyl cyanide)phenylhydrazone. J Biol Chem. 1968;243:2843–2848. [PubMed] [Google Scholar]
- Ricquier D, Nechad M, Mory G. Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. J Clin Endocrinol Metab. 1982;54:803–807. doi: 10.1210/jcem-54-4-803. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of Isolated Fat Cells. I. Effects of Hormones on Glucose Metabolism and Lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Ross SR, Graves RA, Greenstein A, Platt KA, Shyu HL, Mellovitz B, Spiegelman BM. A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proc Natl Acad Sci U S A. 1990;87:9590–9594. doi: 10.1073/pnas.87.24.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Combined effects of cafeteria and tube-feeding on energy balance in the rat. Proc Nutr Soc. 1979;38:5A. doi: 10.1079/pns19790026. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Effects of denervating brown adipose tissue on the responses to cold, hyperphagia and noradrenaline treatment in the rat. J Physiol. 1984;355:457–463. doi: 10.1113/jphysiol.1984.sp015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, Rudnicki MA. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Soukas A, Socci ND, Saatkamp BD, Novelli S, Friedman JM. Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J Biol Chem. 2001;276:34167–34174. doi: 10.1074/jbc.M104421200. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR. Activation of nuclear receptor coactivator PGC-1alpha by arginine methylation. Genes Dev. 2005;19:1466–1473. doi: 10.1101/gad.1295005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem. 2003;278:33370–33376. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid- activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kriauciunas KM, Kokkotou E, Kahn CR. Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol Cell Biol. 2004;24:1918–1929. doi: 10.1128/MCB.24.5.1918-1929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- Tvrdik P, Asadi A, Kozak LP, Nedergaard J, Cannon B, Jacobsson A. Cig30, a mouse member of a novel membrane protein gene family, is involved in the recruitment of brown adipose tissue. J Biol Chem. 1997;272:31738–31746. doi: 10.1074/jbc.272.50.31738. [DOI] [PubMed] [Google Scholar]
- Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha. Mol Cell. 2003;12:1137–1149. doi: 10.1016/s1097-2765(03)00391-5. [DOI] [PubMed] [Google Scholar]
- Westerberg R, Mansson JE, Golozoubova V, Shabalina IG, Backlund EC, Tvrdik P, Retterstol K, Capecchi MR, Jacobsson A. ELOVL3 is an important component for early onset of lipid recruitment in brown adipose tissue. J Biol Chem. 2006;281:4958–4968. doi: 10.1074/jbc.M511588200. [DOI] [PubMed] [Google Scholar]
- Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


