Summary
We compared the effects of bilateral amygdala, hippocampal or orbital frontal cortex lesions on emotional and hormonal reactivity in rhesus monkeys (Macaca mulatta). Experiment 1 measured behavioral reactivity to an unfamiliar human intruder before and after surgery. Animals with amygdala lesions demonstrated decreases in one passive defensive behavior (freezing), whereas animals with hippocampal lesions showed decreases in a more stimulus-directed defensive behavior (tooth grinding). Orbital frontal cortex lesions also reduced these two defensive behaviors, as well as decreased cage-shaking dominance displays. Animals with amygdala, hippocampal or sham lesions also demonstrated increased tension-related behaviors after surgery, but those with orbital frontal lesions did not. Finally, all three lesions diminished the operated animals' ability to modulate tension-related behaviors depending on the magnitude of threat posed by the human intruder. Experiment 2 measured circulating levels of cortisol and testosterone when a subset of these same animals were at rest and following physical restraint, temporary isolation, exposure to threatening objects and social interactions with an unfamiliar conspecific. None of the lesions impacted on testosterone levels in any condition. Amygdala or orbital frontal lesions blunted cortisol reactivity during isolation from peers, but not during any other condition. Hippocampal lesions did not alter circulating levels of cortisol under any conditions. These results indicate that the amygdala, hippocampus and orbital frontal cortex play distinct, yet complimentary roles in coordinating emotional and hormonal reactivity to threat.
Keywords: macaque, threat, stress response, amygdala, orbital frontal cortex, hippocampal formation
1. Introduction
Upon encountering danger, a host of internal physiological responses and external behavioral reactions are modulated to promote survival. Physiological modulation is orchestrated by the autonomic nervous system (both the sympathetic and parasympathetic systems) and hypothalamic-pituitary-adrenal (HPA) axis to facilitate cardiovascular and neuroendocrine adaptations, respectively. Autonomic responses are under direct control of brainstem nuclei and the cerebellum, which receive innervation from higher cortical and subcortical structures involved in the control of emotional behavior (Herman et al., 2003). Although the extent of this higher regulatory network is not fully understood, the amygdala, hippocampus, lateral hypothalamus, orbital frontal cortex, anterior cingulate cortex and insular cortex play key roles (Cechetto and Saper, 1990; Porges, 1995; Benarroch, 1997).
Previous studies, mostly with rodents, indicate that the amygdala and ventromedial or orbital frontal cortex stimulate the HPA axis (Mandell et al., 1963; Frankel and Jenkins, 1975; Frankel et al., 1978; Gallagher et al., 1987; Feldman et al., 1995; Weidenfeld et al., 1997; Feldman and Weidenfeld, 1998; Kalin et al., 2004), whereas the hippocampus is more critical for negative feedback (Mandell et al., 1963; Feldman and Conforti, 1976; 1980; Dunn and Orr, 1984; Sapolsky et al., 1984; 1991; Feldman and Weidenfeld, 2001; Goursaud et al., 2006). Behavioral reactivity to danger also appears to involve the amygdala, hippocampus and orbital frontal cortex. For example, inactivation or permanent lesions of the amygdala (Maren et al., 1996; Muller et al., 1997; Maren, 1999; Wilensky et al., 1999; 2000; Goosens and Maren, 2001; Koo et al., 2004) or hippocampus (Maren and Fanselow, 1997; Maren et al., 1997; Holt and Maren, 1999; Hobin et al., 2006) in rodents result in diminished fear behaviors (freezing, avoidance, etc.) towards stimuli or contexts paired with aversive consequences. By contrast, the rodent ventromedial frontal cortex seems to be more involved in modulating fear behavior given a change in contingency (Lebron et al., 2004; Santini et al., 2004; Akirav et al., 2006).
Nonhuman primate studies have also linked the amygdala (Aggleton and Passingham, 1981; Zola-Morgan et al., 1991; Meunier et al., 1999; Kalin et al., 2001; Prather et al., 2001; Stefanacci et al., 2003; Izquierdo and Murray, 2004; Kalin et al., 2004; Mason et al., 2006; Antoniadis et al., 2007), hippocampus (Chudasama et al., 2008) and orbital frontal cortex (Butter et al., 1968; Butter et al., 1970; Butter and Snyder, 1972; Izquierdo et al., 2005; Kalin et al., 2007) to normal emotional reactivity, especially towards potential predators or a staring human. We also found that bilateral amygdala or orbital frontal lesions in monkeys altered behavioral responses to threatening social signals (Machado and Bachevalier, 2006).
Although much evidence exists to implicate the amygdala, hippocampus and orbital frontal cortex in facilitating appropriate fear-related behaviors and modulating physiological responses when danger is detected, several important questions remain unanswered. First, the effects of amygdala, hippocampal or orbital frontal lesions have never been studied simultaneously with respect to behavioral and hormonal reactivity to danger. Cross-species differences, along with small deviations in methods and inter-animal variability across laboratories can have a large impact upon the specific behavioral and physiological deficits observed following brain lesions (Kling, 1972; Kling and Steklis, 1976; Bachevalier and Málková, 2006). Second, the impact of frontal or temporal lobe lesions on physiological and behavioral reactivity to danger has rarely been investigated with knowledge of each animal's disposition before surgery (except Kalin et al., 2007). Without such knowledge, it is difficult to know with certainty whether group differences observed after surgery are due to the lesion or to or the animal's normal temperament.
To address these issues, we conducted two experiments with young-adult rhesus monkeys (Macaca mulatta) that measured the effects of amygdala, hippocampal or orbital frontal lesions on behavioral and hormonal reactivity to threatening stimuli. In Experiment 1, we used a well-established paradigm (Kalin and Shelton, 1989; Kalin et al., 1991; 2001; Izquierdo and Murray, 2004; Kalin et al., 2004; Mason et al., 2006; Kalin et al., 2007) to measure the effect of each lesion on behavioral reactivity to an unfamiliar human displaying either his profile or direct eye contact. The latter condition readily elicits fear, aggression and/or generalized tension, since direct eye contact is a highly threatening gesture among macaque monkeys. In Experiment 2, we measured the effects of each lesion on levels of circulating hormones associated with stress (cortisol) or social dominance (testosterone) in a baseline state and when animals were faced with physical restraint, temporary isolation, aversive objects or an unfamiliar conspecific.
2. General Methods
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center, Houston and carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used, as well as their pain and suffering. No alternatives currently exist for the in vivo techniques described here.
2.1. Animals
Subjects were 36 young-adult male rhesus monkeys (Macaca mulatta), weighing 3 - 6 kg and ranging between 2.4 and 3.3 years old at the beginning Experiment 1. Animals were randomly assigned to one of the following five experimental groups, which were balanced with respect to pre-surgical social dominance rank and age: Sham-operated control (C; n = 9), bilateral neurotoxic hippocampal lesion (H-ibo; n = 9), bilateral neurotoxic amygdala lesion (A-ibo; n = 9), bilateral neurotoxic orbital frontal lesion (O-ibo; n = 3) and bilateral aspiration orbital frontal lesion (O-asp; n = 6). Animals were housed individually at the University of Texas Medical School Animal Care Facility, given water ad libitum and fed daily with fresh fruit, vegetables and high-protein monkey chow (Lab Diet #5045, PMI Nutrition International Inc., Brentwood, MO). Animal housing rooms were maintained on a 12h light/dark cycle.
2.2. Neuroimaging
Magnetic resonance imaging (MRI) procedures have been detailed in four previous studies from our laboratory (Nemanic et al., 2002; 2004; Machado and Bachevalier, 2006; 2007b). Briefly, animals were maintained under general anesthesia throughout the scanning procedure. Two scanning sessions were performed with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI). The first session occurred 1 – 3 weeks before surgery, and included one T1-weighted structural scan (1 mm thick) and three Fluid Attenuated Inversion Recovery (FLAIR, 3 mm thick, each offset by 1 mm) scans. The second session occurred 7 – 10 d after surgery and included the same two MRI series. Pre-surgical T1-weighted images were used to select stereotaxic coordinates for neurotoxin injections (Saunders et al., 1990) or to visualize sulcal landmarks for orbital frontal cortex lesions. Post-surgical T1-weighted images were used to quantify the extent of orbital frontal cortex aspiration lesions (Group O-asp). Post-surgical FLAIR images were used to identify localized areas of edema indicative of neurotoxin-induced cell death, and were used to quantify lesion extent for Groups H-ibo, A-ibo and O-ibo (Málková et al., 2001; Nemanic et al., 2002).
2.3. Surgery
A detailed description of all surgical procedures can be found elsewhere (Nemanic et al., 2002; 2004; Machado and Bachevalier, 2006). Briefly, surgical procedures were performed under deep general anesthesia using aseptic techniques. Vital signs were monitored throughout the surgical procedure until the animal recovered fully from anesthesia. The scalp and connective tissue were incised and gently retracted together with the temporalis muscles. Each group then underwent lesion-specific procedures.
Neurotoxic hippocampal formation lesions were intended to damage all ammonic fields, the dentate gyrus, the prosubiculum and subiculum. Neurotoxic amygdala lesions were intended to damage all amygdaloid nuclei. For these two operated groups, small bilateral craniotomies were created above the injection sites and slits were cut in the dura bilaterally to allow the needle of the 10 μl Hamilton syringe, held by a Kopf electrode manipulator (David Kopf Instruments, Tujunga, CA), to be lowered to the appropriate injection coordinates. Two Hamilton syringes were filled with ibotenic acid (Biosearch Technologies, Novato, CA, 10 mg/ml in phosphate buffered saline, pH 7.4) and delivered the neurotoxin to each hemisphere simultaneously.
Orbital frontal cortex lesions (both ibotenic and aspiration) were intended to damage those areas of the ventral frontal cortex that are heavily interconnected with the amygdala (Amaral et al., 1992), namely areas 11 and 13 (as defined by Carmichael and Price, 1994). The bone of the supra-orbital ridge was opened, followed by incision and retraction of the dura. The surface landmarks used to approximate areas 11 and 13 were as follows. The anterior border was a line joining the anterior tips of the medial and lateral orbital sulci. The posterior border was a line joining the medial bank of the lateral orbital sulcus and the olfactory stria just anterior to its division into the medial and lateral olfactory tracts. The medial border followed the olfactory stria, and the lateral border followed the medial bank of the lateral orbital sulcus from its anterior tip to the posterior border of the lesion. For Group O-ibo, ibotenic acid was injected manually in a 2 mm × 2 mm square matrix within these borders. For animals in Group O-asp, 21 and 23 gauge suckers were used to aspirate the cortical tissue contained within these limits until the white matter beneath could be seen.
For sham lesions, bilateral craniotomies (similar to those used for hippocampal formation or amygdala lesions) were made. For eight of the nine cases, the dura was cut bilaterally, but no needle penetrations occurred. The remaining animal (case C-1-inj) was prepared to serve as a control animal for one of the hippocampal-operated animals that sustained inadvertent damage to the putamen. Case C-1-inj received ibotenic acid injections into the section of the putamen, which lies dorsal to the posterior one-third of the amygdala and the anterior one-third of the hippocampal formation.
Following these group-specific procedures, the wound was closed in anatomical layers and the animal was removed from anesthesia. During recovery from surgery, none of the animals displayed any changes in food and water consumption, or arousal state. Reduced locomotor behaviors and weakness of the limbs were temporarily observed in the two cases that sustained additional damage to the ventral putamen (i.e., cases C-1-inj and H-ibo-1, see Machado and Bachevalier, 2006). Because behavioral and hormonal results of case C-1-inj did not differ from Group C, its data were pooled with those obtained from the other animals in Group C.
2.4. MRI-based lesion evaluation
All lesions were evaluated using MRI techniques since 24 of the 36 animals used in these studies died in the flooding of Tropical Storm Allison in June 2001. These techniques provide an accurate estimate of cell loss following neurotoxic hippocampal lesions in nonhuman primates (Málková et al., 2001; Nemanic et al., 2002) and have been described in detail previously (Machado and Bachevalier, 2006, 2007a, 2007b). Lesion extent results for all animals have been previously reported (Machado and Bachevalier, 2007b), but see Table 1 for a summary of the extent of intended and unintended damage for each animal.
Table 1.
Intended and Unintended Damage for All Experimental Groups
| Cases | Hippocampal Formation |
Amygdala |
TH |
TF |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | |
| H-ibo-1 | 76.3 | 97.9 | 87.1 | 74.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-ibo-2 | 75.7 | 81.3 | 78.5 | 61.6 | 0 | 5.9 | 2.9 | 0 | 53.1 | 20.1 | 36.6 | 10.7 | 60.3 | 27.6 | 43.9 | 16.6 |
| H-ibo-3 | 67.5 | 74.1 | 70.8 | 50.0 | 0 | 0 | 0 | 0 | 26.7 | 15.3 | 21.0 | 4.1 | 29.9 | 44.0 | 37.0 | 13.2 |
| H-ibo-4 | 56.2 | 76.2 | 66.2 | 42.9 | 0 | 0 | 0 | 0 | 13.6 | 27.8 | 20.7 | 3.8 | 18.5 | 19.4 | 18.9 | 3.6 |
| H-ibo-5 | 98.8 | 99.3 | 99.1 | 98.1 | 0 | 0 | 0 | 0 | 15.2 | 15.9 | 15.6 | 2.4 | 38.8 | 8.5 | 23.7 | 3.3 |
| H-ibo-6 | 88.8 | 94.8 | 91.8 | 84.3 | 0 | 0 | 0 | 0 | 29.6 | 45.6 | 37.6 | 13.5 | 21.2 | 17.2 | 19.2 | 3.6 |
| H-ibo-7 | 12.7 | 59.0 | 35.9 | 7.5 | 0 | 11.0 | 5.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-ibo-8 | 79.8 | 98.4 | 89.1 | 78.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-ibo-9 | 91.0 | 89.4 | 90.2 | 81.3 | 12.9 | 1.0 | 6.9 | 0.1 | 0 | 0 | 0 | 0 | 0.9 | 1.3 | 1.1 | 0 |
| X | 71.9 | 85.6 | 78.7 | 64.3 | 1.4 | 2.0 | 1.7 | 0 | 15.4 | 13.9 | 14.6 | 3.8 | 18.8 | 13.1 | 16.0 | 4.5 |
| Cases |
Amygdala |
Hippocampal Formation |
ERh |
PRh |
||||||||||||
| L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | |
| A-ibo-1 | 20.6 | 82.2 | 51.4 | 17.0 | 10.6 | 1.6 | 6.1 | 0.2 | 0 | 1.8 | 0.9 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-2 | 48.9 | 88.1 | 68.5 | 43.1 | 1.2 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-3 | 27.1 | 73.1 | 50.1 | 19.8 | 15.7 | 13.6 | 14.6 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-4 | 79.1 | 92.5 | 85.8 | 73.2 | 3.4 | 3.0 | 3.2 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-5 | 88.7 | 91.3 | 90.0 | 81.0 | 1.5 | 0.1 | 0.8 | 0 | 0 | 5.5 | 2.8 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-6 | 70.3 | 90 | 80.2 | 63.3 | 21.1 | 10.3 | 15.7 | 2.2 | 0.8 | 0 | 0.4 | 0 | 0.1 | 0 | 0.1 | 0 |
| A-ibo-7 | 80.8 | 96.4 | 88.6 | 77.9 | 5.4 | 3.6 | 4.5 | 0.2 | 0.9 | 9.7 | 5.3 | 0.1 | 0.2 | 3.7 | 2.0 | 0 |
| A-ibo-8 | 29.6 | 44.3 | 37.0 | 13.1 | 0 | 0 | 0 | 0 | 0 | 3.6 | 1.8 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-9 | 43.9 | 75.2 | 59.6 | 33.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X | 54.3 | 81.5 | 67.9 | 46.8 | 6.5 | 3.6 | 5.1 | 0.5 | 0.2 | 2.3 | 1.2 | 0 | 0 | 0.4 | 0.2 | 0 |
| Cases |
Orbital Frontal Cortex (Areas 11 & 13) |
Area 12 |
Area 14 |
Ia |
||||||||||||
| L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | |
| O-ibo-1 | 37.1 | 19.9 | 28.5 | 7.4 | 2.3 | 0.9 | 1.6 | 0 | 49.0 | 23.8 | 36.4 | 11.7 | 37.0 | 25.3 | 31.2 | 9.4 |
| O-ibo-2 | 33.9 | 37.5 | 35.7 | 12.7 | 28.1 | 41.7 | 34.9 | 11.7 | 8.3 | 5.9 | 7.1 | 0.5 | 28.2 | 34.3 | 31.3 | 9.6 |
| O-ibo-3 | 43.0 | 47.3 | 45.2 | 20.3 | 9.9 | 20.9 | 15.4 | 2.1 | 25.4 | 11.7 | 18.5 | 3.0 | 44.9 | 38.0 | 41.4 | 17.0 |
| X | 38.0 | 34.9 | 36.5 | 13.5 | 13.4 | 21.2 | 17.3 | 4.6 | 27.6 | 13.8 | 20.7 | 5.1 | 36.7 | 32.5 | 34.6 | 12.0 |
| O-asp-1 | 88.4 | 95.3 | 91.8 | 84.2 | 3.9 | 28.9 | 16.4 | 1.1 | 15.2 | 21.7 | 18.5 | 3.3 | 20.6 | 21.1 | 20.9 | 4.3 |
| O-asp-2 | 83.0 | 92.0 | 87.5 | 76.3 | 7.0 | 9.3 | 8.2 | 0.7 | 10.7 | 5.9 | 8.3 | 0.6 | 21.4 | 23.7 | 22.6 | 5.1 |
| O-asp-3 | 90.3 | 87.6 | 88.9 | 79.1 | 4.5 | 10.2 | 7.4 | 0.5 | 0.7 | 0 | 0.3 | 0 | 5.5 | 5.9 | 5.7 | 0.3 |
| O-asp-4 | 86.8 | 85.8 | 86.3 | 74.5 | 21.5 | 30.1 | 25.8 | 6.5 | 1.0 | 1.2 | 1.1 | 0 | 37.5 | 28.3 | 32.9 | 10.6 |
| O-asp-5 | 82.5 | 84.1 | 83.3 | 69.4 | 17.5 | 25.6 | 21.5 | 4.5 | 0.7 | 1.4 | 1.0 | 0 | 12.2 | 9.8 | 11.0 | 1.2 |
| O-asp-6 | 94.4 | 97.4 | 95.9 | 92.0 | 7.7 | 16.5 | 12.1 | 1.3 | 6.6 | 7.9 | 7.3 | 0.5 | 8.8 | 7.7 | 8.2 | 0.7 |
| X | 87.6 | 90.4 | 89.0 | 79.3 | 10.4 | 20.1 | 15.2 | 2.4 | 5.8 | 6.4 | 6.1 | 0.7 | 17.7 | 16.1 | 16.9 | 3.7 |
Data are the estimated percentage of normal volume as assessed from MR images. Areas 11, 12, 13, and 14 – cytoarchitectonic subregions of the macaque frontal lobe as defined by Carmichael and Price (1994); Ia – agranular insular area as defined by Carmichael and Price (1994); ERh – entorhinal cortex and PRh – perirhinal cortex as defined by Amaral and colleagues (Amaral et al., 1987; Insausti et al., 1987); L – percentage of damage to the left hemisphere; R – percentage of damage to the right hemisphere; Avg – average of L and R; W = (L × R)/100 [weighted index as defined by Hodos and Bobko (1984)]; X – group mean.
3. Experiment 1: Behavioral reactivity to threat
Experiment 1 investigated the effects of selective hippocampal, amygdala and orbital frontal lesions on emotional reactivity to two levels of threat using the Human Intruder paradigm. For laboratory-housed rhesus monkeys, the presence of a human can have either reinforcing (i.e., food distribution) or punishing consequences (i.e., a hypodermic injection). If the human averts his or her gaze, as in the No Eye Contact (NEC) condition in the Human Intruder paradigm, the animal typically displays heightened vigilance and defensive freezing, as well as ceases exploration, self-directed activities and vocalizations (Kalin et al., 1991; 2001; 2004; 2007). Conversely, direct eye contact, whether from a human or another monkey, constitutes an unequivocal threat for rhesus macaques. The Stare (ST) condition in the Human Intruder paradigm typically results in defensive hostility, generalized tension, submission and/or retreat away from the intruder (Kalin et al., 1991; 2001; 2004; 2007).
3.1. Subjects
All animals participated in this experiment. No behavioral testing occurred before this experiment. Animals were assessed both before and after surgery. Between these two replications, animals received assessments of social behavior (Machado and Bachevalier, 2006), food preference (Machado and Bachevalier, 2007a; 2007b), their specific lesion surgery and testing in the Visual Paired Comparison task (Bachevalier and Nemanic, 2007). Approximately eight months intervened between the two replications.
3.2. Methods
Procedures for the Human Intruder paradigm were adapted from several previous studies (Kalin and Shelton, 1989; Kalin et al., 1991; 2001; 2004; Mason et al., 2006). Testing occurred during two consecutive days. The threatening stimulus before surgery was an unfamiliar human male. After surgery that same intruder's face was rendered unfamiliar by wearing a vinyl mask of a human male.
3.2.1. Apparatus
Animals were tested in a stainless steel cage (47 cm wide × 56 cm tall × 47 cm deep) attached to a hydraulic platform (Sugiyasu Corporation, Takaham, Aichi, Japan, model BX30S). The test cage was raised so that the animal was at the human intruder's eye level. This testing apparatus was located in a room adjacent to the animal housing quarters.
3.2.2. Testing procedures
On each day, the experimenter placed the animal in the test cage and left the room. Behavior was continuously recorded using a Sony Handycam video recorder (model # CCD-FX710) as the animal experienced five conditions. First, baseline emotional reactivity to the testing environment was recorded for 10 min while the animal remained alone (Pre-stimulus Alone). Next, the intruder entered the room and displayed his right profile to the monkey, without making direct eye contact, for 10 min (NEC). The animal was then left alone for 3 min. The intruder re-entered the room, but this time maintained direct eye contact with the animal for 10 min (ST). The animal was then left alone again for 10 min (Post-stimulus Alone) to assess its return to baseline reactivity. To prevent habituation to the intruder during the 10 min NEC and ST conditions, the intruder stood 2.5 m from the animal for the first 5 min and then moved to 1.25 m for the remaining 5 min.
To control for circadian effects on reactivity, all testing occurred between 0900h and 1500h. Animal testing order was generated randomly and that order remained consistent across the two replications. The presentation order for the NEC and ST conditions was counter-balanced across test days to control for any long-term effects of the ST condition on subsequent conditions. Video records of the Pre-stimulus Alone, NEC, ST and Post-stimulus Alone conditions were coded by one previously-trained observer using The Observer Video Pro software package (Noldus et al., 2000). Table 2 lists and defines all behaviors scored for this experiment. This ethogram is different from those used in previous reports from our laboratory (Meunier et al., 1999; Meunier and Bachevalier, 2002; Meunier et al., 2003; 2006). The number of specific behaviors has been expanded to detect subtle changes in behavior for each operated group. Several behaviors have also been grouped into different general categories. For example, we previously classified yawns as mildly aggressive, but in the current study this behavior is included in a general category of tension-related behaviors. While the categorization of macaque monkey behavior is inherently subjective, the current ethogram and behavioral categories were specifically designed to differentiate between aggressive or defensive behaviors that typically occur only in the presence of the human intruder (Defensive Behaviors) and behaviors that convey more generalized fear or anxiety and do not occur exclusively in the presence of a threatening stimulus (Tension Behaviors).
Table 2.
Behavioral Ethogram
| Behavior category & specific behavior |
Brief Definition |
|---|---|
| Defensive Behaviors | |
| Bark Vocalization | Low pitched, high intensity vocalization |
| Cage Aggression | Vigorous shaking of cage walls |
| Crooktail | Tail held in a “?” shape |
| Freezing* | Rigid, tense, motionless posture except slight head movements |
| Full Threat | Two or more of the following: open mouth stare, head-bobbing, ear flaps or lunges |
| Mild Threat | One of the following: open mouth stare, head-bobbing, ear flaps, or lunges |
| Tooth Grinding | Audible rubbing together of teeth |
| Tension Behaviors | |
| Coo Vocalization | High pitched, low intensity “oooooh” vocalization |
| Covert Look | The animal looks toward the camera from between his legs or under his arm |
| Fear Grimace | Exaggerated grin exposing teeth |
| Motor Stereotypy* | Repetitive, abnormal motor movements such as bucking, bouncing or twirling |
| Pacing* | Repetitive circular pacing around the test cage |
| Scratch* | Rapid scratching of body with hands or feet |
| Scream Vocalization | High intensity, high pitched vocalization |
| Yawn | Open mouth, exposing teeth |
| Affiliative Behaviors | |
| Groom Solicitation* | Shoulder, back, rump or flank held stationary towards stimulus |
| Grunt Vocalization | Low pitched, low intensity bubbly vocalization |
| Lipsmack | Rapid lip movement with pursed lips |
| Mount Solicitation* | Rump oriented towards stimulus, tail up and all four legs straight |
| Self-directed Behaviors | |
| Self-bite | Hair-plucking, self-biting, or other self-mutilation |
| Self-clasp | Abnormal grasping of the torso |
| Self-groom | Picking or licking at one's own fur or non-fur body part |
| Self-sex | Manual or oral manipulation of one's own genitals |
| Exploratory Behaviors | |
| Oral Exploration* | Oral manipulation of the test setting |
| Tactile Exploration* | Manual manipulation of the test setting |
| Vertical Positions | |
| Bipedal* | Standing on hind limbs, hands on walls or ceiling |
| Crouch* | Head on or near floor, front limbs bent |
| Hang* | Hanging on walls or ceiling, all limbs off floor |
| Sit* | Sitting with callosities on floor |
| Stand* | Four point stance, all on floor |
| Horizontal Positions | |
| Back* | Animal's head in the back half of the cage |
| Front* | Animal's head in the front half of the cage |
| Gaze Direction | |
| Look Away* | Animal's head is pointing in any direction other than forward |
| Look Forward* | Animal's head is pointing within 45° of the front-center of the test cage |
List of all behaviors recorded along with brief definitions. All behaviors were analyzed for frequency (total number of occurrences).
Behavior for which total duration was also measured.
NOTE: An additional general category of Facial Expressions was also analyzed, which included Lipsmack, Fear Grimace, Mild Threat and Full Threat.
3.3. Data analysis
Because the current study involved many factors and sample size for each lesion group was relatively small, we performed preliminary data analyses to ascertain if orbital frontal lesion method, the two alone conditions, the two test days per phase and the two intruder distances in the NEC and ST conditions had any profound effects on the data. To investigate if orbital frontal lesion method impacted differentially on Groups O-asp and O-ibo, these two groups were compared for all behaviors using General Linear Model ANOVAs with Group (2) as the between subjects factor and Phase (2; Pre- and Post-surgery) and Condition (4; Pre-stimulus Alone, NEC, ST and Post-stimulus Alone) as within subjects factors with repeated measures using the SPSS 12.0 statistical analyses package. A Huynh-Feldt correction was used to adjust the degrees of freedom if sphericity could not be assumed. No significant main effects of Group or interactions between Group and Phase or Group and Condition were found. Therefore, in the results section below, Groups O-asp and O-ibo are pooled into a single Group O.
Similar analyses were conducted with all four experimental groups to compare baseline emotional reactivity during the Pre-stimulus Alone and Post-stimulus Alone conditions (4 Groups × 2 Phases × 2 Conditions ANOVAs). Relative to testing before surgery, none of the groups demonstrated any profound changes in baseline emotional reactivity, position in the test cage or gaze direction during the Pre- and Post-stimulus Alone conditions. Therefore, data from these two conditions were averaged to create a single Alone (A) condition.
Measuring emotional reactivity to the human intruder across two testing days and at the two distances (2.5 m and 1.25m) were included to generate enough behavioral data to differentiate the lesion groups despite pronounced inter-animal behavioral differences. Therefore, data from test days 1 and 2 and from the 2.5 m and 1.25 m distances were summed in the A, NEC and ST conditions. The main comparison was therefore across these three conditions during the two testing replications to ascertain the effects of amygdala, hippocampal or orbital frontal lesions on modulation of emotional reactivity. Repeated-measures ANOVAs (4 Groups × 2 Phases × 3 Conditions) were again used to compare the groups. Significant main effects of Group were investigated using two-sided Dunnett tests to investigate differences between Group C and the three operated groups and Tukey tests when comparing the three operated groups. Significant interactions between factors were investigated with paired-sample t-tests and one-way ANOVAs. If any variable contained a majority of zero values within one or more groups (as was the case for Freezing), nonparametric versions of the preceding tests (such as the Wilcoxon signed-rank test and Mann-Whitney U test) were used. In all cases, statistical significance was set at p < .05. However, given the small number of animals in each experimental group and the heterogeneity of lesion extent across groups, we occasionally report results for which p values fall above this threshold. Results are identified as “marginally significant” if their p value is greater than .05 but less than .10. Pearson product moment correlation matrices were also generated to determine if the extent of damage to any brain region (intended or unintended) significantly influenced the behavioral parameters measured.
3.4. Results
The groups did not differ in gaze direction, position within the test cage or any behavior from the categories of Exploratory Behaviors, Affiliative Behaviors, Self-Directed Behaviors or Facial Expressions. Pre- versus post-surgery differences were detected for the frequency of behaviors associated with generalized tension, and the groups also differed in their modulation of these behaviors between the NEC and ST conditions.
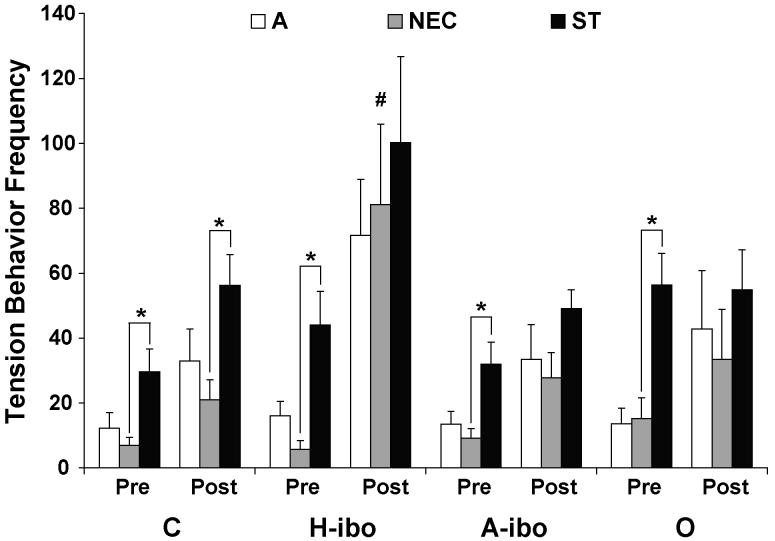
For Tension Behaviors, Groups C, H-ibo and A-ibo showed a significant increase between the pre- and post-surgery testing phases, but Group O did not [Group × Phase: F(3,32) = 2.909, p = .05; post-hoc: t = −3.359 for Group C, t = −2.707 for Group H-ibo and t = −3.491 for Group A-ibo, all ps < .05]. The groups also displayed differences in how they modulated Tension Behaviors across the three conditions [Group × Phase × Condition: F(6,64) = 2.729, p < .05; Figure 1]. The groups did not differ in the frequency of Tension Behaviors during the A, NEC or ST conditions, except that Group H-ibo displayed more of these behaviors than Group C in the NEC condition after surgery [F(3,35) = 3.135, post-hoc: Dunnett p < .05]. Before surgery all groups showed significantly more Tension Behaviors in the ST than in the NEC condition [pre-surgery NEC vs. ST: t = −4.269 for Group C, t = −3.471 for Group H-ibo, t = −4.216 for Group A-ibo and t = −4.869 for Group O, all ps < .05]. After surgery, only Group C showed this same modulation of Tension Behaviors between the two levels of threat (post-surgery NEC vs. ST: t = −3.608, p < .05).
Figure 1.
Total frequency of Tension Behaviors initiated by each group during the Alone (A), No Eye Contact (NEC) and Stare (ST) conditions when tested before (Pre) and after (Post) surgery. Vertical bars represent Standard Errors of the Mean. * p < .05, difference between NEC and ST conditions as indicated. # p < .05, difference relative to same condition for Group C.
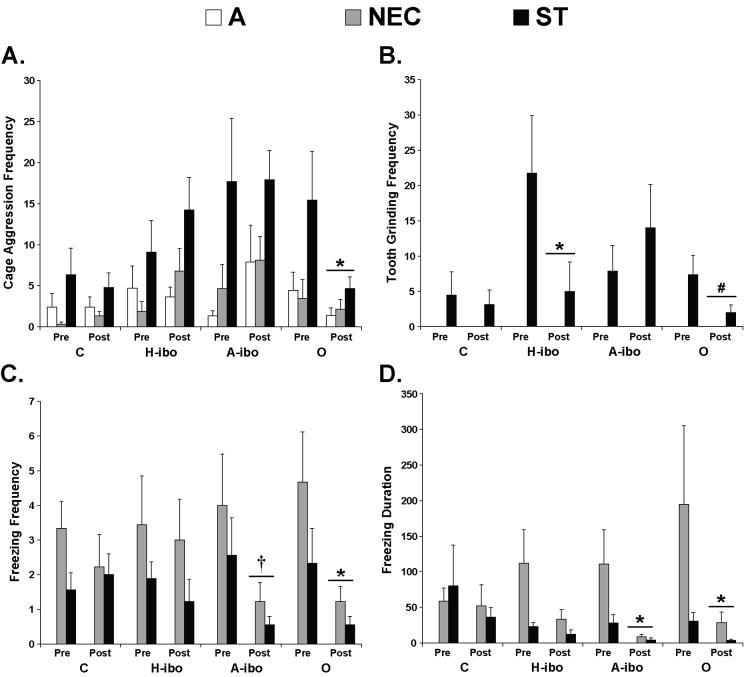
Three defensive behaviors were also differentially affected by the lesions, but those differences transcended the three conditions. After surgery, Group O initiated less Cage Aggression [Group × Phase: F(3,32) = 2.404, p = .086, post-hoc: t = 2.361; p < .05; Figure 2A], and both Groups H-ibo and O initiated less Tooth Grinding [Group × Phase: F(3,32) = 4.170, p < .05; post-hoc: t = 2.325, p < .05 for Group H-ibo and t = 2.219, p = .06 for Group O; Figure 2B]. By contrast, Groups A-ibo and O showed less Freezing after surgery across the three conditions [Frequency - Wilcoxon: Z = −2.371, p < .05 for Group O, Z = −1.862, p = .09 for Group A-ibo; Duration – Wilcoxon: Z = −2.521, p < .05 for Group O, Z = −1.955, p = .05 for Group A-ibo; Figures 2C & D].
Figure 2.
Average frequency of Cage Aggression (A), Tooth Grinding (B) and Freezing (C), along with the duration of Freezing (D) for each group during the A, NEC and ST conditions when animals were assessed before (Pre) and after (Post) surgery. Vertical bars represent Standard Errors of the Mean. † p = .09, # p = .06, * p < .05, mean of A, NEC and ST conditions relative to corresponding pre-surgery mean.
The only noteworthy correlations between behavioral reactivity and lesion extent were found in Group O. Across all three conditions, there was a negative correlation between intended damage to the orbital frontal cortex and the frequency of Tooth Grinding (r = −.879, p < .01). Similarly, a negative correlation was also detected for unintended damage to the agranular insular area and the frequency (r = −.809, p < .01) and duration (r = −.832, p < .01) of Freezing after surgery.
3.5. Summary
One advantage of the Human Intruder paradigm is that the animal's ability to modulate its behavioral reactivity depending on the magnitude of a threat (A < NEC < ST) can be measured. A second advantage built into Experiment 1 was to establish a baseline of emotional reactivity before surgery, to which post-lesion measurements could be compared. Before surgery, all groups showed modulation of tension-related behaviors between the NEC (low threat) to the ST (high threat) conditions. After surgery, only sham-operated control animals continued to demonstrate this ability.
Sham-operated monkeys also demonstrated consistent levels of most behaviors when assessed before and after surgery, especially those related to defense. Hippocampal lesions decreased tooth grinding, whereas amygdala lesions reduced the frequency and duration of defensive freezing. Orbital frontal cortex lesions also diminished intruder-directed defensive behaviors (cage aggression and tooth grinding) and freezing. Animals with orbital frontal lesions were also the only group that did not show increased tension-related behaviors after surgery.
4. Experiment 2 – Hormonal reactivity to threat
Experiment 1 demonstrated that the amygdala, hippocampus and orbital frontal cortex make distinct, yet complementary contributions to behavioral reactivity in the presence of threat. These behavioral modifications are mirrored by internal physiological changes. Therefore, the goal of Experiment 2 was to contrast the effects of each lesion on the regulation of hormones associated with stress (cortisol) and social dominance (testosterone).
4.1. Subjects
Due to the loss of many monkeys in Tropical Storm Allison in 2001, only a subset of the animals tested in Experiment 1 was available for Experiment 2 (cases 7 – 9 in Groups C, H-ibo and A-ibo, and cases 4 – 6 for Group O-asp). None of the animals in Group O-ibo participated in Experiment 2. The animals weighed 3.8 – 5.8 kg and were 2.9 – 3.3 years old at the beginning of Experiment 2. Between the pre-surgery phase of Experiment 1 and the pre-surgery component of Experiment 2, these monkeys received only food preference testing (Machado and Bachevalier, 2007b). The post-surgical conditions of this experiment occurred between three and 12 months after surgery in the order described below. Other post-surgical behavioral testing that occurred between, but not concurrently with these conditions included: Post-surgery replication of Experiment 1 (above) and post-surgical assessment of food preferences with the addition of primary reinforcer devaluation (Machado and Bachevalier, 2007b). All housing and feeding conditions were held constant throughout.
4.2. Methods
Blood samples were obtained while animals were either in their living quarters or in a rolling cage of similar dimensions. These cages were equipped with a squeeze-back panel that the experimenter could pull forward to restrain the animal and inject a chemical immobilizing agent (10-15 mg/kg; 100 mg/ml Ketamine Hydrochloride, IM) to expedite sample collection.
4.2.1. Baseline conditions
Baseline samples were collected approximately two weeks before and three months after lesion surgery. To control for circadian effects, sampling procedures always began at 1300h. On the day of sampling, animals received their normal morning feeding by 0800h, but no other behavioral testing occurred. The animals' housing room was not disturbed after 1100h to ensure that samples reflected resting levels as much as possible.
The experimenter and a veterinarian entered the housing room, calmly approached the animal, restrained it with the cage's squeeze mechanism and immobilized it with Ketamine Hydrochloride. The experimenter recorded the room entry time, the room temperature and the time when each animal was sedated. The veterinarian obtained a 2.5 ml blood sample via femoral vein puncture. The time when the veterinarian finished collecting the blood sample was also recorded. The blood sample was transferred to a 4 ml sterile Vacutainer tube (SSTTM Tube with Clot Activator and Gel; Becton, Dickenson & Co., Franklin Lakes, NJ) and allowed to clot at room temperature for at least 60 min before processing (see below).
4.2.2. Conspecific conditions
These conditions occurred approximately eight months after surgery. Hormonal responses to social interactions with an unfamiliar peer were measured under two conditions on different days. To control for hormonal responses to the testing apparatus itself, the first sample was taken after a 20 min exposure to a large rectangular arena (3.1 m long × 1.5 m wide × 2.0 m tall; see details in Machado and Bachevalier, 2006) used for social behavior testing (Social Enclosure Alone). Animals were previously trained to reliably enter and exit within 15 sec of the door opening. The second blood sample was taken after the animal received 20 min of free interactions with an unfamiliar member of Group C (Social Enclosure + Conspecific). The Social Enclosure Alone sample preceded the Social Enclosure + Conspecific sample by at least 6 d for all animals.
Both conditions began with the experimenter calmly entering the housing room (disturbance time was recorded) and transporting the participating animal(s) to the Social Enclosure. The animal to be sampled was released into the cage, followed by its unfamiliar partner (Social Enclosure + Conspecific condition only). Once each condition was completed, the animal was placed in a sample collection cage, immobilized and a blood sample was collected.
4.2.3. Aversive object conditions
These conditions occurred approximately 10 months after surgery. Blood samples to measure hormonal responses to aversive objects were collected under two conditions on different days. To control for hormonal responses to the testing apparatus, one sample was taken after 20 min of isolation in a Wisconsin General Testing Apparatus (WGTA Alone). In the WGTA + Aversive Object condition, a blood sample was taken after 20 min in the same apparatus with exposure to six unfamiliar, aversive objects. These two samples were separated by 3 – 4 d. The specific aversive items were: 1) Mr. Potato Head (plastic toy with large eyes, height = 20 cm; Hasbro, Inc., Pawtucket, RI), 2) Girl Doll (human-like facial features and hair, sitting height = 35 cm; Zapf Creation, Inc., Orlando, FL), 3) Handling Gloves (brown leather, used for nonhuman primate restraint), 4) Capture Net (1.75 m aluminum pole with brown nylon netting, used for nonhuman primate capture), 5) Mop (1.5 m wooden handle, cotton rope head) and 6) Rubber Snake (green and black, coiled, 20 cm diameter).
Both conditions began with the experimenter entering the housing room (disturbance time recorded) and transporting the animal to the WGTA in an adjacent room. Animals received six 3-min trials during which it could view the testing tray, each separated by 20-sec intervals where it could not see the tray. For the WGTA Alone condition, the tray was empty during all six trials. For the WGTA + Aversive Object condition, a different aversive object was positioned on the tray during each 3-min trial. The order of object presentation was generated randomly and balanced across Groups C and O-asp or across Groups H-ibo and A-ibo. The order of the WGTA Alone and the WGTA + Aversive Object conditions were also similarly counter balanced. At the end of both conditions, the animal was removed from the WGTA, placed into a sample collection cage, immobilized and a blood sample was drawn.
4.2.4. Physical Restraint condition
This final condition occurred approximately 12 months after surgery. The animal was placed in a primate restraint chair (Crist Instruments, Hagerstown, MD) and secured with the adjustable neck hole and padded head restraints. The experimenter recorded this disturbance time, rolled the animal to a dimly-lit adjacent room, and left the animal alone for 20 min. Once the 20 min had elapsed, the animal was placed into a sample collection cage, immobilized and a blood sample was drawn.
4.2.5. Sample processing and radioimmunoassays
Vacutainer tubes were centrifuged at 3000 RPM for 10 min (MSE Mistral 2000, GMI Inc., Ramsey, MN). Serum (750 μl) was placed into each of two plastic microcentrifuge tubes (Brinkmann Instruments, Inc., Westbury, NY); one for cortisol assay, one for testosterone assay. These samples were stored in a −80° C freezer (Harris Manufacturing Co., Asheville, N.C.) within 2 h of collection. Concentrations of cortisol and testosterone were measured by radioimmunoassay (RIA). Each serum sample was divided into two aliquots (25 μl each for cortisol and 50 μl each for testosterone) which were analyzed within the same RIA. The mean concentration in the two aliquots was used for statistical analyses. Cortisol concentrations (μg/dl) were obtained from an I125 RIA kit (Diagnostic Products Corporation, Los Angeles, CA; cat. # TKCO-2) with a lowest detectable dose of 0.5 μg/dl. Testosterone concentrations (ng/dl) were also measured with an I125 RIA kit (Diagnostic Products Corporation, Los Angeles, CA; cat. # TKTT2) with a lowest detectable dose of 1 ng/dl. The intra- and inter-assay variability for both hormonal analyses were within expected levels.
4.3. Data Analysis
All cortisol measurements were above the lowest detectable dose. Five testosterone measurements were below the lowest detectable dose (case C-7 – Pre- and Post-surgery Baseline; case C-9 – WGTA Alone; case H-ibo-8 – Post-surgery Baseline; case A-ibo-7 – Pre-surgery Baseline). These five data points were omitted from statistical analyses. Data from both assays were subjected to a Shapiro-Wilk test of normality. A majority of the data for each group were not normally distributed. All data were therefore log10 transformed prior to parametric statistical analyses, but non-transformed values were used for illustration purposes. Group differences were analyzed using repeated-measures General Linear Model ANOVAs (4 Groups × 2 or 3 Conditions) in SPSS. Post-hoc tests and correlations with lesion extents were conducted as for Experiment 1.
4.4. Results
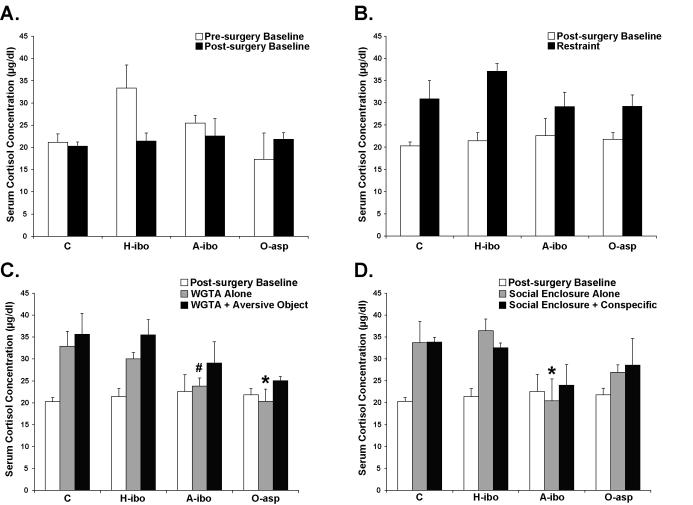
None of the lesions altered levels of testosterone under any conditions. Comparison of cortisol levels between the Pre- and Post-surgery Baseline samplings (Figure 3A) also did not produce any significant main effects of Group, Condition or interactions (all ps > .10). Similarly, all groups showed higher cortisol concentrations during the Restraint condition relative to the Post-surgery Baseline condition [Condition effect: F(1,8) = 22.912, p = .001, Figure 3B].
Figure 3.
Serum cortisol concentrations (μg/dl) for each group in the Pre- and Post-surgery Baseline conditions (A), Post-surgery Baseline and Restraint conditions (B), Post-surgery Baseline, WGTA Alone and WGTA + Aversive Object conditions (C) and Post-surgery Baseline, Social Enclosure Alone and Social Enclosure + Conspecific conditions (D). Vertical bars represent Standard Errors of the Mean. # p = .06 * p < .05, relative to same condition for Group C.
When the Post-surgery Baseline, WGTA Alone and WGTA + Aversive Object conditions were compared, Group O-asp demonstrated lower cortisol levels than Group C when all three conditions were collapsed [Group effect: F(3,8) = 3.749, p = .06; post-hoc: Dunnett p < .05, Figure 4C]. Inspection of the group means for each condition indicated that the blunted cortisol levels for Group O-asp and also Group A-ibo occurred primarily during the WGTA Alone and WGTA + Aversive Object conditions. However, this cortisol reduction for both groups relative to Group C reached significance only for the WGTA Alone condition [F(3,8) = 5.428, p < .05; post-hoc: Dunnett p = .06 for Group A-ibo and p < .01 for Group O-asp).
Comparison of the Post-surgery Baseline, Social Enclosure Alone and Social Enclosure + Conspecific conditions again revealed a significant main effect of Group. However, in this case, Group A-ibo demonstrated lower cortisol levels than Group C across all three conditions [Group effect: F(3,8) = 3.056, p = .09; post-hoc: Dunnett p < .05, Figure 3D]. A closer inspection of the group means for these three conditions revealed that Group A-ibo demonstrated lower cortisol levels relative to Group C primarily in the Social Enclosure Alone and the Social Enclosure + Conspecific conditions. Comparison of the four groups in each condition separately revealed that cortisol concentrations for Group A-ibo were significantly lower than Group C only in the Social Enclosure Alone condition [F(3,8) = 3.895, p = .06; post-hoc: Dunnett p < .05).
To investigate whether these results could have been influenced by differential durations of stress across the groups, the total disturbance time (time when blood sampling was completed – time when housing room was entered) and the total time required to collect the blood sample (time when blood sampling was completed – time when blood sampling started) were calculated (in sec) for each condition and compared across groups using one-way ANOVAs. We also calculated Pearson product moment correlation matrices for these times and testosterone or cortisol concentrations in each condition. There were no significant group differences for any of these variables or correlations between hormone concentrations and disturbance or sample collection times. There were also no significant correlations between intended or unintended damage and any hormone measure.
4.5. Summary
None of the lesions appreciably altered circulating levels of testosterone, whether measured at rest or after provocation with aversive situations. None of the lesions impacted significantly on resting levels of cortisol or impaired animals' ability to show elevated cortisol levels following physical restraint, exposure to aversive objects or an unfamiliar conspecific. By contrast, amygdala lesions resulted in lower cortisol concentrations (relative to sham-operated monkeys) following temporary isolation in a small testing apparatus (WGTA) and in a larger arena. Animals with orbital frontal lesions also demonstrated lower cortisol levels after being isolated, but only in the WGTA.
5. Discussion
This was the first study to compare the effects of amygdala, hippocampal or orbital frontal cortex lesions on both behavioral and hormonal reactivity to threatening stimuli in adult monkeys. Each lesion produced a distinct pattern of behavioral and hormonal deficits, indicating complementary roles for each structure in the regulation of appropriate responses to threat.
5.1. Marshalling appropriate behavioral responses to threat
Defending oneself requires accurate assessment of both the immediacy and potential severity of danger, followed by appropriate modulation of “fight or flight” behaviors. Experiment 1 measured the effect of three brain lesions on a range of behaviors when monkeys were faced with two levels of threat (i.e., averted gaze or direct eye contact). Within this context, none of the lesions appreciably changed the frequency or duration of facial expressions of emotion, affiliative gestures, self-directed behaviors, cage exploration, position in the cage or gaze direction. These observations for animals with neurotoxic amygdala or aspiration orbital frontal lesions are consistent with previous nonhuman primate studies that examined reactivity to humans (Meunier et al., 1999; Kalin et al., 2001; 2004; Izquierdo et al., 2005; Mason et al., 2006; Kalin et al., 2007), with one exception. Mason and colleagues (2006) found that, relative to control monkeys, animals with neurotoxic amygdala lesions spent more time at the front of the cage when confronted by a human intruder, especially during initial encounters. Two factors could account for this discrepancy. Compared to Mason and colleagues (2006), the amygdala lesions for our animals were, on average, less complete (group means = 67.9% for current study and 77.8% for Mason et al. (2006)). Further, our animals experienced the human intruder twice after surgery, whereas those studied by Mason and colleagues (2006) received six presentations. Admittedly, our design does not afford the same opportunity to detect changes over time. Although the amygdala, hippocampus and orbital frontal cortex do not appear to be responsible for the initiation of normal facial expressions of emotion, affiliative gestures, self-directed behaviors, cage exploration or gaze direction, the amygdala seems to play a role in recoiling or retreating away from danger. The orbital frontal cortex and hippocampus seem to play less of a role in establishing a safe distance from danger since we and others (Izquierdo et al., 2005;Kalin et al., 2007) have not observed any abnormal approach or avoidance behavior in response to direct eye contact following these lesions.
5.1.1. Regulation of tension and anxiety
Despite many normal aspects of behavior, the operated groups differed from control animals with respect to modulation of Tension Behaviors depending on the magnitude of threat (i.e., A < NEC < ST) and experience with the paradigm. This particular category includes behaviors that demonstrate fear, submission and generalized anxiety. Before surgery, all animals were capable of displaying significantly more tension-related behaviors when confronted with the staring human intruder than when alone or when the intruder averted his gaze. After surgery, animals with amygdala, hippocampal or orbital frontal cortex lesions lost this ability, but sham-operated animals did not. Although this inability to modulate tension has not been demonstrated before in the Human Intruder paradigm, it is again reminiscent of the study by Mason and colleagues (2006). Besides confronting animals with a staring human, monkeys with amygdala lesions also experienced several animal-like objects that formed a continuum of threat, from mild to strong. Animals with amygdala lesions showed lower levels of avoidance and longer duration of physical contact for all items, but control animals were more avoidant and wary of the most threatening items. The amygdala and orbital frontal cortex (Málková et al., 1997; Izquierdo et al., 2004; Machado and Bachevalier, 2007a; 2007b), but not the hippocampus (Machado and Bachevalier, 2007a; 2007b; Chudasama et al., 2008), also appear to modulate behavior towards rapidly changing positive (food reward) stimuli. Our results expand upon these previous findings and indicate that the amygdala, hippocampal formation and orbital frontal cortex each contribute a specific cognitive process essential for appropriate modulation of tension or anxiety in response to rapidly changing levels of threat.
When tension-related behaviors were compared across the two testing replications, control animals and those with amygdala or hippocampal lesions displayed a greater frequency after surgery. Animals with orbital frontal lesions were the only group that did not show this change with experience in the paradigm. Although this is a novel finding for the Human Intruder task, we reported previously that sham-operated monkeys and those with hippocampal or amygdala lesions show increases in anxious and fearful personality qualities in a social context after surgery. By contrast, animals with orbital frontal lesions did not demonstrate such changes (Machado and Bachevalier, 2006). Taken together, these findings indicate that in addition to rapidly regulating behavior depending on the level of an immediate threat, the orbital frontal cortex may also be critical for integrating past experience into the modulation of generalized tension or anxiety.
5.1.2. Regulation of defensive behaviors
Specific changes in defensive behaviors were also observed when all conditions were collapsed. Neurotoxic hippocampal lesions resulted in decreased tooth grinding after surgery. This audible defensive signal occurs when rhesus monkeys grind their large upper canine teeth on lower premolars to display and sharpen them. Since this is the first time monkeys with hippocampal lesions have been tested in the Human Intruder paradigm and these animals showed the highest levels of tooth-grinding before surgery (see Figure 2), this result should be taken with caution. However, hippocampal lesions are known to blunt defensive behaviors and increase tendencies to approach when monkeys are exposed to a fake snake (Chudasama et al., 2008). Rats with hippocampal lesions (especially the ventral component) also demonstrate reduced defensive behaviors and aggression when exposed to threatening stimuli and contexts (Gray and McNaughton, 1983; Blanchard et al., 2005; Pentkowski et al., 2006). It is possible that the decrease in defensive responses in each of these cases is related to the well-established role of the hippocampal formation in memory for previous contextual experiences (Eichenbaum, 2003). Alternatively, similar defensive changes could also result from the hippocampal formation increasing the weight of affectively negative information in cases of conflict (Gray and McNaughton, 2000). The paucity of controlled nonhuman primate studies in this domain warrants future attention, especially because these behavioral changes were not associated with significant changes in cortisol levels.
Amygdala lesions, on the other hand, decreased both the frequency and duration of defensive freezing. Neurotoxic amygdala lesions result in generalized deficits in defensive behavior when monkeys are exposed to direct human eye contact (Meunier et al., 1999) or to snakes (Meunier et al., 1999; Kalin et al., 2001; 2004; Izquierdo et al., 2005) . Diminished freezing was not observed following complete neurotoxic lesions of the amygdala in the Human Intruder paradigm (Kalin et al., 2001; Izquierdo et al., 2005) until neurotoxic lesions only included the central amygdaloid nucleus and adjacent portions of the basal forebrain (Kalin et al., 2004). The central nucleus has strong connections to the periaqueductal gray matter (Amaral et al., 1992), which is crucial for freezing behavior in rodents (Walker et al., 1997). The amygdala, and particularly the central nucleus, also has strong, reciprocal connections with the basal forebrain (Amaral et al., 1992). Glucose metabolism in the nonhuman primate basal forebrain correlates positively with the duration of freezing in the Human Intruder paradigm (Kalin et al., 2005). Basal forebrain lesions (Knox and Berntson, 2006) or inactivation (Fendt et al., 2003) also reduce conditioned and unconditioned freezing in rodents. The lesions for our animals targeted the entire amygdala, but may have also resulted in some inadvertent damage to the basal forebrain due to its proximity to the amygdala. Unfortunately, post-mortem histological analysis of lesion extent was not possible for all animals described here. Nevertheless, this confluence of findings from rodent and nonhuman primate studies emphasizes that the central amygdaloid nucleus and basal forebrain are critical neural substrates for inhibiting behavior in the face of danger.
Orbital frontal lesions produced the most diffuse pattern of behavioral changes. After surgery, animals with orbital frontal lesions showed fewer stimulus-directed defensive behaviors, namely cage aggression (rapid cage shaking to display dominance) and tooth grinding. The frequency of tooth grinding recorded after surgery also correlated negatively with the amount of damage to areas 11 and 13. These deficits are consistent with several previous experiments in monkeys with large ventral frontal lobe lesions that examined reactivity to a human intruder (Butter et al., 1968; 1970; Butter and Snyder, 1972), although these studies did not specifically report tooth grinding. Our results are also consistent with human functional neuroimaging studies demonstrating heightened orbital frontal activity to angry facial expressions (an unambiguous indicator of threat, similar to direct eye contact for macaques) relative to expressions of sadness (Blair et al., 1999). Monkeys with aspiration lesions of the orbital frontal cortex have also been reported to show heightened mild aggression (a category including frowning, ears back and yawning) in the Human Intruder paradigm (Izquierdo et al., 2005). Our results did not corroborate that finding. The most likely explanations for this discrepancy again relate to differing experimental design, extent of lesion and perhaps even animals' age at time of lesion. The study by Izquierdo and colleagues (2005) was conducted only after surgery, damaged areas 11, 13 and 14 of the orbital frontal cortex and included animals that were comparatively older (based on weight) than ours.
Animals with orbital frontal lesions, like those with amygdala lesions, also froze less after surgery. This finding is consistent with a recent report by Kalin and colleagues (2007) that also tested animals before and after surgery. Other similar studies (Butter et al., 1968; Izquierdo et al., 2005) measured emotional reactivity only after surgery and did not find freezing deficits for monkeys with orbital frontal lesions. It is possible that freezing deficits in the current study and in the experiment by Kalin and colleagues (2007) were significantly influenced by unintended damage to the agranular insular area (Carmichael and Price, 1994). We found significant negative correlations between damage to this cortical region and the frequency and duration of freezing after surgery. Damage to this posterior sector of the ventral frontal lobe occurred for all animals with orbital frontal lesions, but was especially profound for those that received ibotenic acid lesions (see Table 1). Similar damage to the agranular insular area is also apparent in the cases studied by Kalin and colleagues (2007; see Figure 1 in that report). Therefore, beyond indicating that the orbital frontal cortex is involved in the initiation of both stimulus-directed and passive defensive behaviors, our study may also indicate a regional specificity within the ventral frontal lobe for the mediation of defensive behaviors. Stimulus-directed defensive behaviors may be mediated by anterior orbital frontal regions, such as areas 11 and 13. Passive defensive behaviors, such as freezing, may be predominantly mediated by an inter-play between the agranular insular area and the amygdala, two areas that show robust and bidirectional anatomical connections (Amaral et al., 1992; Ghashghaei et al., 2007). This conclusion is supported by two recent meta-analyses of human functional neuroimaging studies indicating distinct neural networks involved in the perception, interpretation or generation of anger, disgust or fear (Murphy et al., 2003), as well as regional specificity within the orbital frontal cortex for interpretation of positive and negative reinforcers based on complexity or ambiguity (Kringelbach and Rolls, 2004).
5.2. Hormonal modulation in response to isolation, physical and emotional stressors
The main goal of Experiment 2 was to assess the impact of each lesion on a hormone related to social dominance (testosterone) and another associated with stress (cortisol) when animals were at rest and following a range of threatening situations. None of the lesions had any appreciable affect on testosterone levels under any condition. These results conflict with the rodent literature since neurotoxic amygdala lesions result in lower basal serum testosterone concentrations (Banczerowski et al., 2003). Although cross-species comparisons should be made with caution, the lack of significant lesion effects in our study could represent an important difference between rodent and primate regulation of testosterone. It is also possible that our study lacked sufficient statistical power (n = 3 in each group) to detect subtle changes in testosterone following each lesion. Our testosterone findings are consistent, however, with a previous study from our laboratory that did not find any changes in social dominance following selective amygdala, hippocampal or orbital frontal lesions (Machado and Bachevalier, 2006). However, the current study cannot reasonably rule out a role for the amygdala, hippocampus or orbital frontal cortex in regulation of testosterone in adult rhesus monkeys. Continued research in this area is certainly warranted.
As discussed above, the amygdala, hippocampal formation and orbital frontal cortex appear to play specific, yet complimentary roles in behavioral reactivity to stress. Previous rodent, nonhuman primate and human studies indicate that the amygdala and orbital frontal cortex may stimulate the HPA axis (Mandell et al., 1963; Frankel and Jenkins, 1975; Frankel et al., 1978; Feldman and Conforti, 1980; Gallagher et al., 1987; Feldman et al., 1995; Weidenfeld et al., 1997; Feldman and Weidenfeld, 1998; Kalin et al., 2004), whereas hippocampal formation is more critical for negative feedback (Mandell et al., 1963; Feldman and Conforti, 1976; 1980; Dunn and Orr, 1984; Sapolsky et al., 1984; 1991; Feldman and Weidenfeld, 2001; Goursaud et al., 2006). None of the lesions studied here had any appreciable affects on cortisol levels when animals were at rest or during three conditions presumed to be stressful for laboratory rhesus monkeys (physical restraint, social interactions with an unfamiliar partner and threatening objects). The lack of a hippocampal lesion effect seems to directly contrast with two previous studies in nonhuman primates. Sapolsky and colleagues (1991) found that aspiration lesions of the hippocampal formation resulted in hypersecretion of glucocorticoids after surgery. Similarly, Goursaud and colleagues (2006) demonstrated that neonatal neurotoxic hippocampal lesions diminished negative feedback control of HPA activity. At least six important differences in experimental design could explain why the current results do not concur with these previous studies. The current study and the previous two differed in lesion method, the age at which lesions occurred, the total number of animals per group, gender ratio within experimental groups and the time(s) after lesion when samples were collected. Any combination of these differences could account for the differing results. Admittedly, the current study was also limited in statistical power with only three animals in each group. This also may account for not finding an effect of hippocampal lesions. Nevertheless, the role of the primate hippocampus in HPA axis regulation appears to be extremely complex and deserves more systematic study.
Animals with amygdala or orbital frontal lesions demonstrated lower cortisol levels when temporarily isolated in a WGTA. A similar effect was produced by amygdala lesions when animals were isolated in a larger social behavior enclosure. These were unexpected results since the two “alone” conditions were included in the study simply as testing context control conditions and animals were familiar with both settings. Further, amygdala or orbital frontal lesions did not produce any appreciable behavioral changes during the Alone conditions of the Human Intruder task when animals were similarly isolated. For control animals and those with hippocampal lesions, being temporarily isolated in these enclosures was nearly as stressful as experiencing aversive objects or a novel conspecific in the same settings (see Figure 3C & D). Animals with amygdala or orbital frontal lesions produced significantly lower cortisol responses than control animals in each setting. Such effects of amygdala or orbital frontal lesions have rarely been reported in the nonhuman primate literature. Kalin and colleagues measured plasma cortisol concentrations in unoperated monkeys and animals with lesions of the amygdaloid central nucleus (2004) or the orbital frontal cortex (2007). In those studies, animals were physically restrained within a squeeze-back cage for 10 min in the presence of humans, but were then moved to a transport cage in a quiet room away from humans and other animals for an additional 20 min (N.H. Kalin and S.E. Shelton, personal communication). Similar to our results, Kalin and colleagues (2004) found that the amount of central nucleus damage was negatively correlated with cortisol levels in this stressful condition. However, orbital frontal lesions did not alter basal cortisol concentrations or levels measured after temporary isolation (Kalin et al., 2007). Our results replicate the null result in the baseline state, but provide new information that the orbital frontal cortex can exert some control over HPA axis functioning. As opposed to the amygdala, influence of the orbital frontal cortex may be context dependent, since we observed blunted cortisol reactivity for animals with orbital frontal lesions when they were isolated in a small testing enclosure, but not when isolated in a larger arena.
5.3. Concluding comments
The results presented here provide further evidence that the nonhuman primate amygdala, hippocampus and orbital frontal cortex all play a role in expressing normal fear and anxiety under threatening conditions. We have also shown that each of these three structures is essential for the normal regulation of anxiety in response to different magnitudes of danger. The amygdala and orbital frontal cortex also exert control over hormonal responses to stress, but the hippocampal formation may be more involved in negative feedback. These findings merge well with recent assessments of other forms of goal-directed behavior (i.e., food selection, social behavior, etc.) in rodents and monkeys following amygdala or orbital frontal lesions (see for review Murray, 2007; Murray and Izquierdo, 2007). Our interpretation of this converging evidence is that the amygdala, orbital frontal cortex and hippocampus are part of a specialized, and evolutionarily conserved, neural network that is charged with evaluating the meaning of external environmental stimuli with regards to current motivational state, level of threat and past experience. The output of this network thereby guides the selection of behavioral and physiological responses adaptively. Further study of this network, along with identifying additional components and their unique contributions, will greatly accelerate our ability to develop specific and long-lasting treatments for anxiety and affective disorders in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) J Comp Physiol Psychol. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J Comp Neurol. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. John Wiley & Sons, Inc.; New York: 1992. pp. 1–66. [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: Effects of chronic lesions in the rhesus monkey. J Neurosci. 2007;27:7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Málková L. The amygdala and development of social cognition: theoretical comment on Bauman, Toscano, Mason, Lavenex, and Amaral (2006) Behav Neurosci. 2006;120:989–991. doi: 10.1037/0735-7044.120.4.989. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2007 doi: 10.1002/hipo.20369. in press, DOI 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Banczerowski P, Csaba Z, Csernus V, Gerendai I. Lesion of the amygdala on the right and left side suppresses testosterone secretion but only left-sided intervention decreases serum luteinizing hormone level. J Endocrinol Invest. 2003;26:429–434. doi: 10.1007/BF03345198. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Central autonomic network: Functional organization and clinical correlations. Futura Publishing Company, Inc.; Armonk, NY: 1997. Functional anatomy of the central anutonomic network; pp. 29–60. [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev 2005. 2005;29(8):1243–53. doi: 10.1016/j.neubiorev.2005.04.019. Epub 2005 Aug 9 29: 1243-1253. [DOI] [PubMed] [Google Scholar]
- Butter CM, Mishkin M, Mirsky AF. Emotional responses toward humans in monkeys with selective frontal lesions. Physiology & Behavior. 1968;3:213–215. [Google Scholar]
- Butter CM, Snyder DR. Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiol Exp (Wars ) 1972;32:525–565. [PubMed] [Google Scholar]
- Butter CM, Snyder DR, McDonald JA. Effects of orbital frontal lesions on aversive and aggressive behaviors in rhesus monkeys. J Comp Physiol Psychol. 1970;72:132–144. doi: 10.1037/h0029303. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Cechetto DR, Saper CB. Role of cerebral cortex in autonomic function. In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. Oxford University Press; New York: 1990. pp. 208–223. [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.11.012. epub ahead of print; doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Orr SE. Differential plasma corticosterone responses to hippocampal stimulation. Exp Brain Res. 1984;54:1–6. doi: 10.1007/BF00235813. [DOI] [PubMed] [Google Scholar]
- Eichenbaum HB. Learning and memory: Brain systems. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. Academic Press; New York, NY: 2003. pp. 1299–1328. [Google Scholar]
- Feldman S, Conforti N. Inhibition and facilitation of feedback influences of dexamethasone on adrenocortical responses to ether stress in rats with hypothalamic deafferentations and brain lesions. Acta Endocrinol (Copenh) 1976;82:785–791. doi: 10.1530/acta.0.0820785. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N. Participation of the dorsal hippocampus in the glucocorticoid feedback effect on adrenocortical activity. Neuroendocrinology. 1980;30:52–55. doi: 10.1159/000122974. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Weidenfeld J. Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neurosci Biobehav Rev. 1995;19:235–240. doi: 10.1016/0149-7634(94)00062-6. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45:389–393. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. Electrical stimulation of the dorsal hippocampus caused a long lasting inhibition of ACTH and adrenocortical responses to photic stimuli in freely moving rats. Brain Res. 2001;911:22–26. doi: 10.1016/s0006-8993(01)02538-0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel RJ, Jenkins JS. Pituitary hormone response to brain stimulation in man. J Endocrinol. 1975;67:113–117. doi: 10.1677/joe.0.0670113. [DOI] [PubMed] [Google Scholar]
- Frankel RJ, Jenkins JS, Wright JJ. Pituitary-adrenal response to stimulation of the limbic system and lateral hypothalamus in the rhesus monkey (Macacca mulatta) Acta Endocrinol (Copenh) 1978;88:209–216. doi: 10.1530/acta.0.0880209. [DOI] [PubMed] [Google Scholar]
- Gallagher BB, Flanigin HF, King DW, Littleton WH. The effect of electrical stimulation of medial temporal lobe structures in epileptic patients upon ACTH, prolactin, and growth hormone. Neurology. 1987;37:299–303. doi: 10.1212/wnl.37.2.299. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud AP, Mendoza SP, Capitanio JP. Do neonatal bilateral ibotenic acid lesions of the hippocampal formation or of the amygdala impair HPA axis responsiveness and regulation in infant rhesus macaques (Macaca mulatta)? Brain Res. 2006;1071:97–104. doi: 10.1016/j.brainres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. Oxford University Press; Oxford, UK: 2000. [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions assoicated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context- dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kling A. Effects of amygdalectomy on socio-affective behavior in non-human primates. In: Eleftheriou BE, editor. Neurobiology of the Amygdala. Plenum; New York: 1972. pp. 511–536. [Google Scholar]
- Kling A, Steklis HD. A neural substrate for affiliative behavior in nonhuman primates. Brain Behav Evol. 1976;13:216–238. doi: 10.1159/000123811. [DOI] [PubMed] [Google Scholar]
- Knox D, Berntson GG. Effect of nucleus basalis magnocellularis cholinergic lesion on fear-like and anxiety-like behavior. Behav Neurosci. 2006;120:307–312. doi: 10.1037/0735-7044.120.2.307. [DOI] [PubMed] [Google Scholar]
- Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex or hippocampal formation lesions on established social relationships in monkeys. Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Measuring reward assessment in a semi-naturalistic context: The effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience. 2007a;148:599–611. doi: 10.1016/j.neuroscience.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007b;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Málková L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Lex CK, Mishkin M, Saunders RC. MRI-Based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Mandell A, Chapman L, Rand R, Water R. Plasma corticosteroids: Changes in concentration after stimulation of the hippocampus and amygdala. Science. 1963;139:1212–1214. doi: 10.1126/science.139.3560.1212. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys: Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J. Comparison of emotional responses in monkeys with rhinal cortex or amygdala lesions. Emotion. 2002;2:147–161. doi: 10.1037/1528-3542.2.2.147. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Meunier M, Cirilli L, Bachevalier J. Responses to affective stimuli in monkeys with entorhinal or perirhinal cortex lesions. J Neurosci. 2006;26:7718–7722. doi: 10.1523/JNEUROSCI.1949-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Nalwa V, Bachevalier J. Reactions to familiar and novel objects in infant monkeys with neonatal temporal lesions. Hippocampus. 2003;13:489–493. doi: 10.1002/hipo.10143. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann N Y Acad Sci. 2007;1121:273–296. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Noldus LP, Trienes RJ, Hendriksen AH, Jansen H, Jansen RG. The Observer Video-Pro: new software for the collection, management, and presentation of time-structured data from videotapes and digital media files. Behav Res Methods Instrum Comput. 2000;32:197–206. doi: 10.3758/bf03200802. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neurosci Biobehav Rev. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena dO, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Zola-Morgan S, Squire LR. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. J Neurosci. 1991;11:3695–3704. doi: 10.1523/JNEUROSCI.11-12-03695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: use for stereotactic neurosurgery. Exp Brain Res. 1990;81:443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behav Neurosci. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Walker DL, Cassella JV, Lee Y, De Lima TC, Davis M. Opposing roles of the amygdala and dorsolateral periaqueductal gray in fear-potentiated startle. Neurosci Biobehav Rev. 1997;21:743–753. doi: 10.1016/s0149-7634(96)00061-9. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Itzik A, Feldman S. Effect of glucocorticoids on the adrenocortical axis responses to electrical stimulation of the amygdala and the ventral noradrenergic bundle. Brain Res. 1997;754:187–194. doi: 10.1016/s0006-8993(97)00078-4. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]