Abstract
Background
The late durability of endovascular aneurysm repair (EVAR) has been limited by progressive aortic degeneration believed to be mediated by matrix metalloproteases (MMP). The goal of this study was to evaluate the effect of a MMP inhibitor, doxycycline, on EVAR.
Methods and Results
Patients undergoing EVAR were randomized to doxycycline (100mg twice daily) or placebo for 6 months following the procedure. Clinical data, blood samples and CT scans were obtained pre-operatively, post-operatively (blood only), and at 1 and 6 month follow-up. Forty-four subjects were analyzed based on intention-to-treat. Plasma MMP-9 decreased significantly below baseline in the doxycycline (N=20) treated patients at 6 months (−16.4±20.7%, P<0.05) while there was a non-significant increase in the placebo (N=24) group (128.1±73.5%). This was primarily related to changes between one and six months. In patients with endoleaks at 6 months, plasma MMP-9 increased in 83% of the placebo treated patients, but in only 14% of the doxycycline treated group (P<.03). Among endoleak-free patients with AneuRx or Excluder endografts, doxycycline treatment resulted in greater decreases in maximum aortic diameter than placebo treatment (−13.3±3.3% vs. −3.8±3.0%, P<.05). Furthermore, doxycycline treatment significantly reduced the aortic neck dilatation at six months in Excluder treated patients.
Conclusion
There is evidence of persistent MMP release representing ongoing aortic degradation after endografting which can be inhibited by doxycycline therapy. In analyses based on the endograft used, treatment with doxycycline also demonstrated evidence of increased aortic dimensional stability, a surrogate marker for long-term success of EVAR. Although encouraging, these results require confirmation in larger patient populations. Doxycycline should undergo more thorough evaluation as a potential adjuvant treatment to improve the results of EVAR, particularly in certain subgroups.
Abdominal aortic aneurysms (AAA) develop as a result of arterial wall matrix degeneration, and pose a significant risk for fatal rupture when the diameter exceeds 5.5 cm. The development of endoluminal exclusion of abdominal aortic aneurysms (EVAR) has become widely adopted as a means of reducing the risk of aneurysm rupture, but late loss of effective aneurysm exclusion and need for costly follow-up and reintervention has tempered the enthusiasm for the procedure.
Ongoing aortic wall degeneration and dilatation may result in failure of AAA exclusion. Studies in human tissue and animal models strongly suggest that enzymes of the matrix metalloprotease (MMP) family play a central role in the matrix degeneration of the aorta. There is evidence both in the animal model and in clinical trials that doxycycline, a known metalloprotease inhibitor, can inhibit AAA formation and progression.1–5
We hypothesized that the durability of endovascular aneurysm repair might be improved by inhibiting the process of aortic degeneration with adjuvant doxycycline therapy. We designed this study to determine the short-term effects of doxycycline on both radiographic and serologic markers of aneurysm degeneration and endograft stability.
Methods
Patient Enrollment
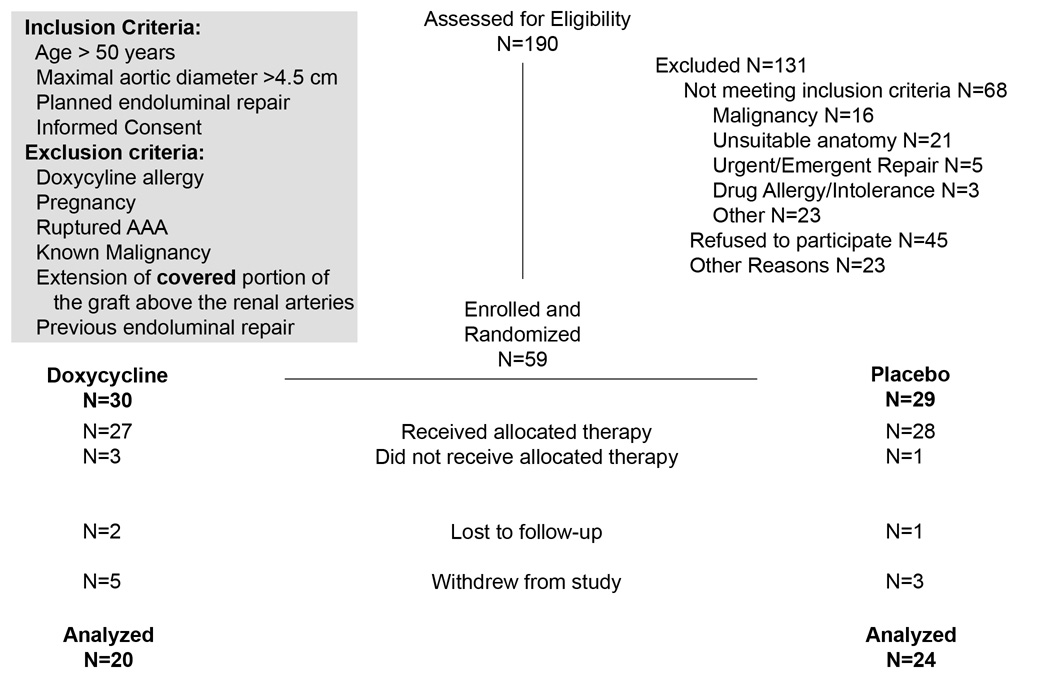
Patients were consented and enrolled at the time of their clinic visit or admission to the hospital for a planned EVAR under IRB guidelines. The inclusion and exclusion criteria are listed in Figure 1. Demographic, risk factor and medication regimen data was obtained from the patient’s clinical chart at the time of enrollment. Anticipated enrollment was 76 patients.
Figure 1. Study enrollment data.
Inclusion and exclusion criteria for enrollment are as listed at the conclusion of the study. During the first year of enrollment, the maximal aortic diameter necessary for enrollment was 5.0 cm; this resulted in most of the anatomic exclusions. After randomization, but before dispensing the study medications we had 4 withdrawals: 1 developed a postoperative ileus and was unable to take the medication. 1 patient had an open AAA repair, and 1 patient from each group decided not to accept the study medications after surgery and withdrew. After the medications were dispensed we had an additional 8 withdrawals before 6 months of follow-up, 1 Doxy and 1 Placebo patients withdrew claiming medication GI side effects, 4 developed other illnesses and declined further participation, and 2 withdrew without stating a reason, 1 person withdrew due to a change in insurance coverage and did not return to our facility, 1 patient in the placebo group died from complications of COPD and there was 1 patient whom we could not contact and was lost to subsequent follow-up.
Randomization and Follow-up
Patients were randomized to doxycycline therapy (100 mg taken twice daily) or a placebo which was identical in physical appearance. Enrollment of women and minorities was proportional to the frequency of the disease in these populations. Randomization was performed in the pharmacy utilizing a pre-assigned table of codes. The patients received their first dose of study medication on the day following surgery, and continued the study therapy for 6 months. Clinical data and peripheral blood samples were collected pre-operatively, immediately post-operatively and at scheduled 1 and 6 month follow-up visits. Cross-sectional imaging was obtained for all patients pre-operatively and at the one and six month follow-up visits.
GI side effects occurred with similar frequency in both the doxycycline (6) and placebo (4) treated patients, and only one patient from each arm withdrew as a result of these (P=NS). Photosensitivity developed in significantly more doxycycline treated patients (6 vs. 0, P<.01), however, was not severe enough to cause study withdrawal in any of the patients. One patient in the placebo group died of an unrelated illness (pulmonary) during the study period.
Assays for Circulating Biomarkers
Serum and plasma were separated into aliquots and stored at −80°C until assayed. Measurements of the circulating biomarkers were performed with commercially available assays for plasma MMP-9, plasma MMP-2, serum IL-6, and serum IL-8 (R&D Systems, Minneapolis). Analysis was performed in a multiplex bead analysis format (Liquichip, Qiagen). We also measured C-reactive protein (CRP) using a highly-sensitive ELISA (hs-CRP, Bio-Check, Burlingame, CA). The primary biomarker endpoint was the change in plasma MMP-9 concentration at 6 months compared to baseline. Secondary endpoints evaluated were the change in circulating MMP-2, IL-6, IL-8 and CRP at 6 months compared to baseline. Based on the pattern of circulating proteases seen during analysis of the data, we also elected to perform analysis of the effects of doxycycline treatment on the interval assessments.
Analysis of Imaging Data
Data were collected from the CT scans, by individuals blinded as to treatment group, regarding maximal aneurysm diameter and transverse neck diameter at the level of the most inferior renal artery and at 5 mm, 10 mm and 15 mm below that level. The mean of these four diameters was considered the neck diameter. “Centerline-of-flow” measurements were used to assess these diameters when digital data was available. All documented endoleaks and their type were recorded. The primary imaging endpoint was the change in maximal aortic diameter at 6 months. Secondary analysis was performed on the percent graft oversizing at the aortic neck and the presence or absence of endoleak at 6 months.
Statistical Analysis
Data are reported as the mean ± standard error of the mean (SE). Power calculations were performed a priori on the primary endpoints of MMP-9 for circulating markers and maximum aneurysm diameter which indicated analysis of about 40 individuals per group would be necessary to have a β-error of less than .1 with an α-error of less than .05. Data representing aortic diameters and measured plasma and serum values were analyzed for the normality of the data distribution with the Shapiro-Wilk W Test. Statistical analyses for non-normally distributed data sets (MMP-9, MMP-2, IL-6, IL-8 and CRP) were performed on log transformations of those data sets.6–8 Associations between plasma measurements and aneurysm size were analyzed with Pearson correlation coefficients. Comparisons of means were performed with one-way analysis of variance. Repeated measures MANOVA for change in measurements over time was performed where appropriate. The Chi-squared test was used to compare categorical data (Fisher’s Exact Test for average cell counts less than 5). A P<.05 was considered statistically significant.
We also identified unanticipated important effects on the imaging endpoints based on the endograft device used as well as the presence or absence of an endoleak. Therefore we performed post-hoc sub-group analyses of both the primary and secondary endpoints by the endograft device. Due to the small size of the female and minority groups in this study, meaningful independent statistical analysis of these groups was not informative.
Results
Patient Population and Baseline Studies
Patients were enrolled over a 2 year period and evaluated at 1 and 6 months after elective placement of an aortic endograft for an infrarenal AAA. Of the 59 patients who met the enrollment criteria and were randomized into the study, 44 subjects had either imaging or plasma marker data available at 6 months of follow-up, and were included in the data analyses (Figure 1). All patients with available data were analyzed by assigned treatment group regardless of compliance with the medication regimen. The demographic characteristics of those who were randomized and completed follow-up are listed in Table 1.
Table 1.
Demographics of Doxycycline Treated and Control Study Populations
| Doxycycline n=20 |
Placebo n=24 |
P-value | |
|---|---|---|---|
| Age (years) | 68.9±1.68 | 74.0±1.5 | 0.0249 |
| Gender (%Males) | 80% | 79.2% | |
| Body Mass Index (kg/m2) | 29.6±1.5 | 27.5±0.8 | |
| Hypertension | 18 (90%) | 19 (79.2%) | |
| Diabetes Mellitus | 2 (10%) | 3 (12.5%) | |
| Coronary Disease | 12 (60%) | 11 (45.8%) | |
| Inflammatory AAA | 0 | 1 (4.2%) | |
| Family History AAA | 1 (5%) | 3 (12.5%) | |
| Peripheral Vascular Disease | 8 (40%) | 7 (29.2%) | |
| Chronic Obstructive Pulmonary Disease |
6 (30%) | 10 (41.7%) | |
| Current Smoker | 13 (65%) | 7 (29.2%) | 0.0324 |
| Renal Insufficiency | 2 (10%) | 6 (25%) | |
| Intestinal Bleed | 4 (20%) | 3 (12.5%) | |
| Aortic Diameter (mm) | 57.2 ± 2.1 | 57.2 ± 2.4 | |
| Hemoglobin (g/dL) | 13.9 ± 0.5 | 13.9 ± 0.3 | |
| Creatinine (mg/dL) | 1.4 ± 0.3 | 1.3 ± 0.2 |
Although there were no significant differences between doxycycline and placebo treated subjects in the distribution of most of the demographic features, the patients in the doxycycline treatment group tended to be slightly younger and were more likely to be current smokers at the time of enrollment. Analysis of circulating markers and maximal aneurysm size did not demonstrate any significant association with either of these demographic features.
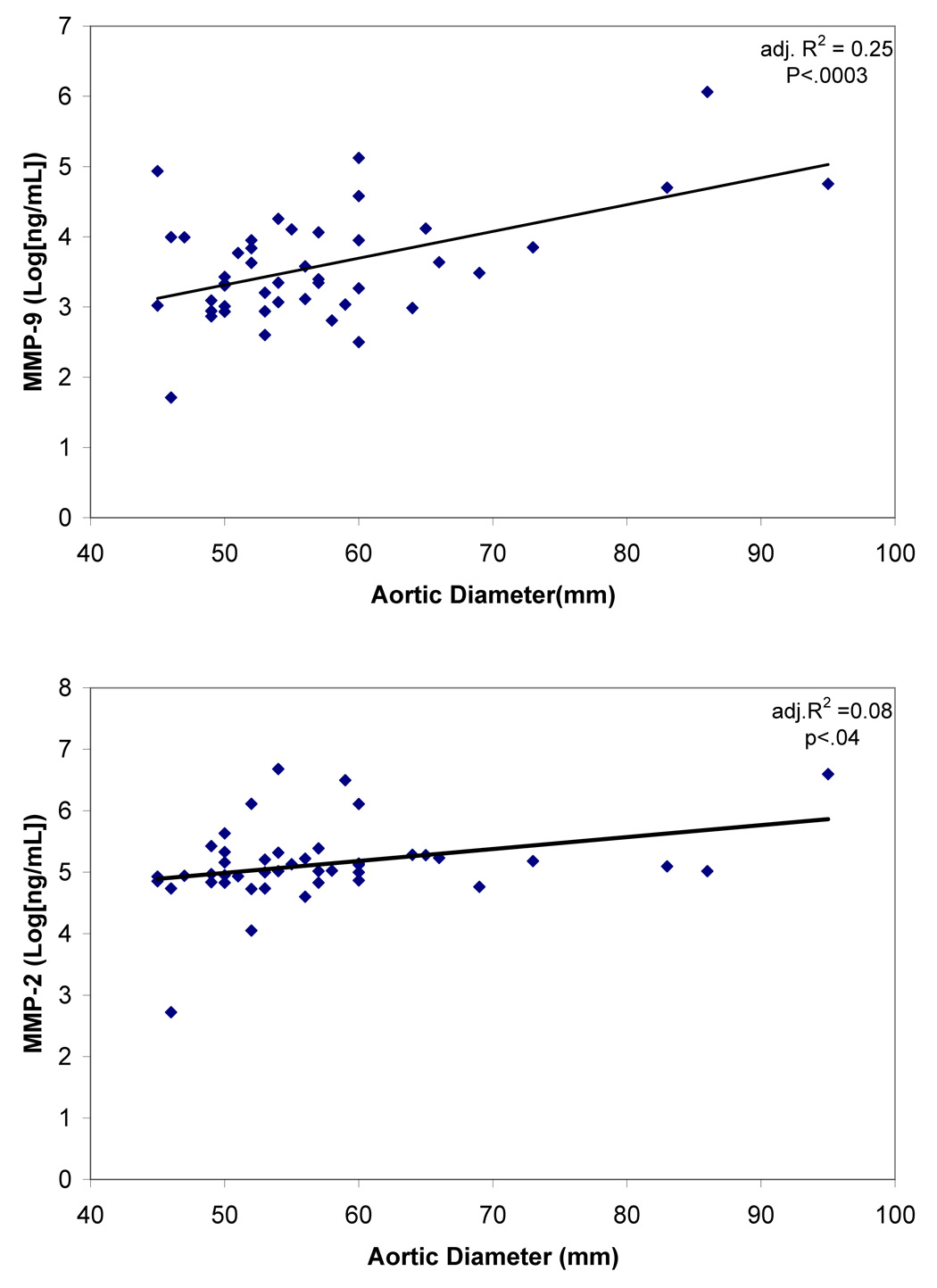
There was no significant difference in maximal aortic diameter or the diameters of the aorta measured at the “neck” of the aneurysm. There were also no differences in pre-operative circulating markers (Table 2). As shown in Figure 2, there was a significant positive correlation between the pre-operative maximum aortic diameter and the pre-operative plasma MMP-9 level (adj. R2=0.25, P<.0003), as well as a weak correlation with plasma MMP-2 (adj. R2=0.08, P<.04). We saw no similar correlation between aneurysm size and any of the other serologic markers measured.
Table 2.
Study Parameters at Baseline
| Doxycycline n=20 |
Placebo n=24 |
|
|---|---|---|
| Aortic Diameters (mm) | ||
| Maximum | 57.2±2.1 | 57.2±2.4 |
| At Lowest Renal Artery | 22.5±0.6 | 23.4±0.5 |
| 5mm inferior | 22.5±0.9 | 23.6±0.7 |
| 10mm inferior | 24.1±0.7 | 23.4±0.6 |
| 15mm inferior | 24.6±0.7 | 24.0±0.7 |
| Circulating Markers (pg/ml) | ||
| MMP-9 | 45380±15238 | 57860±13910 |
| MMP-2 | 199570±36919 | 211492±33702 |
| IL-6 | 3.8±3.1 | 6.6±2.9 |
| IL-8 | 1.9±1.1 | 3.0±1.0 |
| CRP | 56.0±13.3 | 47.5±12.2 |
Figure 2. Correlation between pre-operative MMP-9 and MMP-2 and maximum aortic diameter.
Although the adjusted correlation coefficient (adj. R2) for MMP-9 is considerably greater than that for MMP-2, circulating levels of both enzymes significantly correlate with the magnitude of aneurysmal dilatation.
The device used for the endoluminal repair was left to the discretion of the operating surgeon. Eight patients (18.2 %) were treated with an AneuRx endograft (Medtronic), 19 (43.2%) with Excluder (Gore) and 17 (38.6%) with Zenith (Cook). There were no significant differences in the device used and the baseline pre-operative aortic measurements.
Procedural and Peri-procedural Events
There were no significant differences in the proximal nominal diameter of the device used, procedural time, blood loss or post-operative clinical laboratory values between the subjects randomized to doxycycline or placebo (Table 3). Four patients required hypogastric artery occlusion. Two patients had a Type II endoleak documented on the completion arteriogram. There were no peri-operative mortalities, and one patient developed post-operative atrial fibrillation and myocardial ischemia evidenced by an increase in troponin.
Table 3.
Procedural parameters and peri-procedural demographic data
| Doxycycline n=20 |
Placebo n=24 |
|
|---|---|---|
| Hemoglobin (g/dL) | 11.7±0.4 | 11.1±0.3 |
| Creatinine (mg/dL) | 1.3±0.3 | 1.2±0.3 |
| Procedure Time | 2:44±0:16 | 3:05±0:16 |
| Proximal Device Diameter (mm) |
27.4±0.4 | 26.4±0.6 |
| Blood Loss (mL) | 350.0±64.0 | 406.3±51.6 |
| Fluids (mL) | 2400.0±209.1 | 2620.8±224.2 |
| Device Brand | AneuRx=3 (15%) Excluder=10 (50%) Zenith=7 (35%) |
AneuRx=5 (20.8%) Excluder=9 (37.5%) Zenith=10 (41.7%) |
Compared to baseline, on the day following EVAR, we found that there were significant increases in serum IL-8 (5.8±1.1 pg/ml vs. 2.5±1.1 pg/ml, P<.003), IL-6 (46.3±4.5 pg/ml vs. 5.3±4.4 pg/ml, P<.0001), and CRP (137.9±12.5 pg/ml vs. 51.4±12.3 pg/ml, P<.0001). The increase in MMP-9 (90.7±12.1 ng/ml vs. 52.2±11.9 ng/ml, P<.06) did not quite reach significance. There was no significant change in plasma MMP-2 levels, and there was no correlation between aneurysm size and post-operative plasma MMP-9 or MMP-2 levels.
Effect of Doxycycline Therapy on Endoleak and Maximum Aortic Diameter
For all patients, at one month, the mean AAA size was 57.1±1.5 mm and the median change in AAA diameter was 0.5 mm (mean: 0.02±0.6 mm). At six months of follow-up, the mean maximum diameter of the AAA in the study was 52.6±1.7 mm. Overall, the median absolute decrease in the aortic diameter at 6 months compared to pre-operatively was −2.6 mm (mean: −4.7±1.0 mm, P<.0001), and compared to 1 month was −4.0 mm (mean: −4.8±0.8 mm, P<.0001). The absolute changes in aortic diameter following endograft placement were dependant on the initial aortic diameters(adj. R2 = 0.34, P<.0005).9 At 1 and 6 month follow-up there were no identified Type I, III or IV endoleaks, and there were 11 patients at one month and 12 patients at 6 months who had Type II endoleaks identified.
When analyzed by treatment group, there was no significant effect of doxycycline on the primary outcome measure of change in maximal aneurysm size between baseline and 6 month measurements. There was also no effect of doxycycline therapy on the presence of an endoleak at 6 months, one of our secondary outcome measures.
It has recently been demonstrated that aneurysm shrinkage rates may be affected by the presence of an endoleak or the specific endograft chosen which may have obscured an effect of drug therapy.10 While our study was underpowered to identify effects of these factors, device-dependent differences in the maximal diameter change between 1 and 6 months were consistent with published comparisons,11 tending toward a greater decrease in AAA diameter for those patients treated with a Zenith endograft (−10.3±2.5%) than those with an AneuRx (−8.7±3.4%) or Excluder (−6.7±2.2%, P=NS).
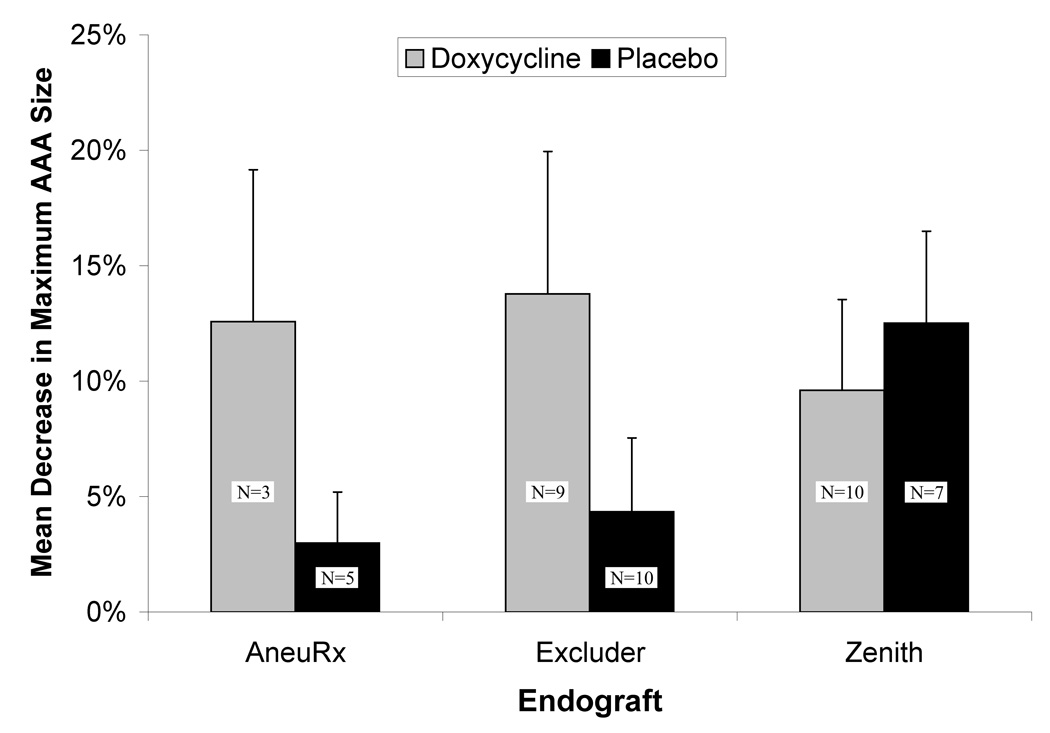
Based on these studies, we performed post-hoc subgroup analysis, excluding patients with the Zenith endograft or an endoleak at 6 months (Figure 3). We found that between 1 and 6 months the doxycycline treated patients who had either an AneuRx or Excluder placed had significantly greater decreases in aortic diameter than the placebo treated patients (−13.3±3.3% vs. −3.8±3.0%, P<.05).
Figure 3. Effect of doxycycline treatment on maximal aneurysm diameter change after endoluminal exclusion of an abdominal aortic aneurysm.
Change in maximal diameter was calculated as a percentage change in the maximum infrarenal aortic diameter between the 1 and 6 month CT scans. Patients with evidence of a Type II endoleak on the 6 month CT follow-up were excluded from this analysis. Doxycycline treatment resulted in a significantly greater decrease in maximal aneurysm size among the combined group of AneuRx and Excluder treated patients (P<.05).
Effect of Doxycycline Therapy on Aortic Neck Diameters and Graft Migration
The aortic neck size was expressed as the percentage by which the nominal proximal graft diameter exceeded the measured aortic diameter on imaging. The mean oversizing of the endograft at baseline overall was 15.2% and decreased to 8.3% at 6 months (P<.0001). We found no difference in the planned oversizing at baseline based on endograft used: 18.3±1.5%, 16.5±1.5% and 12.6±1.9% for the AneuRx, Zenith and Excluder grafts, respectively (P=NS).
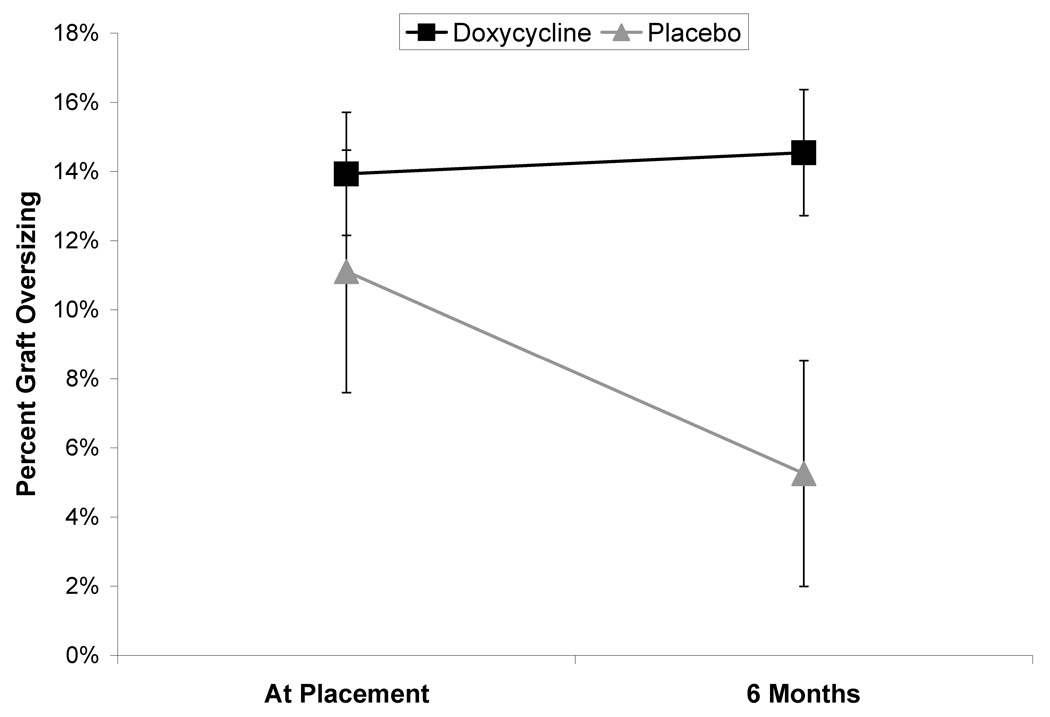
There was no overall difference in change in the mean graft oversizing at the aortic neck based on doxycycline therapy. At six months, in the placebo group, relative residual oversizing had been reduced to 8.6±3.1%, 7.6±2.2% and 5.3±3.3%, for AneuRx, Zenith and Excluder endografts respectively. This represented a significant increase in neck diameter over that interval (P<.0002) without any significant difference between devices. Among the patients treated with doxycycline, there was an overall significant increase in neck diameter over the course of the study (P<.02), however, there was also a significant difference based on the device used (P<.01). Specifically, we found that there was no difference in the mean residual oversizing at 6 months between subjects treated with either the Zenith (4.5±2.9%) or AneuRx (6.3±4.7%) grafts but significantly less dilatation of the neck in the Excluder treated patients (14.5±1.8%, P<.04). Among the individuals with an Excluder graft, there was a significant increase in neck diameter by 6 months in the placebo treated patients (P<.02), while there was no significant difference between baseline and 6 months with doxycycline treatment (Figure 4).
Figure 4. Effect of doxycycline treatment on aortic neck diameter among patients with Excluder endograft.
In the placebo treated group all aortic necks significantly increased in diameter between the pre-operative and 6 month CT scan measures. Among the patients treated with an Excluder endograft, doxycycline prevented this increase in aortic neck diameter. The treated patients demonstrated a significantly smaller increase in aortic diameter than the untreated patients for this sub-group (P<.05). Data is expressed as a percentage of the aortic neck diameter as measured on cross-sectional imaging relative to the nominal endograft diameter (percentage oversized).
No significant difference in the migration between the different endograft devices or based on treatment group was identified.
Effect of Doxycycline Therapy on Plasma Proteases
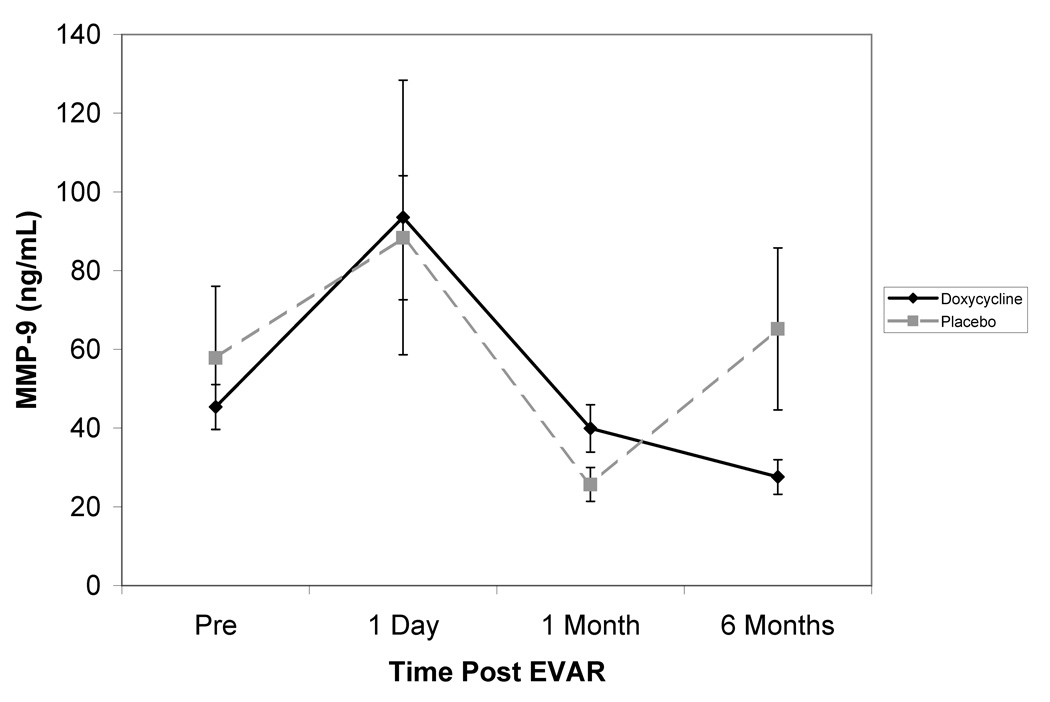
The primary endpoint with respect to circulating biomarkers was achieved in the doxycycline treated group where MMP-9 levels were found to decrease significantly from pre-operative levels (−16.4±20.7%, P<.03) at six months (Figure 5). There was no significant difference between pre-operative and 6 month MMP-9 levels among the placebo treated patients (128.1±73.5%).
Figure 5. Effect of doxycycline therapy on the circulating levels of MMP-9.
The mean levels of plasma MMP-9 are plotted before and at all follow-up intervals after placement of an aortic endograft. While the surgical procedure caused an initial increase in plasma MMP-9, these levels decreased over the first post-operative month in both groups. While plasma MMP-9 subsequently increased by 6 months in the placebo treated patients, doxycycline treatment resulted in a continued decrease of circulating MMP-9 in this interval. At 6 months plasma MMP-9 decreased significantly below pre-operative levels in the doxycycline treated group (P<.03), but not in the placebo treated patients. The overall change in MMP-9 during the study was significantly different between treatment groups (P<.009)
Following the initial increase in levels the day following endograft placement, there was an overall reduction in mean plasma MMP-9 levels for all patients between post-operative day 1 and 1 month (P<.0006). While between one and 6 months the mean MMP-9 levels in the placebo treated subjects significantly increased (206±98.5%, P<.04), in the doxycycline treated subjects, the plasma MMP-9 levels were stable or decreased (−11.8±12.5%, P<.06) – a significant difference (P<.009) between treatment groups. Although there was a generally positive correlation between the change in MMP-9 and the change in aortic diameter, this was not statistically significant.
In the placebo treated group, the presence of a Type II endoleak was associated with a greater likelihood of increased plasma MMP-9 levels at 6 months compared to baseline in patients without an endoleak (83% vs. 35%, P<.04). Treatment with doxycycline reduced the frequency of increase in MMP-9 associated with an endoleak to 14% (P<.03 vs. placebo), which was similar to the incidence without an endoleak (17%). Plasma MMP-2 levels, on the other hand, showed no significant difference based on treatment group or endoleak status, and were stable throughout the study period.
Effect of Doxycycline on Circulating Inflammatory Markers
Following the acute post-operative increase, the levels of IL-6, IL-8 and CRP all significantly decreased by 1 month. The serum levels of IL-6 and CRP were not significantly different than pre-operative levels at both one month (7.8±3.4 pg/ml and 76.8±11.5 pg/ml, respectively) and 6 months (4.5±1.8 pg/ml, 51.1±8.0 pg/ml, respectively), although the mean concentrations continued to drop in that interval. This pattern was identical for the doxycycline and placebo treated groups. The IL-8 levels remained significantly elevated at one month compared to pre-operative levels (6.6±1.4 pg/ml, P<.02). By 6 months the mean IL-8 levels were no longer significantly different (4.5±1.2 pg/ml). The responses of the treated and untreated patients were also similar with respect to IL-8.
Discussion
Endoluminal aneurysm repair relies on the radial force of the stent against non-dilated segments of aorta to maintain the proper apposition of the graft at its attachment sites. The loss of close apposition can result in the development of a leak into the aneurysm sac, re-pressurization of the sac, and risk of rupture. Continued degeneration of the proximal attachment site has been demonstrated after EVAR, and threatens the durability of the repair.12 Overall, long term follow-up has demonstrated the need for occasional secondary interventions after EVAR, although the frequency is likely shrinking due to modifications and improvements in endoluminal device design. Nevertheless, it continues to be necessary to follow these patients with serial imaging.13, 14
Matrix degrading enzymes of the MMP group, and especially MMP-9, are known to be present and active in aneurysm wall.15 Two studies have demonstrated that plasma MMP-9 is increased in the presence of an endoleak.8, 16 It has been proposed that doxycycline therapy may slow or halt the progression of small aneurysms because of its ability to inhibit enzymes of the MMP family.17 It currently is approved for use in gingivitis to reduce the activity of these enzymes on periodontal tissues.18, 19 Doxycycline can inhibit aneurysm formation in animal models,1–3 inhibit MMP activity and production in human aneurysm tissue,4 and is well tolerated in patients with AAA.5 An effective medical therapy, such as doxycycline, has promise to reduce or eliminate secondary interventions and thereby the need for imaging follow-up after endograft placement, potentially improving the cost-effectiveness of this procedure. This pilot study was designed to determine whether doxycycline is an effective means to reduce circulating MMP-9 levels, and accelerate aneurysm shrinkage following endografting.
In this study, the baseline levels of circulating MMP-9 were significantly correlated with aneurysm size, consistent with prior observations.20 Like IL-6, IL-8, and CRP, MMP-9 increased immediately following placement of the endograft from an apparent generalized inflammatory response which had essentially resolved by 1 month.21 Between 1 and 6 months following endograft placement the natural history of the mean plasma MMP-9 in the placebo treated patients was to increase. Remarkably, adjuvant therapy with doxycycline prevented the increase during this interval, and significantly reduced plasma MMP-9 below pre-operative levels at 6 months – one of the study’s primary endpoints.
As expected, in our placebo treated patients, the presence of an endoleak resulted in increasing levels of circulating MMP-9 significantly more frequently than in endoleak free patients. Treatment with doxycycline appeared to inhibit that effect, potentially signaling an inhibition of the matrix degradation response to the pressurization of the sac. Other markers of aneurysm degeneration including CRP, IL-8 and IL-6 did not appear to be significantly affected by doxycycline therapy.
The salutatory effects of doxycycline treatment on plasma MMP-9 levels may imply beneficial effect on aneurysm growth. However, none of the primary imaging endpoints was significantly affected by doxycycline treatment, overall. This may have been due to a lack of power related to not meeting our projected recruitment goals. There was also unanticipated variation based on the specific endograft placed.11 This study was not adequately powered to identify differences based on graft type.
We performed a post-hoc reanalysis of the data by endograft, in particular examining the effects on the patients not treated with a Zenith endograft. In those endoleak-free patients with either AneuRx or Excluder grafts, the sac size decrease with doxycycline therapy was significantly greater than placebo. This is encouraging, albeit weak, evidence suggesting that the effects of doxycycline on MMP-9 may be directly affecting the aneurysmal dilatation.
Potentially of even more clinical significance, we also found that doxycycline therapy can significantly reduce the progressive dilatation of the aortic neck. Others have demonstrated that progressive neck dilatation occurs after endografting22 (and open repair23), is device dependent22 and is associated with risk of migration22 and endoleak.24 In this study, among the placebo-treated patients there was progressive dilatation of the proximal neck of the aneurysm such that there was a significant decrease in the residual oversizing based on the nominal diameter of the endograft. The inhibition of this progressive dilatation in the Excluder patients treated with doxycycline may have been a consequence of lower radial force in that graft, allowing for small neck stabilization effects to be clinically evident.
These findings suggest that protease activity within the AAA wall continues despite endograft exclusion, and that doxycycline therapy may inhibit this degenerative process. The greatest response to doxycycline treatment was seen with the placement of the Excluder where there was evidence of accelerated aneurysm shrinkage and decreased proximal neck dilatation. This may be partially related to the covering material used with this endograft which may have transmitted more force to the aortic wall after graft implantation.25 Prior studies have demonstrated decreased aneurysm sac shrinkage and higher endoleak rates for this endograft, and the covering of this endograft was subsequently modified to address this.26 However, there is evidence that aneurysms treated with other endografts may undergo similar changes albeit at a more prolonged interval.13 Therefore, the beneficial effects of doxycycline therapy seen prominently with the Excluder device in this short study may also have the potential to benefit the durability of all endografts in the long-term.
This trial is the first to use doxycycline in conjunction with aortic endografting. Although the results are exciting, there are many limitations of this study design including, small size, single-institution enrollment, and short follow-up. The limited enrollment resulted in a diminished ability to assess our primary endpoints with adequate statistical power. Although we identified potentially important effects of doxycycline on the plasma protease MMP-9, the physical aneurysm effects demonstrated were based on post-hoc sub-groups. The short follow-up did not allow us to evaluate the clinically meaningful endpoint of secondary interventions.
Overall, these results demonstrate that circulating MMP-9 can be inhibited following endografting and that this may result in improved endograft performance. This study should serve as the basis for further well-controlled and designed studies to improve the treatment of AAA.
Acknowledgements
The authors wish to express their great appreciation for the study coordination assistance provided by Sandy L. Dolan, RN and the data organizational assistance provided by Daniel Roshevsky, BA
Supported by grants from the Barnes-Jewish Hospital Foundation (JAC), National Institutes for Health (5K08HL084004-02, JAC), Department of Veteran’s Affairs (JAC), Flight Attendants Medical Research Institute (JAC), and the American Heart Association (0765432Z, JAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competition of interest: none.
Clinical Trials Registration: NCT00126204; http://clinicaltrials.gov/show/NCT00126204
References
- 1.Boyle JR, McDermott E, Crowther M, Wills AD, Bell PR, Thompson MM. Doxycycline inhibits elastin degradation and reduces metalloproteinase activity in a model of aneurysmal disease. Journal of Vascular Surgery. 1998;27(2):354–361. doi: 10.1016/s0741-5214(98)70367-2. [DOI] [PubMed] [Google Scholar]
- 2.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. Journal of Vascular Surgery. 1996;23(2):336–346. doi: 10.1016/s0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 3.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, Sicard GA, Thompson RW. Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg. 2000;31(2):325–342. doi: 10.1016/s0741-5214(00)90163-0. [DOI] [PubMed] [Google Scholar]
- 5.Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, Upchurch GR, Jr, Chaikof EL, Mills JL, Fleckten B, Longo GM, Lee JK, Thompson RW. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. Journal of Vascular Surgery. 2002;36(1):1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 6.McMillan WD, Pearce WH. Increased plasma levels of metalloproteinase-9 are associated with abdominal aortic aneurysms. J Vasc Surg. 1999;Vol. 29:122–127. doi: 10.1016/s0741-5214(99)70363-0. discussion 127-9. [DOI] [PubMed] [Google Scholar]
- 7.Rohde LE, Arroyo LH, Rifai N, Creager MA, Libby P, Ridker PM, Lee RT. Plasma concentrations of interleukin-6 and abdominal aortic diameter among subjects without aortic dilatation. Arteriosclerosis, Thrombosis & Vascular Biology. 1999;19(7):1695–1699. doi: 10.1161/01.atv.19.7.1695. [DOI] [PubMed] [Google Scholar]
- 8.Lorelli DR, Jean-Claude JM, Fox CJ, Clyne J, Cambria RA, Seabrook GR, Towne JB. Response of plasma matrix metalloproteinase-9 to conventional abdominal aortic aneurysm repair or endovascular exclusion: implications for endoleak. Journal of Vascular Surgery. 2002;35(5):916–922. doi: 10.1067/mva.2002.123676. [DOI] [PubMed] [Google Scholar]
- 9.Fairman RM, Nolte L, Snyder SA, Chuter TA, Greenberg RK. Factors predictive of early or late aneurysm sac size change following endovascular repair. Journal of Vascular Surgery. 2006;43(4):649–656. doi: 10.1016/j.jvs.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Wolf YG, Hill BB, Rubin GD, Fogarty TJ, Zarins CK. Rate of change in abdominal aortic aneurysm diameter after endovascular repair. Journal of Vascular Surgery. 2000;32(1):108–115. doi: 10.1067/mva.2000.107754. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg RK, Deaton D, Sullivan T, Walker E, Lyden SP, Srivastava SD, Ouriel K, Ivanc T, Burton T, Mayo J. Variable sac behavior after endovascular repair of abdominal aortic aneurysm: analysis of core laboratory data. J Vasc Surg. 2004;39(1):95–101. doi: 10.1016/j.jvs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura JS, Chaikof EL. Continued expansion of aortic necks after endovascular repair of abdominal aortic aneurysms. EVT Investigators. EndoVascular Technologies, Inc. Journal of Vascular Surgery. 1998;28(3):422–430. doi: 10.1016/s0741-5214(98)70127-2. discussion 430-1. [DOI] [PubMed] [Google Scholar]
- 13.Conners MS, 3rd, Sternbergh WC, 3rd, Carter G, Tonnessen BH, Yoselevitz M, Money SR. Endograft migration one to four years after endovascular abdominal aortic aneurysm repair with the AneuRx device: a cautionary note.[comment] Journal of Vascular Surgery. 2002;36(3):476–484. doi: 10.1067/mva.2002.126561. [DOI] [PubMed] [Google Scholar]
- 14.Hobo R, Buth J. Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. Journal of Vascular Surgery. 2006;43(5):896–896. doi: 10.1016/j.jvs.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms: An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sangiorgi G, D'Averio R, Mauriello A, Bondio M, Pontillo M, Castelvecchio S, Trimarchi S, Tolva V, Nano G, Rampoldi V, Spagnoli LG, Inglese L. Plasma levels of metalloproteinases-3 and -9 as markers of successful abdominal aortic aneurysm exclusion after endovascular graft treatment. Circulation. 2001;104 12 Suppl 1:I288–I295. doi: 10.1161/hc37t1.094596. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RW, Liao S, Curci JA. Therapeutic potential of tetracycline derivatives to suppress the growth of abdominal aortic aneurysms. Adv Dent Res. 1998;12(2):159–165. doi: 10.1177/08959374980120011301. [DOI] [PubMed] [Google Scholar]
- 18.Crout RJ, Lee HM, Schroeder K, Crout H, Ramamurthy NS, Wiener M, Golub LM. The "cyclic" regimen of low-dose doxycycline for adult periodontitis: a preliminary study. Journal of Periodontology. 1996;67(5):506–514. doi: 10.1902/jop.1996.67.5.506. [DOI] [PubMed] [Google Scholar]
- 19.Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, Giannobile WV. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflammation Research. 1997;46(8):310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- 20.McMillan WD, Tamarina NA, Cipollone M, Johnson DA, Parker MA, Pearce WH. Size matters: the relationship between MMP-9 expression and aortic diameter. Circulation. 1997;96(7):2228–2232. doi: 10.1161/01.cir.96.7.2228. [DOI] [PubMed] [Google Scholar]
- 21.Gerasimidis T, Sfyroeras G, Trellopoulos G, Skoura L, Papazoglou K, Konstantinidis K, Karamanos D, Filaktou A, Parapanisiou E. Impact of endograft material on the inflammatory response after elective endovascular abdominal aortic aneurysm repair. Angiology. 2005;56(6):743–753. doi: 10.1177/000331970505600612. [DOI] [PubMed] [Google Scholar]
- 22.Tonnessen BH, Sternbergh WC, III, Money SR. Mid- and long-term device migration after endovascular abdominal aortic aneurysm repair: A comparison of AneuRx and Zenith endografts. Journal of Vascular Surgery. 2005;42(3):392–401. doi: 10.1016/j.jvs.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 23.Sonesson B, Resch T, Lanne T, Ivancev K. The Fate of the Infrarenal Aortic Neck After Open Aneurysm Surgery. Journal of Vascular Surgery. 1998;28(5):889–894. doi: 10.1016/s0741-5214(98)70066-7. [DOI] [PubMed] [Google Scholar]
- 24.Litwinski, Donayre, Chow, Song, Kopchok, Walot, White The role of aortic neck dilation and elongation in the etiology of stent graft migration after endovascular abdominal aortic aneurysm repair with a passive fixation device. Journal of Vascular Surgery. 2006;44(6):1176–1181. doi: 10.1016/j.jvs.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Haider S-e-a, Najjar SF, Cho J-S, Rhee RY, Eskandari MK, Matsumura JS, Makaroun MS, Morasch MD. Sac behavior after aneurysm treatment with the Gore Excluder low-permeability aortic endoprosthesis: 12-month comparison to the original Excluder device. Journal of Vascular Surgery. 2006;44(4):694–700. doi: 10.1016/j.jvs.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Ouriel K, Clair DG, Greenberg RK, Lyden SP, O'Hara PJ, Sarac TP, Srivastava SD, Butler B, Sampram ESK. Endovascular repair of abdominal aortic aneurysms: Device-specific outcome. Journal of Vascular Surgery. 2003;37(5):991–998. doi: 10.1067/mva.2003.170. [DOI] [PubMed] [Google Scholar]