Summary
Bioactive lysophospholipids include lysophosphatidic acid (LPA), sphingosine 1-phosphate (S1P), cyclic-phosphatidic acid (CPA) and alkyl glycerolphosphate (AGP). These lipid mediators stimulate a variety of responses that include cell survival, proliferation, migration, invasion, wound healing, and angiogenesis. Responses to lysophospholipids depend upon interactions with biomolecular targets in the G protein-coupled receptor (GPCR) and nuclear receptor families, as well as enzymes. Our current understanding of lysophospholipid interactions with these targets is based on a combination of lysophospholipid analog structure activity relationship studies as well as more direct structural characterization techniques such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and experimentally-validated molecular modeling. The direct structural characterization studies are the focus of this review, and provide the insight necessary to stimulate structure-based therapeutic lead discovery efforts in the future.
Keywords: Phospholipid, G protein-coupled receptor, lysophosphatidic acid, sphingosine 1-phosphate, autotaxin, PPAR
1. Introduction
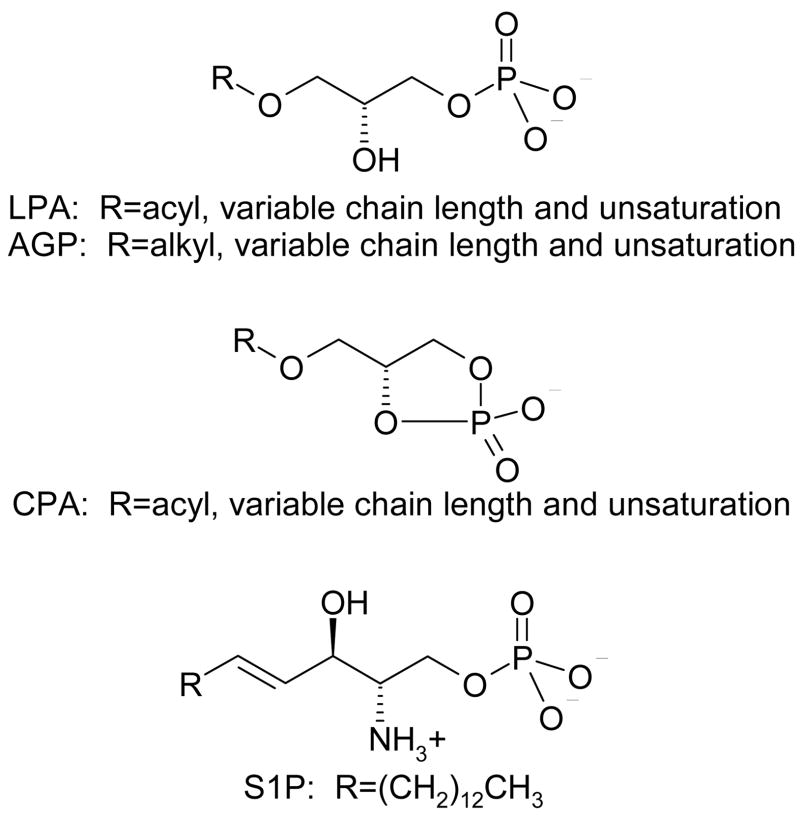
Lysophospholipids, including lysophosphatidic acid (LPA), alkyl glycerol phosphate (AGP), cyclic phosphatidic acid (CPA), and sphingosine 1-phosphate (S1P) (Figure 1), play critical and incompletely elucidated roles in development [1] and disease [2–5]. Lysophospholipids share a minimal set of structural features, in particular a phosphate headgroup and a single hydrophobic chain. Other structural features, such as the linkage between the headgroup and tail, substituents and unsaturation, vary. The relative simplicity and flexibility of lysophospholipid structures result in interactions at a diverse array of biomolecular targets. Lysophospholipid targets have been confirmed to include both soluble and integral membrane spanning receptors and enzymes [6]. These targets have demonstrated roles in signaling, lysophospholipid production and degradation, and lysophospholipid availability to other targets. Details on the interactions between lysophospholipids and their targets are of interest to guide the discovery and optimization of therapeutic lead compounds that mimic or interfere with lysophospholipid function. Mimicking or inhibiting lysophospholipid recognition may have therapeutic relevance to a vast array of pathophysiological situations including immune disorders, cardiovascular disease, cancer, diabetes, and neural disease [2, 3, 7–11]. Details about lysophospholipid recognition can be obtained by indirect studies of lysophospholipid analogs to identify the structure activity relationships (SAR) at a particular biomolecular target. In fact, the earliest studies aimed at identifying the characteristics of lysophospholipid recognition by biomolecular targets relied on analog SAR. SAR information continues to be useful, with reports of new analog series appearing at regular intervals. Early SAR studies focused on the tolerance for changes to the lysophospholipid headgroup, length of the lipid tail, as well as the influence of degrees of unsaturation on lysophospholipid recognition using commercially available natural analogs [12–16]. More recent SAR studies have applied synthetic organic chemistry to the problem [17–38], generating series of molecules with broader variability at the headgroup, changes in the linker between the headgroup and tail, as well as stereochemical differences. Detailed knowledge of the ligand-based SAR has facilitated the development and application of pharmacophore models, resulting in new leads acting at individual lysophospholipid targets [39–41]. SAR studies at lysophospholipid targets have been previously reviewed [42–44], and will not be described in further detail here. In contrast, atomic-level insights of lysophospholipid interactions with their biomolecular targets can be obtained by combinations of more direct methods that include crystallography, spectroscopy, and computational modeling coupled with site-directed mutagenesis. Insights gained from these techniques are the focus of this review.
Figure 1.
Chemical structures of lysophospholipids.
2. Lysophospholipid Protein Targets
Direct interactions between lysophospholipids and multiple protein classes have been demonstrated. These direct protein targets include transmembrane receptors in the G protein-coupled receptor (GPCR) or seven transmembrane (7TM) receptor superfamily, the soluble nuclear peroxisome proliferator activated receptor gamma (PPARγ), the soluble extracellular enzyme autotaxin (ATX, nucleotide pyrophosphatase/phosphodiesterase-2: NPP2), and members of the transmembrane enzyme family of lipid phosphate phosphohydrolases (LPP). Each of these target types poses unique challenges to researchers seeking to elucidate the factors responsible for lysophospholipid recognition.
2.1. G protein-coupled receptors
Among the approximately 950 [45] human GPCR, eleven show responses to LPA or S1P. The first eight receptors discovered to have responses to LPA and S1P, LPA1–3 and S1P1–5, are members of the endothelial differentiation gene (EDG) family of GPCR [6, 46]. The more recently discovered LPA receptors, LPA4–6 [47–49], were discovered among orphan GPCR in the purinergic receptor cluster of GPCR. Sequences within the EDG family are more closely related to each other, with amino acid identities ranging from 25–52%, than they are to LPA4–6, with which they share only 13–16% identical amino acids. Figure 2 shows a phylogenetic tree representing the relationships among the GPCR responsive to lysophospholipids.
Figure 2.
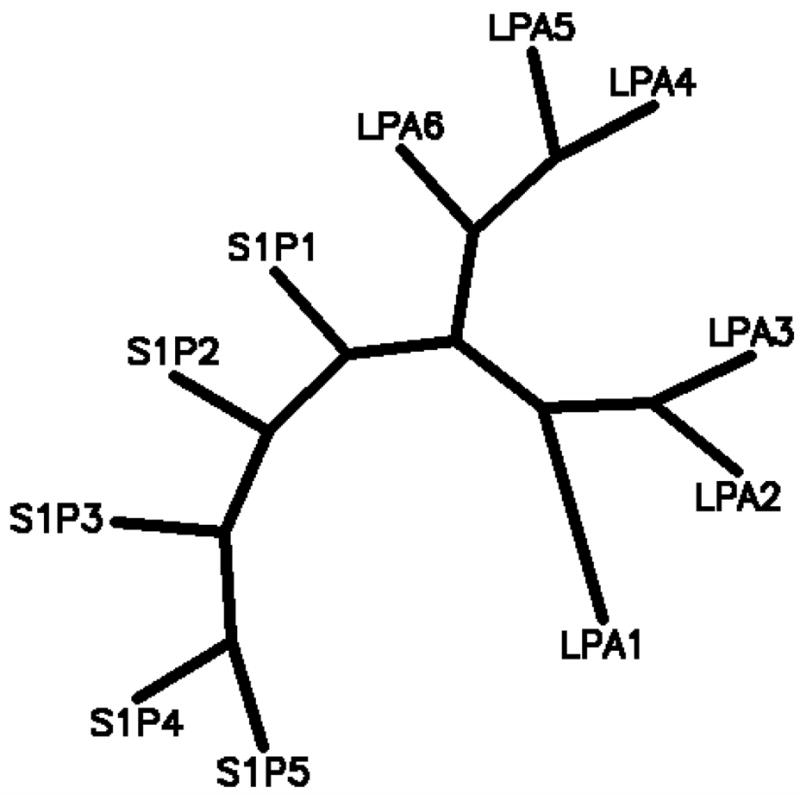
Phylogenetic tree indicating evolutionary distances between human lysophospholipid receptors computed by PHYLIP 3.6 based on protein sequence parsimony.
GPCR present numerous challenges to researchers interested in investigating ligand recognition. GPCR are integral membrane proteins, with low solubility and poor function in aqueous solution. Structural characterization of GPCR by crystallography must therefore be preceded by careful optimization of conditions for expression, protein engineering, purification, reconstitution and crystallization. These challenges are sufficiently daunting that high resolution crystallographic structures of only two members of the GPCR family, rhodopsin [50–53] and the β2-adrenergic receptor [54–57], have been reported to date. Challenges are also faced by NMR spectroscopists due to the line broadening observed with large lipid/protein assemblies. Insights into lysophosphospholipid recognition by GPCR targets therefore come predominantly from indirect studies of these interactions based on ligand SAR and the more direct experimentally validated modeling studies.
2.2. Nuclear receptors
A single nuclear receptor, the peroxisome proliferator-activated receptor gamma (PPARγ), has been demonstrated to interact with LPA [58]. LPA displaces the full agonist, rosiglitazone, from PPARγ and stimulates expression of genes under the control of the peroxisome proliferator response element (PPRE). Rosiglitazone is a member of the thiazolidinedione class of anti-diabetes drugs that act through PPARγ. Recent evidence indicates that LPA induces neointima formation through the PPARγ receptor [5].
Unlike the GPCR targeted by lysophospholipids, multiple crystallographic structures of PPARγ and two other PPAR isoforms sharing more than 50% identical amino acids have been reported. The first PPARγ crystallographic structure included only the isolated ligand-binding domain [59], but this structure was quickly followed by that of the heterodimer formed by the ligand binding domains of PPARγ and the retinoic acid receptor RXR-alpha with their respective agonists, rosiglitazone and 9-cis-retinoic acid, as well as co-activator peptides [60]. Additional comparative crystallographic studies of both full and partial agonists have been performed [61, 62]. No antagonist-bound structure of PPARγ has yet been reported, however, a crystal structure of an antagonist bound to the alpha isoform has been reported [63]. The closest analogs to a PPARγ complex with LPA are complexes of the delta isoform of PPAR with fatty acids [64, 65]. Nevertheless, the availability of numerous relevant crystallographic complexes has stimulated direct studies of lysophospolipid interactions with its nuclear receptor target.
2.3. Enzymes
Many enzymes play roles in the biosynthesis and degradation of lysophospholipids. However, few enzymes demonstrate feedback regulation by lysophospholipids. Autotaxin (ATX, nucleotide pyrophosphatase/phosphodiesterase-2, NPP2) is the serum lysophospholipase D enzyme that converts lysophosphatidylcholine into LPA [66, 67]. LPA, in turn, inhibits ATX-catalyzed hydrolysis of LPC [68]. The temporal and compartmental regulation of this inhibition is likely to involve circulating factors such as other proteins that have already demonstrated interaction with [69] and modulation of [70] LPA function. Neither full-length ATX nor isolated domains have been crystallized. However, the catalytic domain of ATX is 30% identical to a crystallized bacterial NPP [71]. Insights from catalytic domain models of ATX are limited due to the essential role of the nuclease domain in enzyme function [72] and the reported mixed-mode inhibition of ATX by LPA [68]. The majority of studies examining lysophospholipid recognition by ATX have therefore utilized indirect methods based on lysophospholipid analogs, although lysophospholipid complexes with an ATX catalytic domain model have also been reported.
3. Lysophospholipid Recognition
3.1. Crystallographic studies
3.1.1. Crystallographic GPCR structures
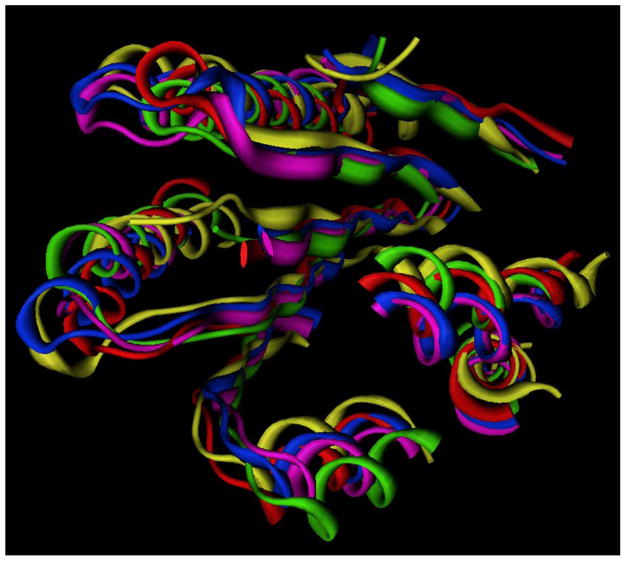
Crystal structures have been solved for only two different members of the GPCR family, rhodopsin [50–53, 73–76] and the β2-adrenergic receptor [54, 57]. These structures represent either inactive conformational states or early intermediates in the rhodopsin activation process. Surprisingly, the nine crystallographic structures of bovine rhodopsin in the Protein Data Bank show not only similarity to each other, with only 0.5 Å root mean square deviation on alpha carbon positions of residues defined in all structures, but also show similarity to the two crystal structures of the β2-adrenergic receptor (Figure 3). The most substantial differences between the crystallized GPCR structures is found by comparison of the backbone conformations of the extracellular and intracellular loops, as well as in finer details of individual amino acid sidechain conformations and interactions. These structures provide an excellent starting point to understand lysophospholipid recognition by GPCR targets, although lysophospholipid receptor characteristics such as the lack of a disulfide bridge between the top of the third transmembrane domain and the second extracellular loop, the lack of proline in the fifth transmembrane domain, and other differences from the two crystallized GPCR must be considered. The anticipated differences between inactive and active GPCR conformations [77, 78] are also important, as the natural lysophospholipids are agonists at their GPCR targets, rather than inverse agonists or neutral antagonists. Thus the active conformation is most relevant to their function. Even considering these differences from the available crystallographic structures, remarkably detailed atomic interactions have been determined for lysophospholipid interactions with GPCR in the LPA1–3 and S1P1–5receptor families as described in the modeling subsection.
Figure 3.
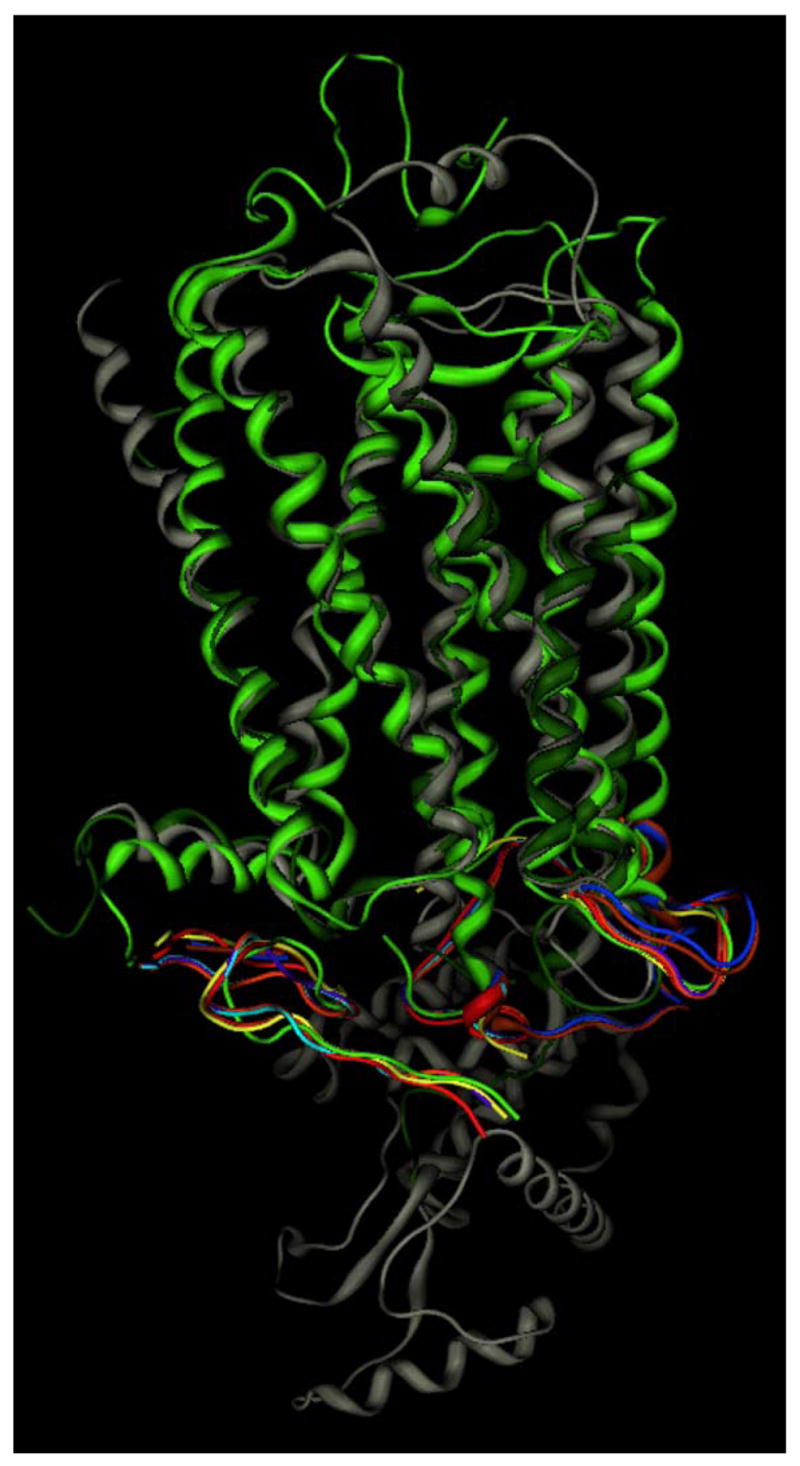
Comparison of all available crystallographic GPCR structures. Rhodopsin structures 1F88 [53] (lt. green), 1HZX [76] (magenta), 1L9H [75] (yellow), 1GZM [103] (blue), 1U19 [52] (orange), 2HPY [74] (purple), 2G87 [74] (cyan), 2I35 [51] (rust) are superposed based on amino acid residues resolved in all structures, and shown only by the lt. green ribbon in these common areas to simplify the image. β2-adrenergic receptor structures 2RH1 [54] (grey) and 2R4R [57] (dk green) were superposed on the rhodopsin structures based on residues in the first three transmembrane domains.
3.1.2. Crystallographic PPAR structures
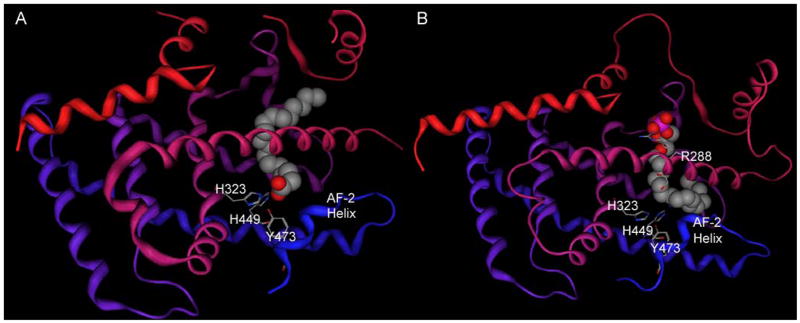
LPA has not yet been crystallized bound to the PPARγ ligand binding domain. However, crystallographic complexes of fatty acids bound to PPARδ [64], which shares 65% identical amino acids with the PPARγ ligand binding domain, are available. These analogs provide insights from which hypotheses concerning LPA recognition by PPARγ can be generated. Fatty acids bound to PPARδ are located near the activation factor helix-2 (AF-2). The anionic carboxylate group of the fatty acid interacts with H323, H449 and Y473 (Figure 4A) [64]. These interactions are analogous for synthetic agonists, such as rosiglitazone, interacting with PPARγ [60]. These studies suggest a natural hypothesis, that the phosphate group of LPA is likely to stabilize the AF-2 helix of PPARγ by hydrogen bonding to Y473, as well as having additional interactions with H323 and H449. However, model-driven mutagenesis studies described in section 4.3 indicate that this hypothesis is not valid.
Figure 4.
PPAR structures. Protein backbones are shown as ribbons shaded from red at the amino terminus to blue at the carboxy terminus A. Crystallographic complex of PPARδ with vaccenic acid (Protein Data Bank [104] entry 2baw [64]). Vaccenic acid (11-Z-octadecenoic acid) is shown as a spacefilling model, select residues are shown as stick models. Discontinuities represent regions that were not assigned. B. Modeled complex of PPARγ with AGP [100]. AGP is shown as a spacefilling model, select residues are shown as stick models.
3.1.3. Crystallographic structures related to ATX
Two ATX segments (or the corresponding segments of the highly homologous NPP1) have been compared with crystallographic structures. The catalytic domain was first identified to share catalytic residues and metal-ligating residues with members of the alkaline phosphatase superfamily [79], and more recently with a bacterial NPP [71]. These comparisons indicate that the residues interacting with the first metal ion are located after the first and fourth strand of a central 6-strand beta sheet, and those interacting with the second metal ion are located after the fifth strand. The catalytic residue is also located near the metal ions. These findings are consistent with mutagenesis studies in the mammalian NPP enzyme family [79, 80]. This structural core is highly conserved in even weakly homologous members of the alkaline phosphatase superfamily [6] as shown in Figure 5. It is likely that the active site metal ions, which position the phosphate group of LPC, are also involved in recognition of LPA. The C-terminal domain of NPP1, and by inference that of the highly homologous NPP2/ATX, has been compared to a crystallized endonuclease from Serratia [81]. The crystallographic structure displays a five strand beta sheet surrounded by four alpha helices and two crossed beta strands. A magnesium ion is bound between the two crossed beta strands and a neighboring alpha helix [82]. The Serratia endonuclease shares only 17% identical amino acids with the C-terminal regions of NPP1-3, which lack conserved amino acids that are essential for endonuclease function, including those involved in interactions with the magnesium ion. The nuclease-like domain of NPP1, instead, plays a role in stability and protein localization [81]. This domain is therefore unlikely to play a direct role in lysophospholipid recognition, although direct evidence to support this speculation is not yet available.
Figure 5.
Superposition of alkaline phosphatase superfamily members showing geometrically conserved structural core. AlkP family members shown as ribbons, 1ALK (alkaline phosphatase: red) [105],1EJJ (phosphoglycerate mutase: green) [106], 1AUK (arylsulfatase: blue) [107], 1FSU (arylsulfatase: magenta) [108], 2GSN (Xac. NPP: yellow) [109], available in the Protein Databank [104].
3.2. Spectroscopic studies
S1P headgroup recognition by the S1P4 first extracellular loop and the extracellular end of TM3 has been examined using NMR spectroscopy [83]. This study provided additional evidence of a direct interaction between R3.28 and the phosphate group and between E3.29 and the ammonium group first proposed based on modeling studies described in section 3.3. Ligand titration additionally affected chemical shifts of residues in the third loop between Arg109 and Pro115, some of which had displayed conformational variability in the absence of ligand. This study was the first to pinpoint specific residues in a lysophospholipid receptor involved in the dynamic conversion between the inactive and active forms of the receptor.
3.3. Modeling studies
The relative scarcity of experimentally characterized structures relevant to lysophospholipid recognition compelled the application of modeling methods coupled with alternative experimental validation studies in order to provide direct, atomic-resolution insights into lysophospholipid recognition. Modeling studies have been applied to study lysophospholipid recognition by the EDG-family GPCR, LPA1–3 [84–90] and S1P1–5 [40, 84, 91–99], the nuclear receptor PPARγ [100], and the enzyme ATX [42].
3.3.1. Modeling Lysophospholipid Interactions with GPCR
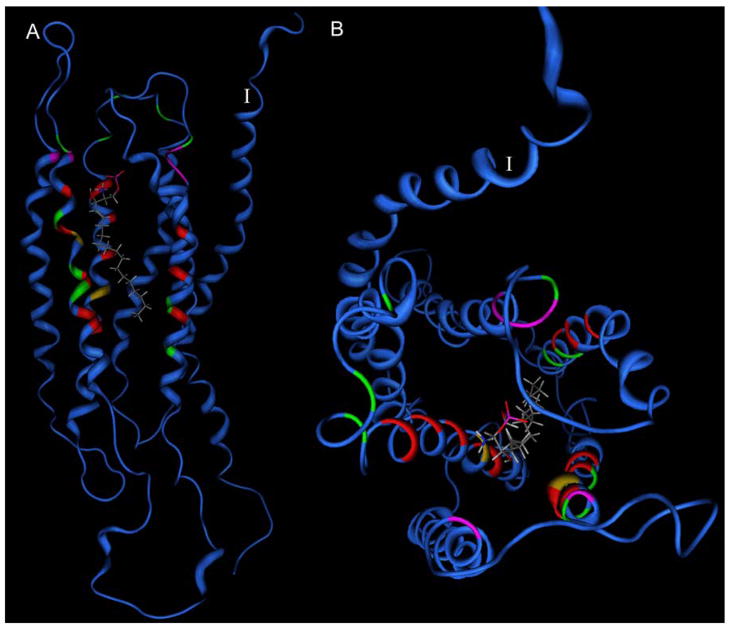
The earliest modeling studies of S1P interactions with S1P1 [94–96] were reported concurrently with the first atomic-resolution crystallographic structure of rhodopsin. Even without a high-resolution crystal structure to provide a structural template, key ion-pairing interactions from amino acids R3.28, E3.29 and R7.34 to the phosphate and ammonium moieties in the S1P headgroup were proposed. Experimental binding assays confirmed that mutant receptors bearing alanine at these positions showed no specific binding of S1P. An alternative model was proposed that reiterated these interactions, but also suggested Y2.57 as a hydrogen bonding partner for the S1P hydroxyl group [99]. This hypothesis has yet to be experimentally validated. Position 3.29 was later predicted by modeling to shift relative recognition of S1P and LPA depending on whether glutamate or glutamine were present, a finding confirmed by experimental characterization of E3.29Q mutants of S1P1 and S1P4 as well as the Q3.29E mutant of LPA1 [84, 97]. These results contradict an alternative model of S1P complexed with S1P4 that suggested interactions predominantly in the extracellular loops [98]. Further modeling and mutagenesis studies identified W4.64 and K5.38 as positions that show variable importance in the S1P receptors [93, 101]. Investigations of both LPA and S1P receptor indicate that a cationic residue in TM7 is often, but not universally, involved in ligand recognition [40, 88, 93]. The residues that surround the hydrophobic tail of S1P in the S1P1 receptor have also been confirmed to occur within the transmembrane domain, particularly involving residues in TM3-7 [40]. A leucine near the extracellular end of TM6, L6.55 (276), has recently been confirmed by site-directed mutagenesis to impact agonist selectivity between S1P1 and S1P3.[102] These models have proven capable of explaining not only lysophospholipid recognition, but also of discrimination between agonist and antagonist activity, describing selectivity profiles across multiple receptor subtypes [86–89, 91], and providing insights into binding of small drug-like molecules [92]. Figure 6 shows a composite map of sites in EDG receptor family members that have been investigated by site-directed mutagenesis. This figure demonstrates that the residues shown to strongly impact either receptor activation or agonist binding orient toward the interior of the transmembrane helical bundle. In contrast, residues that fail to impact receptor activation or agonist binding are either located in the extracellular loops, orient away from the interior of the transmembrane helical bundle, or are located near the bottom of the ligand binding site.
Figure 6.
Modeled location of amino acids subjected to site-directed mutagenesis [40, 84, 88, 92, 93, 95–97] in the EDG receptor GPCR family mapped onto the S1P1 receptor model [40]. Blue ribbons indicate sites not subjected to mutational analysis in any member of the EDG family. Red, yellow and green sites indicate mutations that abolished, reduced, or had no impact on receptor activation or ligand binding, respectively. Magenta sites indicate position giving receptor-dependent effects. The first TM is labeled with the Roman numeral I. The modeled position of S1P is shown as a stick model. A. Side view. B. View from extracellular side.
3.3.2. Modeling Lysophospholipid Interactions with PPARγ
The crystallographic structures of PPARγ provide an excellent starting point for modeling lysophospholipid interactions at this target. Docking studies with an ether analog of LPA, alkyl glycerol phosphate (AGP), predicted that the AGP binding site overlaps with that of the full agonist, rosiglitazone, but that the anionic phosphate group interacts with R288 at the opposite end of the binding pocket from H323, H449 and Y473 (Figure 4B) [100]. Experimental characterization of mutants at these positions confirmed that AGP and rosiglitazone bind competitively with each other, but interact with different subsets of amino acid residues in PPARγ [100].
3.3.3. Modeling Lysophospholipid Interactions with ATX
The catalytic domain of ATX was first modeled based on the alkaline phosphatase crystal structure [79]. Although the sequence of alkaline phosphatase shares less than 10% identity with ATX overall, and only 14% identity within the alpha/beta core, the conclusions drawn from the comparison of these structures were proven valid by comparison to the NPP structure, which shares 30% identity with the NPP1 and NPP2 catalytic domains. Two models of the ATX catalytic domain have been reported based on the Xac. NPP crystal structure. The first model was used to interpret the structural context of an essential glycosylation site [71]. The second model was used to predict the binding site of LPC and to identify a set of structurally diverse, non-lipid ATX inhibitors [42].
The C-terminal domain of NPP1, and by inference of the highly homologous NPP2/ATX, has been modeled based on an endonuclease from Serratia [81]. This model indicates that a five strand beta sheet is surrounded by four alpha helices and two crossed beta strands, as seen in the endonuclease template that contains 17% identical amino acids. The modeled regions of the C-terminal and catalytic domains are separated by 39 amino acids, for which a suitable modeling template is not currently available. Experimental data that defines the relative orientation of these two domains is urgently needed in order to determine whether the C-terminal domain directly impacts lysophospholipid recognition.
4. Concluding Remarks
Lysophospholipid recognition by a diverse array of biological targets has been probed by a powerful combination of crystallographic structures, NMR spectroscopy, and molecular modeling coupled with comparisons to experimental data such as site-directed mutagenesis or pharmacological trends. This combination of tools has provided a picture of lysophospholipid interactions at three different types of biomolecular targets, including GPCR, nuclear receptors and enzymes. The insights from these studies have now reached the critical level required to support structure-based therapeutic lead identification efforts.
Acknowledgments
Research support from the National Institutes of Health (HL 0087004) and the Elsa Pardee Foundation in the area of lysophospholipid recognition is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saba JD. Lysophospholipids in development: Miles apart and edging in. J Cell Biochem. 2004;92:967–992. doi: 10.1002/jcb.20128. [DOI] [PubMed] [Google Scholar]
- 2.Murph M, Mills GB. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- 3.Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets. 2007;8:155–167. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- 4.Meyerzu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parrill AL. Structural characteristics of lysophosphatidic acid biological targets. Biochem Soc Trans. 2005;33:1366–1369. doi: 10.1042/BST0331366. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V, Lynch KR. FTY720: Targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol. 2002;14:569–575. doi: 10.1016/s0952-7915(02)00374-6. [DOI] [PubMed] [Google Scholar]
- 8.Siess W, Tigyi G. Thrombogenic and atherogenic activities of lysophosphatidic acid. J Cell Biochem. 2004;92:1086–1094. doi: 10.1002/jcb.20108. [DOI] [PubMed] [Google Scholar]
- 9.Tatsuta M, Iishi H, Baba M, Uedo N, Ishihara R, Higashino K, Mukai M, Ishiguro S. Induction by lysophosphatidic acid of peritoneal and pleural metastases of intestinal cancers induced by azoxymethane in Wistar rats. Cancer Lett. 2005;219:137–145. doi: 10.1016/j.canlet.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Brindley DN. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J Cell Biochem. 2004;92:900–912. doi: 10.1002/jcb.20126. [DOI] [PubMed] [Google Scholar]
- 11.Moolenaar WH. Lysophospholipids in the limelight: autotaxin takes center stage. J Cell Biol. 2002;158:197–199. doi: 10.1083/jcb.200206094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokumura A, Kume T, Fukuzawa K, Tsukatani H. Cardiovascular effects of lysophosphatidic acid and its structural analogs in rats. J Pharmacol Exp Ther. 1981;219:219–224. [PubMed] [Google Scholar]
- 13.Simon MF, Chap H, Douste-Blazy L. Human platelet aggregation induced by 1-alkyl-lysophosphatidic acid and its analogs: a new group of phospholipid mediators. Biochem Biophys Res Commun. 1982;108:1743–1750. doi: 10.1016/s0006-291x(82)80113-7. [DOI] [PubMed] [Google Scholar]
- 14.Jalink K, Hengeveld T, Mulder S, Postma FR, Simon MF, Chap H, van der Marel GA, van Boom JH, van Blitterswijk WJ, Moolenaar WH. Lysophosphatidic acid-induced Ca2+ mobilization in human A431 cells: structure-activity analysis. Biochem J. 1995;307:609–616. doi: 10.1042/bj3070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Structural differences in the ability of lysophospholipids to inhibit endothelium-dependent hyperpolarization by acetylcholine in rat mesenteric arteries. Biochem Biophys Res Commun. 1996;227:479–483. doi: 10.1006/bbrc.1996.1532. [DOI] [PubMed] [Google Scholar]
- 16.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 17.Hopper DW, Ragan SP, Hooks SB, Lynch KR, Macdonald TL. Structure activity relationships of lysophosphatidic acid: conformationally restricted backbone mimetics. J Med Chem. 1999;42:963–970. doi: 10.1021/jm970809v. [DOI] [PubMed] [Google Scholar]
- 18.Hooks SB, Ragan SP, Hopper DW, Honemann CW, Durieux ME, Macdonald TL, Lynch KR. Characterization of a receptor subtype-selective lysophosphatidic acid mimetic. Mol Pharmacol. 1998;53:188–194. doi: 10.1124/mol.53.2.188. [DOI] [PubMed] [Google Scholar]
- 19.Lynch KR, Hopper DW, Carlisle SJ, Catalano JG, Zhang M, MacDonald TL. Structure/activity relationships in lysophosphatidic acid: the 2-hydroxyl moiety. Mol Pharmacol. 1997;52:75–81. doi: 10.1124/mol.52.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Heise CE, Santos WL, Schreihofer AM, Heasley BH, Mukhin YV, Macdonald TL, Lynch KR. Activity of 2-substituted LPA analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol Pharmacol. 2001;60 doi: 10.1124/mol.60.6.1173. in press. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama K, Baker DL, Virag T, Liliom K, Byun H, Tigyi G, Bittman R. Stereochemical properties of lysophosphatidic acid receptor activation and metabolism. Biochim Biophys Acta. 2002;1582:295–308. doi: 10.1016/s1388-1981(02)00184-1. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Prestwich GD. Concise synthesis of acyl migration-blocked 1,1-difluorinated analogues of lysophosphatidic acid. J Org Chem. 2002;67:7158–7161. doi: 10.1021/jo0203037. [DOI] [PubMed] [Google Scholar]
- 23.Clemens JJ, Davis MD, Lynch KR, Macdonald TL. Synthesis of para-alkyl aryl amide analogues of sphingosine-1-phosphate: discovery of potent S1P receptor agonists. Bioorg Med Chem Lett. 2003;13:3401–3404. doi: 10.1016/s0960-894x(03)00812-6. [DOI] [PubMed] [Google Scholar]
- 24.Qian L, Xu Y, Arai H, Aoki J, McIntyre TM, Prestwich GD. Synthesis of migration-resistant hydroxyethoxy analogues of lysophosphatidic acid. Org Letters. 2003;5:4685–4688. doi: 10.1021/ol0358758. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Qian L, Pontsler AV, McIntyre TM, Prestwich GD. Synthesis of difluoromethyl-substituted lysophosphatidic acid analogues. Tetrahedron. 2004;60:47–53. [Google Scholar]
- 26.Clemens JJ, Davis MD, Lynch KR, Macdonald TL. Synthesis of benzimidazole based analogues of sphingosine-1-phosphate: discovery of potent, subtype-selective S1P4 receptor agonists. Bioorg Med Chem Lett. 2004;14:4903–4906. doi: 10.1016/j.bmcl.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Tanaka M, Arai H, Aoki J, Prestwich GD. Alkyl lysophosphatidic acid and fluoromethylene phosphonate analogs as metabolically-stabilized agonists for LPA receptors. Bioorg Med Chem Lett. 2004;14:5323–5328. doi: 10.1016/j.bmcl.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Santos WL, Heasley BH, Jarosz R, Carter KM, Lynch KR, Macdonald TL. Synthesis and biological evaluation of phosphonic and thiophosphoric acid derivatives of lysophosphatidic acid. Bioorg Med Chem Lett. 2004;14:3473–3476. doi: 10.1016/j.bmcl.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 29.Heasley BH, Jarosz R, Carter KM, Van SJ, Lynch KR, Macdonald TL. A novel series of 2-pyridyl-containing compounds as lysophosphatidic acid receptor antagonists: development of a nonhydrolyzable LPA3 receptor-selective antagonist. Bioorg Med Chem Lett. 2004;14:4069–4074. doi: 10.1016/j.bmcl.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Heasley BH, Jarosz R, Lynch KR, Macdonald TL. Initial structure-activity relationships of lysophosphatidic acid receptor antagonists: discovery of a high-affinity LPA1/LPA3 receptor antagonist. Bioorg Med Chem Lett. 2004;14:2735–2740. doi: 10.1016/j.bmcl.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 31.Qian L, Xu Y, Hasegawa Y, Aoki J, Mills GB, Prestwich GD. Enantioselective responses to a phosphorothioate analogue of lysophosphatidic acid with LPA3 receptor-selective agonist activity. J Med Chem. 2003;46:5575–5578. doi: 10.1021/jm034207p. [DOI] [PubMed] [Google Scholar]
- 32.Qian L, Xu Y, Arai H, Aoki J, McIntyre TM, Prestwich GD. Synthesis of migration-resistant hydroxyethoxy analogues of lysophosphatidic acid. Org Lett. 2003;5:4685–4688. doi: 10.1021/ol0358758. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Qian L, Prestwich GD. Synthesis of monofluorinated analogues of lysophosphatidic acid. J Org Chem. 2003;68:5320–5330. doi: 10.1021/jo020729l. [DOI] [PubMed] [Google Scholar]
- 34.Lu X, Cseh S, Byun HS, Tigyi G, Bittman R. Total synthesis of two photoactivatable analogues of the growth-factor-like mediator sphingosine 1-phosphate: differential interaction with protein targets. J Org Chem. 2003;68:7046–7050. doi: 10.1021/jo034828q. [DOI] [PubMed] [Google Scholar]
- 35.Foss FW, Jr, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, Macdonald TL. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang G, Xu Y, Fujiwara Y, Tsukahara T, Tsukahara R, Gajewiak J, Tigyi G, Prestwich GD. Alpha-substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. ChemMedChem. 2007;2:679–690. doi: 10.1002/cmdc.200600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui P, Tomsig JL, McCalmont WF, Lee S, Becker CJ, Lynch KR, Macdonald TL. Synthesis and biological evaluation of phosphonate derivatives as autotaxin (ATX) inhibitors. Bioorg Med Chem Lett. 2007;17:1634–1640. doi: 10.1016/j.bmcl.2006.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, Uchiyama A, Murakami-Murofushi K, Koh E, Bandle RW, Byun HS, Bittman R, Fan D, Murph M, Mills GB, Tigyi G. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281:22786–22793. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koide Y, Hasegawa T, Takahashi A, Endo A, Mochizuki N, Nakagawa M, Nishida A. Development of novel EDG3 antagonists using a 3D database search and their structure-activity relationships. J Med Chem. 2002;45:4629–4638. doi: 10.1021/jm020080c. [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara Y, Osborne DA, Walker MD, Wang DA, Bautista DA, Liliom K, Van Brocklyn JR, Parrill AL, Tigyi G. Identification of the hydrophobic ligand binding pocket of the S1P1 receptor. J Biol Chem. 2007;282:2374–2385. doi: 10.1074/jbc.M609648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koide Y, Uemoto K, Hasegawa T, Sada T, Murakami A, Takasugi H, Sakurai A, Mochizuki N, Takahashi A, Nishida A. Pharmacophore-based design of sphingosine 1-phosphate-3 receptor antagonists that include a 3,4-dialkoxybenzophenone scaffold. J Med Chem. 2007;50:442–454. doi: 10.1021/jm060834d. [DOI] [PubMed] [Google Scholar]
- 42.Parrill AL, Echols U, Nguyen T, Pham TC, Hoeglund A, Baker DL. Virtual screening approaches for the identification of non-lipid autotaxin inhibitors. Bioorg Med Chem. 2008 doi: 10.1016/j.bmc.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Parrill AL, Sardar VM, Yuan H. Sphingosine 1-phosphate and lysophosphatidic acid receptors: agonist and antagonist binding and progress toward development of receptor-specific ligands. Semin Cell Dev Biol. 2004;15:467–476. doi: 10.1016/j.semcdb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Tigyi G, Parrill AL. Molecular mechanisms of lysophosphatidic acid action. Prog Lipid Res. 2003;42:498–526. doi: 10.1016/s0163-7827(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 45.Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 47.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 48.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 49.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13-and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 50.Standfuss J, Xie G, Edwards PC, Burghammer M, Oprian DD, Schertler GF. Crystal structure of a thermally stable rhodopsin mutant. J Mol Biol. 2007;372:1179–1188. doi: 10.1016/j.jmb.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci U S A. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 53.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp R, Yamamoto M, Miyano M. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 54.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 56.Day PW, Rasmussen SG, Parnot C, Fung JJ, Masood A, Kobilka TS, Yao XJ, Choi HJ, Weis WI, Rohrer DK, Kobilka BK. A monoclonal antibody for G protein-coupled receptor crystallography. Nat Methods. 2007;4:927–929. doi: 10.1038/nmeth1112. [DOI] [PubMed] [Google Scholar]
- 57.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 58.McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uppenberg J, Svensson C, Jaki M, Bertilsson G, Jendeberg L, Berkenstam A. Crystal structure of the ligand binding domain of the human nuclear receptor PPARgamma. J Biol Chem. 1998;273:31108–31112. doi: 10.1074/jbc.273.47.31108. [DOI] [PubMed] [Google Scholar]
- 60.Gampe RTJ, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE. Asymmetry in the Ppargamma/Rxralpha Crystal Structure Reveals the Molecular Basis of Heterodimerization Among Nuclear Receptors. Mol Cell. 2000;5:545–555. doi: 10.1016/s1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- 61.Pochetti G, Godio C, Mitro N, Caruso D, Galmozzi A, Scurati S, Loiodice F, Fracchiolla G, Tortorella P, Laghezza A, Lavecchia A, Novellino E, Mazza F, Crestani M. Insights into the mechanism of partial agonism: crystal structures of the peroxisome proliferator-activated receptor gamma ligand-binding domain in the complex with two enantiomeric ligands. J Biol Chem. 2007;282:17314–17324. doi: 10.1074/jbc.M702316200. [DOI] [PubMed] [Google Scholar]
- 62.Bruning JB, Chalmers MJ, Prasad S, Busby SA, Kamenecka TM, He Y, Nettles KW, Griffin PR. Partial agonists activate PPARgamma using a helix 12 independent mechanism. Structure. 2007;15:1258–1271. doi: 10.1016/j.str.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Xu HE, Stanley TB, Montana VG, Lambert MH, Shearer BG, Cobb JE, McKee DD, Galardi CM, Plunket KD, Nolte RT, Parks DJ, Moore JT, Kliewer SA, Willson TM, Stimmel JB. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature. 2002;415:813–817. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- 64.Fyffe SA, Alphey MS, Buetow L, Smith TK, Ferguson MA, Sorensen MD, Bjorkling F, Hunter WN. Recombinant human PPAR-beta/delta ligand-binding domain is locked in an activated conformation by endogenous fatty acids. J Mol Biol. 2006;356:1005–1013. doi: 10.1016/j.jmb.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 65.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 66.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 68.van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, Perrakis A, Nagano T, Moolenaar WH. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280:21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 69.Li Z, Baker DL, Tigyi G, Bittman R. Synthesis of photoactivatable analogues of lysophosphatidic acid and covalent labeling of plasma proteins. J Org Chem. 2006;71:629–635. doi: 10.1021/jo052030w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hama K, Bandoh K, Kakehi Y, Aoki J, Arai H. Lysophosphatidic acid (LPA) receptors areactivated differentially by biological fluids: possible role of LPA-binding proteins in activation of LPA receptors. FEBS Letters. 2002;523:187–192. doi: 10.1016/s0014-5793(02)02976-9. [DOI] [PubMed] [Google Scholar]
- 71.Jansen S, Callewaert N, Dewerte I, Andries M, Ceulemans H, Bollen M. An essential oligomannosidic glycan chain in the catalytic domain of autotaxin, a secreted lysophospholipase-D. J Biol Chem. 2007;282:11084–11091. doi: 10.1074/jbc.M611503200. [DOI] [PubMed] [Google Scholar]
- 72.Cimpean A, Stefan C, Gijsbers R, Stalmans W, Bollen M. Substrate-specifying determinants of the nucleotide pyrophosphatases/phosphodiesterases NPP1 and NPP2. Biochem J. 2004;381:71–77. doi: 10.1042/BJ20040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schertler GFX, Villa C, Henderson R. Projection structure of rhodopsin. Nature. 1993;362:770–772. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- 74.Nakamichi H, Okada T. Local peptide movement in the photoreaction intermediate of rhodopsin. Proc Natl Acad Sci U S A. 2006;103:12729–12734. doi: 10.1073/pnas.0601765103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okada T, Fujiyoshi Y, Silow M, Navarro J, Landau EM, Shichida Y. Functional role of internal water molecules in rhodopsin revealed by X-ray crystallography. Proc Natl Acad Sci U S A. 2002;99:5982–5987. doi: 10.1073/pnas.082666399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teller DC, Okada T, Behnke CA, Palczewski K, Stenkamp RE. Advances in determination of a high-resolution three-dimensional structure of rhodopsin, a model of G-protein-coupled receptors (GPCRs) Biochemistry. 2001;40:7761–7772. doi: 10.1021/bi0155091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. Functionally different agonists induce distinct conformations in the G protein coupling domain of the β2 adrenergic receptor. J Biol Chem. 2001;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- 78.Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the β2-adrenergic receptor: extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- 79.Gijsbers R, Ceulemans H, Stalmans W, Bollen M. Structural and catalytic similarities between nucleotide pyrophosphatases/phosphodiesterases and alkaline phosphatases. J Biol Chem. 2001;276:1361–1368. doi: 10.1074/jbc.M007552200. [DOI] [PubMed] [Google Scholar]
- 80.Koh E, Clair T, Woodhouse EC, Schiffmann E, Liotta L, Stracke M. Site-directed mutations in the tumor-associated cytokine, autotaxin, eliminate nucleotide phosphodiesterase, lysophospholipase D, and motogenic activities. Cancer Res. 2003;63:2042–2045. [PubMed] [Google Scholar]
- 81.Gijsbers R, Ceulemans H, Bollen M. Functional characterization of the non-catalytic ectodomains of the nucleotide pyrophosphatase/phosphodiesterase NPP1. Biochem J. 2003;371:321–330. doi: 10.1042/BJ20021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller MD, Cai J, Krause KL. The active site of Serratia endonuclease contains a conserved magnesium-water cluster. J Mol Biol. 1999;288:975–987. doi: 10.1006/jmbi.1999.2729. [DOI] [PubMed] [Google Scholar]
- 83.Pham TT, Kriwacki RW, Parrill AL. Peptide Design and Structural Characterization of a GPCR Loop Mimetic. Biopolymers. 2007;86:298–310. doi: 10.1002/bip.20745. [DOI] [PubMed] [Google Scholar]
- 84.Wang D, Lorincz Z, Bautista DL, Liliom K, Tigyi G, Parrill AL. A single amino acid determines ligand specificity of the S1P1 (EDG1) and LPA1 (EDG2) phospholipid growth factor receptors. J Biol Chem. 2001;276:49213–49220. doi: 10.1074/jbc.M107301200. [DOI] [PubMed] [Google Scholar]
- 85.Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang D, Baker DL, Bautista D, Parrill AL, Tigyi G. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol Pharmacol. 2001;60:776–784. [PubMed] [Google Scholar]
- 86.Sardar VM, Bautista DL, Fischer DJ, Yokoyama K, Nusser N, Virag T, Wang D, Baker DL, Tigyi G, Parrill AL. Molecular basis for lysophosphatidic acid receptor antagonist selectivity. Biochim Biophys Acta. 2002;1582:309–317. doi: 10.1016/s1388-1981(02)00185-3. [DOI] [PubMed] [Google Scholar]
- 87.Virag T, Elrod DB, Liliom K, Sardar VM, Parrill AL, Yokoyama K, Durgam G, Deng W, Miller DD, Tigyi G. Fatty alcohol phosphates are subtype-selective agonists and antagonists of LPA receptors. Mol Pharmacol. 2003;63:1032–1042. doi: 10.1124/mol.63.5.1032. [DOI] [PubMed] [Google Scholar]
- 88.Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, Tigyi G. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J Biol Chem. 2005;280:35038–35050. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- 89.Durgam GG, Tsukahara R, Makarova N, Walker MD, Fujiwara Y, Pigg KR, Baker DL, Sardar VM, Parrill AL, Tigyi G, Miller DD. Synthesis and pharmacological evaluation of second-generation phosphatidic acid derivatives as lysophosphatidic acid receptor ligands. Bioorg Med Chem Lett. 2006;16:633–640. doi: 10.1016/j.bmcl.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 90.Deng W, Shuyu E, Tsukahara R, Valentine WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M, VanMiddlesworth L, Johnson LR, Parrill AL, Miller DD, Tigyi G. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 2007;132:1834–1851. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pham TC, Fells JI, Sr, Osborne DA, North EJ, Naor MM, Parrill AL. Molecular recognition in the sphingosine 1-phosphate receptor family. J Mol Graph Model. 2008 doi: 10.1016/j.jmgm.2007.11.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo-active agonists from high throughput screening: Off-the-shelf chemical probes of receptor interactions, signaling and fate. Chemistry & Biology. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 93.Inagaki Y, Pham TT, Fujiwara Y, Kohno T, Osborne DA, Igarashi Y, Tigyi G, Parrill AL. Sphingosine-1-phosphate analog recognition and selectivity at S1P4 within the endothelial differentiation gene family of receptors. Biochem J. 2005;389:187–195. doi: 10.1042/BJ20050046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parrill AL, Wang DA, Bautista DL, Van Brocklyn JR, Lorincz Z, Fischer DJ, Baker DL, Liliom K, Spiegel S, Tigyi G. Identification of Edg1 receptor residues that recognize sphingosine 1- phosphate. J Biol Chem. 2000;275:39379–39384. doi: 10.1074/jbc.M007680200. [DOI] [PubMed] [Google Scholar]
- 95.Parrill AL, Baker DL, Wang D, Fischer DJ, Bautista DL, van Brocklyn J, Spiegel S, Tigyi G. Structural features of EDG1 receptor-ligand complexes revealed by computational modeling and mutagenesis. In: Goetzl EJ, Lynch KR, editors. Lysophospholipids and Eicosanoids in Biology and Pathophysiology. Vol. 905. New York Academy of Sciences; New York: 2000. pp. 330–339. [DOI] [PubMed] [Google Scholar]
- 96.Bautista DL, Baker DL, Wang D, Fischer DJ, Van Brocklyn J, Spiegel S, Tigyi G, Parrill AL. Dynamic modeling of EDG1 receptor structural changes induced by site-directed mutations. J Mol Struct THEOCHEM. 2000;529:219–224. [Google Scholar]
- 97.Holdsworth G, Osborne DA, Pham TT, Fells JI, Hutchinson G, Milligan G, Parrill AL. A single amino acid determines preference between phospholipids and reveals length restriction for activation of the S1P4 receptor. BMC Biochem. 2004;5:12. doi: 10.1186/1471-2091-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vaidehi N, Floriano WB, Trabanino R, Hall SE, Freddolino P, Choi EJ, Zamanakos G, Goddard WAI. Prediction of structure and function of G protein-coupled receptors. Proc Nat Acad Sci, USA. 2002;99:12622–12627. doi: 10.1073/pnas.122357199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lim H, Park J, Ko K, Lee M, Chung S. Syntheses of sphingosine-1-phosphate analogues and their interaction with EDG/S1P receptors. Bioorg Med Chem Lett. 2004;14:2499–2503. doi: 10.1016/j.bmcl.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, Yuan H, Baker DL, Li Z, Bittman R, Parrill A, Tigyi G. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281:3398–3407. doi: 10.1074/jbc.M510843200. [DOI] [PubMed] [Google Scholar]
- 101.Naor MM, Walker MD, Van Brocklyn JR, Tigyi G, Parrill AL. Sphingosine 1-phosphate pKa and binding constants: intramolecular and intermolecular influences. J Mol Graph Model. 2007;26:519–528. doi: 10.1016/j.jmgm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng Q, Clemas JA, Chrebet G, Fischer P, Hale JJ, Li Z, Mills SG, Bergstrom J, Mandala S, Mosley R, Parent SA. Identification of Leu276 of the S1P1 receptor and Phe263 of the S1P3 receptor in interaction with receptor specific agonists by molecular modeling, site-directed mutagenesis, and affinity studies. Mol Pharmacol. 2007;71:724–735. doi: 10.1124/mol.106.029223. [DOI] [PubMed] [Google Scholar]
- 103.Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 104.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim EE, Wyckoff HW. Reaction mechanism of alkaline phosphatase based on crystal structures. Two-metal ion catalysis. J Mol Biol. 1991;218:449–464. doi: 10.1016/0022-2836(91)90724-k. [DOI] [PubMed] [Google Scholar]
- 106.Jedrzejas MJ, Chander M, Setlow P, Krishnasamy G. Structure and mechanism of action of a novel phosphoglycerate mutase from Bacillus stearothermophilus. Embo J. 2000;19:1419–1431. doi: 10.1093/emboj/19.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lukatela G, Krauss N, Theis K, Selmer T, Gieselmann V, von Figura K, Saenger W. Crystal structure of human arylsulfatase A: the aldehyde function and the metal ion at the active site suggest a novel mechanism for sulfate ester hydrolysis. Biochemistry. 1998;37:3654–3664. doi: 10.1021/bi9714924. [DOI] [PubMed] [Google Scholar]
- 108.Bond CS, Clements PR, Ashby SJ, Collyer CA, Harrop SJ, Hopwood JJ, Guss JM. Structure of a human lysosomal sulfatase. Structure. 1997;5:277–289. doi: 10.1016/s0969-2126(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 109.Zalatan JG, Fenn TD, Brunger AT, Herschlag D. Structural and functional comparisons of nucleotide pyrophosphatase/phosphodiesterase and alkaline phosphatase: implications for mechanism and evolution. Biochemistry. 2006;45:9788–9803. doi: 10.1021/bi060847t. [DOI] [PubMed] [Google Scholar]